No Evidence of a Homogeneous Frontal Neuropsychological Profile in a Sample of Schizophrenic Subjects
Abstract
The authors obtained a frontal functions profile for 81 schizophrenic patients using six neuropsychological tests that evaluate the dorsolateral prefrontal cortex functions, i.e., working memory, executive functions, and strategic performance. They then analyzed the test performances with a cluster analysis, which produced a four-cluster solution. The results support the hypothesis that neuropsychological dysfunctions in schizophrenia are heterogeneous. The performances on many of the neuropsychological tests were also strongly correlated with verbal and nonverbal IQ, as measured by the Wechsler Adult Intelligence Scale–Revised.
At present, the most authoritative etiopathogenetic hypothesis of schizophrenia involves anomalies of cerebral circuitries in frontal regions, in particular those related to the dorsolateral prefrontal cortex (DLPFC). This hypothesis is supported by a series of data from different lines of research showing that subjects affected by schizophrenia are significantly different in a variety of frontal-related characteristics from subjects not affected by schizophrenia.1,2 Moreover, the hypothesis conceptualizes schizophrenia as a unitary disorder, despite of its clinical heterogeneity, which makes it reasonable to suppose also a functional homogeneity.
The DLPFC seems to be involved in a number of mental activities ranging from set shifting to divergent reasoning, from rule formation to working memory.3 The current literature proposes a number of different neuropsychological tasks, each one designed to evaluate a specific mental activity; however, all of these tests are quite different from each other in theoretical background and in level of difficulty. These two aspects (the heterogeneity of the investigated mental functions and the different levels of difficulty of the tasks) can explain, in part, the heterogeneity of neuropsychological performance in schizophrenia.
This study has been designed to provide answers to the following questions:
| 1. | Will a comprehensive neuropsychological evaluation of DLPFC lead to a unitary profile in a sample of schizophrenic patients? | ||||
| 2. | If not, can we suggest alternative hypotheses to explain the neuropsychology of DLPFC in schizophrenia? | ||||
| 3. | Can other factors like IQ, age, education, or task complexity be important in determining neuropsychological performance? | ||||
We first described the neuropsychological frontal profile of our sample of schizophrenic patients by using a cluster statistical technique, hypothesizing that if an inherent unique cognitive frontal dysfunction could be represented by a clustering function, then such a cluster solution might represent a kind of external validation. A similar approach has been proposed in the recent literature on the field.4,5 Two recent studies have explored the neuropsychological functioning in schizophrenia by using a cluster analysis technique, but both failed to provide a univocal functional assessment in their schizophrenic samples.4,5
For our present purposes, the tasks that we selected to explore DLPFC functioning are the A-not-B Test, Delayed Response Task (DRT), Object Alternation Test (OAT), Word Fluency Test (WFT), Weigl Sorting Test (WST), and Wisconsin Card Sorting Test (WCST). The A-not-B and DRT are simple tests that evaluate working memory functions dependent on the integrity of DLPFC functions.6 Performance on the OAT depends on working memory and executive functions and is impaired when the orbitofrontal cortex (OFC) is lesioned.7 WFT is a word-finding test typically impaired in patients with left hemisphere lesions, particularly in left frontal lobe.8 WST assesses the ability to shift from one cognitive strategy to another—a domain of executive functioning that is strongly impaired when the left frontal lobe anterior to Broca's area is damaged.9 WCST is a strategy-dependent test that mainly evaluates executive functions that are impaired when the DLPFC is lesioned.10
METHODS
Subjects
Eighty-one schizophrenic outpatients participated in the study. Patients were recruited from the outpatient facility of the Psychiatric Branch of the Department of Medicine, Surgery, and Dentistry of the University of Milan Medical School.
Diagnosis was made independently by two of the authors (S.S., O.G.) according to DSM-IV criteria.11 Each patient was included in the study sample only if both the psychiatrists agreed on the diagnosis of schizophrenia. Exclusion criteria were a history of perinatal traumas, organic illnesses involving the central nervous system, substance and/or alcohol abuse, clinical evidence of mental retardation, and marked extrapyramidal symptomatology. All of the patients were already being treated with antipsychotic medications at the time of the recruitment.
