Limbic System Function and Dream Content in University Students
Abstract
This study explored the relationship between limbic system function and threatening dream content. Recently it has been proposed that dreams are an evolutionary mechanism designed to facilitate the rehearsal of coping strategies in dangerous situations. It is known that the limbic system is active both during times of threat and during REM sleep. Therefore, it was hypothesized that individuals with relative limbic hyperfunction, as indexed by increased scores on the Limbic System Checklist (LSCL-33), would report more threatening dream content. The data of the present research confirmed the hypothesis.
A large body of evidence implicates the limbic system in emotional reactions. Apparently, this system is designed to attach emotional significance to environmental input and to generate adaptive responses to emotion-producing stimuli.1 Studies indicate that dysfunction of the limbic system is correlated with clinical disorders of mood and with anxiety states.2–4
Theories and models relating emotional experience to dreaming have had a long and varied history in psychology and psychiatry.5,6 It is surprising, therefore, that little research has been conducted on the putative connection of limbic system functions and dream content. It is known that limbic structures are highly active during rapid-eye movement (REM) sleep.3,7,8
A recent model that provides a novel and unique perspective on the functional significance of dreaming has proposed that dreams are readouts of evolutionary mechanisms designed to improve Darwinian fitness. The model suggests that human evolutionary history was filled with dangerous environments and, therefore, it presumably was adaptive to evolve a mechanism that allowed the rehearsal of threat-avoidance techniques prior to being forced to cope with dangerous situations. Dreams, it was proposed, allowed such coping rehearsals to occur in relatively safe circumstances.6
It has been reported that individuals living in stressful, traumatic, or dangerous environments are more likely to have recurrent dreams pertaining to threat and aggressive situations.9,10 Furthermore, such individuals tend to score highly on measures of mood disorders, such as depression, that are known to involve limbic mechanisms.9
Studies have shown that various limbic structures are differentially active during dreaming. Maquet et al.7 used positron emission tomography (PET) and statistical parametric mapping to ascertain which structures are active during REM sleep. Using 30 healthy, right-handed males, they demonstrated that, over three nights of observed sleep, the hippocampus was relatively inactive, whereas the cingulate gyrus and, especially, the amygdaloid complex demonstrated increased cerebral blood flow and elevated EEG activity. Braun et al.11 also used PET data, with 10 healthy male subjects, to determine that limbic and paralimbic structures are highly active during REM sleep. Amygdalofugal pathways to the right parietal operculum, entorhinal cortex, thalamic nuclei, dorsal mesencephalon, and pontine tegmentum were also found to be activated during REM sleep. These areas, in addition to the visual association cortices, were suggested to function as a “closed unit” during REM sleep.11
In terms of general limbic function, the hippocampus appears to be responsible for the processing of “cool,” episodic, explicit experiences, whereas the amygdala is active during emotional activity that is often implicit and tied to conditional fear and aggression responses.12 It appears that the amygdala and its connections with the hypothalamus and lower brainstem areas become engaged when an individual is under stress. This limbic structure also facilitates the organism's efficiency in processing threatening information and quickly initiates response mechanisms to successfully avoid threat. In other words, the “hot” emotional processing system that is activated during waking hours to assist in the detection of threat is also highly active during REM sleep.12,13
Recently, Teicher et al.14 published a noninvasive instrument designed to measure temporolimbic activity, the Limbic System Checklist (LSCL-33), which was based on functional features associated with this region. The questionnaire is a 33-item checklist measuring somatic, sensory, behavioral, and memory symptoms associated with temporolimbic abnormalities. These symptoms could be generally described as brief hallucinations, paroxysmal somatic disturbances, automatisms, and dissociative disturbances.14 The validity of the LSCL-33 was ascertained by comparing the scores of 10 persons with no history of diagnosed temporolimbic abnormalities with the scores of 10 subjects who had been diagnosed with, and were receiving effective treatment for, temporal lobe epilepsy. Most normal subjects had scores of less than 10, while patients with temporolimbic abnormalities scored between 33 and 60 on the checklist.14 The scores on the checklist were also correlated with other measures of limbic dysfunction, such as the Dissociative Experiences Scale and the Hopkins Symptoms Checklist. All correlations were 0.81 or greater.