Mental state was evaluated with a clinical interview administered by two of the authors (O.G., A.C.) just before the neuropsychological session and was scored by the same two authors using the Brief Psychiatric Rating Scale (BPRS),12 the Scale for the Assessment of Positive Symptoms (SAPS),13 and the Scale for the Assessment of Negative Symptoms (SANS).13 IQ was assessed with the Wechsler Adult Intelligence Scale–Revised (WAIS-R).14
Information about the purpose of the study was provided to each subject, and informed consent was obtained before starting the testing procedure.
Testing Procedures
A trained neuropsychologist administered the tests in a quiet laboratory. WAIS-R verbal and nonverbal IQ were assessed before the neuropsychological session. All patients completed all the tests, which were submitted in a fixed order. The details of the complete procedure for each test are reported elsewhere.6–10 The tests are described briefly below. In the A-not-B test,6 the patient sits facing two identical hiding wells, one on the left and one on the right. The examiner shows an object to the patient and puts it into one of the two hiding wells and the subject has to look for the correct answer immediately, without delay. The subject scores successfully if he or she makes no more than one error.
The Delayed Response Task6 is performed like the A-not-B test, but with a 10-second delay between the moment the object is positioned in one of the wells and the subject's answer. The object is hidden according to a predetermined schedule with a pseudo-random Gellerman sequence.6 The DRT is scored in the same way as the A-not-B test.
In the Object Alternation Test,7 the subject and the examiner are separated by a wooden platform 60 cm wide and 60 cm high. A black curtain is anchored to the platform and can be moved to reveal the stimulus board, where there are two plaques. When the curtain is lowered, the patient can see neither the plaques nor the investigator. When the curtain is raised, the subject can see the two objects and only the hands of the investigator. An object, such a penny, is placed under one of the two black plaques. The subject is asked to choose one of the two. After each response the curtain is lowered, and if the subject has given a correct response the investigator changes the position of the penny. On the first trial both plaques are baited with a penny. For the other trials, the penny is put under the side that was not previously chosen. The penny remains on the same side until the subject finds the coin. After a correct response, the penny is put under the opposite plaque. Each task is composed of 25 complete trials; the learning criterion is 15 consecutive correct responses. OAT performance was scored as the total number of perseverative errors. An error is scored positively if the subject chooses the incorrect object two or more times consecutively before shifting strategy.
The Weigl Sorting Test9 consists of 20 wooden blocks varying in shape, color, thickness, symbol printed on the surface, and size. The subject is requested to form homogeneous groups, according to a common feature, in 3 minutes. The score is based on the number of categories that the subject recognizes, and it ranges from 0 to 5.
The Wisconsin Card Sorting Test is performed according to standardized criteria.10 Four stimulus cards with different symbols (one single red square, two green stars, three yellow crosses, four blue circles) are placed in front of the subject, who is given a pack of 128 response cards, eight of which are identical to the stimulus cards. The subject is instructed to place each response card under one of the four stimulus cards and is told that the examiner will say whether the coupling criterion is right or wrong. According to the examiner's response the subject has to place as many cards as possible with the correct strategy. After coupling 10 cards with the first criterion (color), the subject has to shift to the second criterion (shape) and then to the third (number). This procedure is repeated twice or until all 128 cards have been placed.
Out of the three indices of WCST performances (i.e., number of total errors, number of stages, and number of perseverative errors), only the perseverative errors score (PE) is considered in this paper. A perseverative error is scored when the subject continues to sort the cards in the same way (e.g., i.e., according to color criterion) even when the examiner says the card is wrong or changes the criterion.
The Word Fluency Test8 consists of three word-naming trials. The letters F, A, S are employed, and for each letter there is a one-minute time limit to produce as many words as possible. The score consists of the total number of words recalled and is corrected for age, sex, and education, according to Benton's procedures.
Statistical Analyses
Because all subjects performed correctly on the A-not-B and the DRT tests, those results were not included in the statistical analyses.