The scores on the LSCL-33 were then correlated with stressful life events in a sample of 253 psychiatric patients. Teicher et al.14 found that limbic hyperfunction was significantly more prominent in adults who had been exposed to severe trauma in childhood. In addition, Ito et al.15 studied cortical development and hemispheric asymmetry in abused children. Their results supported the original conclusions of Teicher et al.14 that childhood abuse and other traumatic experiences might adversely effect the development of the maturing limbic system, producing LSCL-33 measures indicative of limbic hyperfunction.
The present study investigated the relationship between limbic functions, as indexed by LSCL-33 measures, and prevalence of fear-related dream content. It was hypothesized that limbic hyperactivity might be related to a greater incidence of such dream material, and also of recurrent dreams, allowing the dreamer to rehearse possible coping strategies for threatening circumstances.
METHODS
Participants
Five hundred and sixty introductory psychology students at St. Francis Xavier University, Canada, voluntarily completed the LSCL-33. Students with scores in the bottom quartile (scores: 4–19) or the top quartile (scores: 36–74) were asked to participate further in the study; 260 students met these criteria, 134 low scorers and 127 high scorers. Eighty-one of these students, comprising 38 (46.9%) from the upper quartile and 43 (53.1%) from the lower quartile, agreed to complete the remaining portion of the study. Of the 81 subjects, 28 were male and 53 were female, and all were between the ages of 18 and 24 years. No independent measures of psychiatric disturbances were obtained. Prior to the study, all participants were informed only that the study was concerned with possible connections between dream content and certain areas within the brain. No hypotheses or interests of the present study were divulged to subjects at any time throughout the experiment.
Procedures
Groups of up to six participants were scheduled to visit the laboratory. Participants were asked to provide a written answer to four questions:
| 1. | Do you ever remember your dreams? | ||||
| 2. | If yes, please write down the content of the last dream you remember, including as much detail as you feel comfortable providing. | ||||
| 3. | Do you ever have recurrent dream(s)? | ||||
| 4. | If yes, describe the content of your recurrent dream(s), including as much detail as you feel comfortable providing. | ||||
The dreams were collected and, under blind conditions, subjected to a content analysis to determine the degree of threatening content. The scoring was based on criteria developed by Gregor,16 modified from Hall and Van de Castle.17 Gregor studied the Mehinaku, an aboriginal ethnic group of Brazil whose daily living is reminiscent of ancestral times. The Mehinaku placed great value on the content of their dreams and developed the habit of recording and discussing them. Gregor was interested in determining if there were differences between the dream content of the average North American and that of a Mehinaku. He found that there were. As compared to Hall and Van de Castle's analysis of American dreams,17 there was significantly more threatening content in the dreams of the aborigines.16 Likewise, pleasant dream ideation was significantly reduced in the aboriginal sample population. Gregor's analysis classified the dreams by content and then coded them as containing either a) objective threat, b) subjective threat, c) peaceful activity, or d) none of the above.
For the dream to be classified as containing an objectively threatening event, the dreamer had to report an event that had the potential to decrease the probability of reproductive success for the dreamer or his or her kinship group or any member their community. Such events could include death or threat of death, injury or threat of injury, or the loss or destruction of social or physical resources. These dreams were fairly self-evident within our sample. One example of an objectively threatening dream is that of a young woman in our study who dreamed she was being stalked in her own home. Trying to escape her assailant, she and her younger brother sneak from room to room, heading for the front door. When they are almost there, the attacker traps them and then, screaming, the subject awakens. The lives of the subject and her brother are clearly threatened in this dream, and the threat therefore clearly has the potential to reduce the reproductive success of the dreamer and her family member.
Subjective threat consisted of events or feelings that did not reduce reproductive success but nonetheless inspired anxiety or fear in the dreamer. One subject in our study reported being chased down the street by his mother, who was brandishing his toothbrush and yelling at him to brush his teeth. The subject recalled feelings of deep anxiety as he ran away from his mother, stemming from an intense fear of brushing his teeth. While the anxiety he felt appeared to be real in the context of his dream, his mother was indeed unlikely to diminish his chances for reproductive success by insisting that he brush his teeth.
The most important difference between objective and subjective threat is that subjective threats cannot inhibit survival potential or reproductive success. By the same token, objectively threatening events must reduce survival capabilities. Whether this fear of death, injury, or social reprise comes from a realistic source, such as the threat of murder by a human being, or a fantastical source, such as a physical attack by a monster, is irrelevant. As long as the potential to hinder reproductive ability could be perceived as realistic and fear-invoking to the dreamer, even after he or she awakens, the dream is classified as objectively threatening.
Peaceful activity encompassed all activity that was judged to be pleasant and nonviolent. For example, one subject reported meeting her friend at the campus bar for a drink; another reported going fishing with his friends. The final category, “none of the above,” was designated only when a dream was unclassifiable into any of the categories listed above. It was not necessary to assign any of the dreams recorded in the present study to this category.
Interrater reliability for two judges was established for one-third of all dreams analyzed. In the total group of 81 subjects, 78 reported their most recent dreams and 51 reported recurrent dreams. The recent dreams of 30 subjects were randomly selected and analyzed for reliability; 18 of the 30 subjects also had recurrent dreams, and these dreams were assessed for reliability as well. These results were then averaged across the whole sample to produce a reliability coefficient. All disagreements were resolved through further discussion.
RESULTS
Interjudge reliability was 93%. A Pearson's chi-square test of independence was conducted to determine whether limbic system function, as measured by the LSCL-33, was related to the content of the last dream remembered by each subject. A high or low level of limbic functioning did not determine whether or not participants remembered the content of their dreams (χ2=0.631, df=1, not significant). However, there was a significant difference between those scoring in the upper and lower quartiles on the LSCL-33 in the reported frequency of the different categories of dream content (objective threat, subjective threat, and pleasant; χ2=7.672, df=1, P=0.02; Figure 1).
A detailed follow-up analysis to explore the specific relationships revealed that quartile placement on the LSCL-33 was related to the proportion of threatening dreams (χ2=7.537, df=1, P=0.006). The high LSCL-33 scorers reported 67.9% of the objective-threat dreams, whereas lower quartile scorers reported only 32.1% of those dreams. In addition, pleasant dream ideation was significantly related to quartile placement on the LSCL-33 (χ2=5.735, df=1, P=0.01): low scorers (66.7%), as compared with high scorers (33.3%), had more frequent pleasant dreams. The relationship between subjective threat and score on the LSCL-33 was not significant (χ2=0.952, df=1).
Results were similar for recurrent dreams. Although the likelihood of having recurrent dreams was not significantly different between groups (χ2=0.156, df=1), the content of such dreams did vary significantly by quartile placement (χ2=6.423, df=2, P=0.04; Figure 2).
Follow-up analysis was also completed for recurrent dreams, with similar results. Recurrent dreamers who scored on the upper end of the LSCL-33 were more likely to have objectively threatening (61.5%), as opposed to pleasant (12.5%), dreams (χ2=6.460, df=1, P=0.01). Low scorers, however, were more likely than high scorers to have pleasant content in their recurrent dreams (87.5% versus 38.5% respectively; χ2=4.221, df=1, P=0.04). Again, the relationship between subjective threat and limbic system function was not significant (χ2=0.899, df=1).
DISCUSSION
The hypothesis of the present study was generally confirmed. Limbic system functioning did appear to be related to the reported threatening or pleasant content in the dreams of the participants. Upper quartile scorers on the LSCL-33 had more objective threat and less pleasant content in their dreams. The inverse was found for the lower quartile scorers, who demonstrated more pleasant and less threatening dream content. However, the relationship between subjective threat and limbic function was not significant.