We first computed z scores for neuropsychological and clinical variables to normalize the distributions. Then we looked for particular patterns of frontal function profiles by evaluating the data with cluster analysis, where OAT, WFT, WST, and WCST z scores were the clustering variables. Clustering techniques were the squared Euclidean distance and the Ward's method. Analysis of variance (ANOVA) with Scheffé's multiple comparison test, chi-square, and correlation analysis were also used when appropriate.15
External validity of the descriptive cluster statistics was obtained by performing ANOVA with clusters as independent variables and OAT, WFT, WST, WCST as dependent variables.
Correlation analysis was performed to evaluate the relationships between neuropsychological performances and verbal IQ, nonverbal IQ, age, and education.
RESULTS
Table 1 shows the clinical and demographic characteristics of the patients and their neuropsychological performance results. All patients were fully successful on A-not-B and DRT.
Upon examination of the cluster analysis dendrogram, a four-cluster solution appeared to be appropriate. Cluster 1 groups 30 patients who are characterized by WFT and WST low scores; Cluster 2 groups 39 patients who are characterized by WST and WFT high scores; Cluster 3 groups 9 patients who are characterized by WCST-PE, WFT, and WST low scores; and Cluster 4 groups 3 patients who are characterized by WST and WCST-PE low scores.
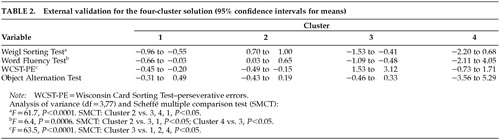
Table 2 shows the results of the external validation. OAT scores were not significantly different between the four clustered groups. WFT was significantly correlated with verbal IQ (r=0.43, P<0.001) and nonverbal IQ (r=0.31, P<0.01). WST also was significantly correlated with verbal IQ (r=0.53, P<0.001) and nonverbal IQ (r=0.43, P<0.001). WCST-PE errors were negatively correlated with verbal IQ (r=–0.36, P<0.001) and nonverbal IQ ( r=–0.42, P<0.001). No significant correlations were found between verbal IQ or nonverbal IQ and A-not-B, DRT, or OAT.
Education was significantly correlated with verbal IQ, nonverbal IQ, and WST. Age was not correlated with any of the neuropsychological variables.
DISCUSSION
This is, to the best of our knowledge, the first report that explores the effect of clustering the frontal neuropsychological performances in a sample of subjects suffering from schizophrenia.
AnonB and DRT tests are authoritatively recognized as a neuropsychological paradigm of working memory and tasks sensitive to DLPFC lesions.16 Our sample of schizophrenic subjects did not show any deficit in this area of mental functioning. It is possible that because of their extreme simplicity, the two tests are not sensitive enough to detect abnormal DLPFC functioning. Moreover, it should be noted that other, more recent working memory tests, despite being more developed from a technological point of view, are also very simple in their procedures and very similar to the DRT paradigm.17
Our major finding is that the external validation of cluster analysis shows a pattern of frontal performance that is heterogeneous and does not support the hypothesis of an inherent unitary pattern of frontal impairment in subjects suffering from schizophrenia.
Our data indicate three profiles of frontal functioning:
| 1. | A profile of good global frontal scores (the 39 subjects of Cluster 2). These subjects show the best performance compared with the rest of the sample: their performance on WST (mean score 4.28±0.51), WFT (27.6±7.8), and WCST-PE (5.8±8.11) was fully in the normal range, according to normative data in normal populations. | ||||
| 2. | A profile of poor scores (the 30 subjects of Cluster 1) on WST and WFT that reflects left frontal malfunctioning without DLPFC involvement. | ||||
| 3. | A pattern of poor scores (the 9 subjects of Cluster 3 and the 3 subjects of Cluster 4) on WCST-PE that reflects DLPFC malfunctioning. In our opinion, the poor performance of these subjects also on WST and WFT suggests a more diffuse and presumably severe malfunctioning in frontal regions. | ||||
OAT performance did not enter in the external validation of the cluster analysis. This result indicates that the orbitofrontal cortex does not have a critical role in the frontal active profile on schizophrenia. This finding is consistent with our previous results.18
We observed also that IQ is significantly correlated with WST, WFT, and WCST-PE, suggesting that performance on frontal neuropsychological tests is dependent on the IQ level.
These data support the hypothesis that the WST, WFT, and WCST are neuropsychological instruments strongly dependent on individual mental capacities (logical? deductive? learning-dependent?) that are not the functional expression of any specifically defined brain area. A previous report from our group showed no significant differences in WCST performance between schizophrenic and normal control subjects when corrected for education, a result that seems to be consistent with this view.19 A critical impact of socioeconomic status and educational level on cognitive performance in schizophrenia has been also reported.20
The results of the present investigation do not support a unitary frontal cognitive model of malfunctioning in schizophrenia. Schizophrenic subjects presented different responses to neuropsychological tests in a pattern that more probably suggests the activation of different frontal areas.
ACKNOWLEDGMENTS
This research was supported by the Ministry of the University and of Scientific Research and Technology (MURST), 1999 ex-60% Grant 12-1-5201001-433.
 |
 |
1 Goldman-Rakic PS, Salomon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:437-458Crossref, Medline, Google Scholar
2 Palmer BW, Heaton RK, Paulsen JS, et al: Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology 1997; 11:437-446Crossref, Medline, Google Scholar
3 Duncan J, Owen AM: Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 2000; 23:475-482Crossref, Medline, Google Scholar
4 Seaton BE, Allen DN, Goldstein G, et al: Cognitive subgroups of patients with schizophrenia are not differentiated by symptom profile. Schizophr Res 1999; 36:153-154Google Scholar
5 Hill SK, Ragland D, Gur RE, et al: Neurocognitives subtypes of schizophrenia: a cluster analytic examination of neuropsychological functions (abstract). Schizophr Res 2001; 49(suppl):109Google Scholar
6 Diamond A, Doar B: The performance of human infants on a measure of frontal cortex function, the Delayed Response Task. Dev Psychobiol 1989; 22:271-294Crossref, Medline, Google Scholar
7 Mishkin M: A memory system in the monkey, in The Neuropsychology of Cognitive Function, edited by Broadbent DE, Weiskantz L. London, The Royal Society, 1982, pp 48-55Google Scholar
8 Benton AL, Hamsher K: Multilingual Aphasia Examination (revised manual). Iowa City, IA, University of Iowa, 1978Google Scholar
9 Weigl E: On the psychology of so-called process of abstraction. J Abnorm Psychol 1941; 36:3-33Crossref, Google Scholar
10 Milner B: Effects of different brain lesions on card sorting: the role of frontal lobes. Arch Neurol 1963; 9:90-100Crossref, Google Scholar
11 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
12 Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
13 Andreasen NC, Flaum M, Swayze VW II, et al: Positive and negative symptoms in schizophrenia: a critical reappraisal. Arch Gen Psychiatry 1990; 47:615-621Crossref, Medline, Google Scholar
14 Wechsler DA: Wechsler Adult Intelligence Scale-Revised. New York, The Psychological Corporation, 1981Google Scholar
15 SPSS Advanced Models 10.0. Chicago, SPSS Inc., 1999Google Scholar
16 Diamond A, Goldman-Rakic PS: Comparison of infants and rhesus monkeys on Piaget's AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res 1989; 74:24-40Crossref, Medline, Google Scholar
17 Baddeley A: Working memory, in The Cognitive Neurosciences. Edited by Gazzaniga M. Cambridge, MA, MIT Press, 1994, pp 755-764Google Scholar
18 Abbruzzese M, Ferri S, Scarone S: The selective breakdown of frontal functions in patients with obsessive-compulsive disorder and in the patients with schizophrenia: a double dissociation experimental finding. Neuropsychologia 1997; 35:907-912Crossref, Medline, Google Scholar
19 Gambini O, Macciardi F, Abbruzzese M, et al: Influence of education on WCST performances in schizophrenic patients. Int J Neurosci 1992; 67:105-109Crossref, Medline, Google Scholar
20 Goldberg TE, Ragland DJ, Fuller Torrey E, et al: Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 1990; 47:1066-1072Crossref, Medline, Google Scholar