It was predicted that recurrent dreams would be more prevalent among upper quartile participants. This hypothesis was not supported by our data; the proportions of recurrent dreamers in the two groups were relatively similar. Robbins and Houshi9 have suggested that a significant number of recurrent dreamers have clinical disorders of the limbic system; however, in our study the relationship between recurrent dreams and quartile placement on the LSCL-33 was not significant. One possibility is that the participants in the present study did not score high enough on the LSCL-33 to demonstrate this relationship. Even the upper quartile scorers were generally within the range of normal scores. It is possible that such a relationship between recurrent dreams and scores on the LSCL-33 might be detected within a clinical sample. Replication of the current study within a clinical population would therefore be relevant.
The present findings also indicate an absence of a relationship between limbic functioning and subjective threat. Subjective threat refers to an event that is perceived by the dreamer as unpleasant or fear-invoking but that has no consequence for evolutionary fitness (such as a subject's dream that his mother was pursuing him to get him to brush his teeth). On the other hand, subjects with limbic hyperfunction were significantly more likely to have dreams that related to objective threat, that is, fears related to interference with reproductive success (such as another subject's dream of being stalked in her own home). The finding that limbic hyperfunction predicts only objective threat, and not subjective threat, in dream content suggests that differential limbic activity is primarily related to the rehearsal of coping strategies in circumstances of evolutionary relevance. If threatening events or feelings exist in the dreams of an individual with relative limbic hyperfunction, they tend to be threats that have evolutionary consequences.
Overall, the results of this study support the idea that the limbic system plays an active role in the generation of specific types of dream content. The results indicate that individuals with relative limbic hyperfunction have dreams in which objective-threat–related content is highly prevalent and pleasant dream content greatly diminished. Numerous studies with various species have shown that the limbic system is highly active in situations that have threatening connotations.9 The limbic system is also known to be active in REM sleep.5,10 In addition, during REM sleep limbic structures are active as a part of a closed-loop system, disengaged from overt behavioral mechanisms.5,10,16 This system would make possible the realistic rehearsal of threatening situations without active bodily responses.
Revonsuo6 suggested that dreams might function as a rehearsal mechanism, wherein threatening situations are rehearsed mentally during sleep in order to increase the organism's efficiency and vigilance in dealing with potentially life-threatening situations. In the presumably danger-ridden environment of our evolutionary ancestors, such a mechanism would have the potential to increase Darwinian fitness because the ability to deal with threatening situations would be enhanced.
This study examined the proposition that limbic involvement in the generation of dream content is beneficial to Darwinian fitness. However, it is possible that in some cases limbic system activity may be dysfunctional. Abnormal limbic activity, possibly related to the kindling effect, has been related to various mood disorders, clinical anxiety states, and psychotic states2–4 that are clearly detrimental to Darwinian fitness.
Our data suggest that limbic system activity, as inferred from LSCL-33 scores, predicts the relative prevalence of such objective-threat-related dreams. Limbic hyperfunction, either due to specific environmental influences, genetic predisposition, or both, would be expected to produce a greater proportion of such threatening dream experiences. The results of the present study provide preliminary support for this conclusion.
Whether or not the apparent relationship between limbic functions and dream content is based on evolutionary adaptations is unclear at this point. Further study may help clarify this issue. In any event, the present data support the idea of a connection between limbic mechanisms, implicated by a large body of evidence in fear and anxiety states, and threat-related dreams in university students.
 |
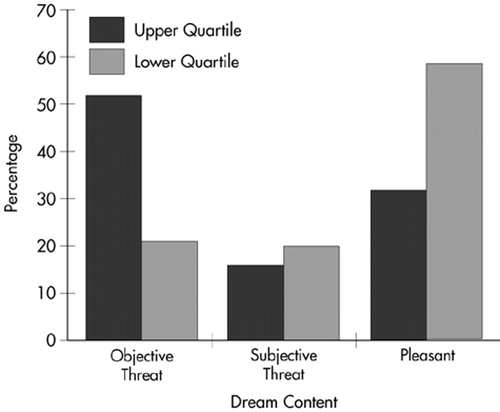
FIGURE 1. Last dream remembered: percentage of objective threat, subjective threat, and pleasant content in last dream remembered in upper and lower quartile LSCL-33 scorers.
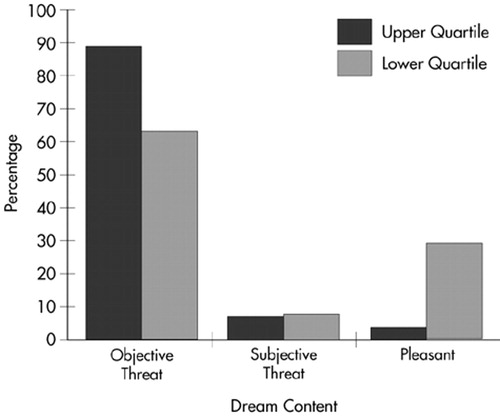
FIGURE 2. Recurrent dreams: percentage of objective threat, subjective threat, and pleasant content in recurrent dreams in upper and lower quartile LSCL-33 scorers.
1 Bear D: Hemispheric asymmetries in emotional function: a reflection of lateral specialization in cortical limbic connections, in The Limbic System: Functional Organization and Clinical Disorders, edited by Doane B, Livingstone K. New York, Raven, 1984, pp 29-42Google Scholar
2 Byrum C, Thompson J, Heinz E, et al: Limbic circuits and neuropsychiatric disorders: functional anatomy and neuroimaging findings. Neuroimaging Clin N Am 1997; 7:79-98Medline, Google Scholar
3 Nofzinger E, Nichols T, Meltzer C, et al: Changes from forebrain function from waking to REM sleep in depression: preliminary analyses of [18F]FDG PET studies. Psychiatry Res Neuroimaging 1999; 19:59-78Crossref, Google Scholar
4 Doane B: Clinical psychiatry and the physiodynamics of the limbic system, in The Limbic System: Functional Organization and Clinical Disorders, edited by Doane B, Livingstone K. New York, Raven, 1984, pp 285-317Google Scholar
5 Berger L: Function of dreams. J Abnorm Psychol 1967; 72:1-28Crossref, Medline, Google Scholar
6 Revonsuo A: The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav Brain Sci 2000; 23:877-901Crossref, Medline, Google Scholar
7 Maquet P, Peters J, Aerts J, et al: Functional neuroanatomy of human rapid eye movement sleep and dreaming. Nature 1996; 383:163-166Crossref, Medline, Google Scholar
8 Grozinger M, Roschke J: Recognition of rapid eye movement sleep from single-channel EEG data by artificial neural networks: a study in depressive patients with and without amitriptyline treatment. Neuropsychobiology 1996; 33:155-159Crossref, Medline, Google Scholar
9 Robbins P, Houshi F: Some observations on recurrent dreams. Bull Menninger Clin 1983; 47:262-265Medline, Google Scholar
10 Zadra A, O'Brien S, Donderi D: Dream content, dream recurrence, and well being: a replication with a younger sample. Imagination, Cognition, and Personality 1998; 17:293-311Crossref, Google Scholar
11 Braun A, Balkin J, Westenfaud J, et al: Dissociated patterns of activity in visual cortices and their projections during human rapid eye movement sleep. Science 1998; 279:91-99Crossref, Medline, Google Scholar
12 Metcalfe J, Jacobs W: Emotional memory: the effects of stress on “cool” and “hot” memory systems. The Psychology of Learning and Motivation 1998; 38:187-222Crossref, Google Scholar
13 Henke PG: Stomach pathology and the amygdala, in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, edited by Aggleton JP. New York, Wiley-Liss, 1992, pp 323-339Google Scholar
14 Teicher M, Glod C, Surry J, et al: Early childhood abuse and limbic system ratings in adult psychiatric outpatients. J Neuropsychiatry Clin Neurosci 1993; 5:301-306Link, Google Scholar
15 Ito Y, Teicher M, Glod C, et al: Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. J Neuropsychiatry Clin Neurosci 1998; 10:298-307Link, Google Scholar
16 Gregor T: A content analysis of Mehinaku dreams. Ethos 1981; 9:353-390Crossref, Google Scholar
17 Hall CS, Van de Castle GW: The Content Analysis of Dreams. New York, Appleton-Century-Crofts, 1966Google Scholar



