Progress of Memory Function After Radiation Therapy in Patients With Nasopharyngeal Carcinoma
Abstract
The authors evaluated the late neurocognitive profile and progress of memory functions in 60 patients with primary nasopharyngeal carcinoma who had been treated with radiation therapy for more than 2 years. Forty had imaging evidence of temporal lobe injury (TLI-positive), and 20 did not (TLI-negative). The patients and 19 healthy control subjects, matched with the patients for age and educational level, underwent extensive memory assessments. Seventeen patients (10 TLI-positive, 7 TLI-negative) were reassessed after an average of 28 months for progress of memory functions. The patient groups performed significantly worse on most memory tests compared with the normal subjects. Patients with memory complaints had lower scores for verbal memory than those without such complaints. The TLI-positive and TLI-negative groups did not differ significantly from each other in cognitive performance. At follow-up, visual memory performance had deteriorated, while verbal memory remained more stable.
With increases in the long-term survival of patients treated with radiation therapy (RT) for malignant tumors of the central nervous system, the late effects of cranial irradiation have attracted much concern.1,2 Follow-up studies of patients who received cranial RT and survived childhood tumors have revealed a high incidence of significant intellectual deficit.3–7 Children treated for medulloblastoma with cranial RT were found to have lower intelligent quotients (IQs) and specific cognitive deficits involving reading, arithmetic, and visuospatial functions.8,9 Similarly, children who had received cranial RT for acute lymphoblastic leukemia suffered impaired performance in IQ tests, reading, arithmetic, attentional ability, and psychosocial functioning.10–13 Gender, age at the time of administration of RT, use of chemotherapy, and radiation dose have been suggested as determinants for neurotoxicity.14–18
Although much work has covered the late effects of RT on the developing brain, information on the effects of irradiation on the adult brain tends to be more limited. Some reports have suggested that the acute effects of RT on neurocognitive functions in adults are usually less significant.19,20 Findings on the late cognitive effects of RT, however, have been more diverse.21–24 Variations in patient selection and methodology have limited the comparability of results. The different neuropsychological instruments used may have had different sensitivities in detecting subtle and specific cognitive deficits.25 Furthermore, the nature of cognitive impairment in patients with focal brain tumors treated by RT is inherently influenced by the location and size of the primary tumor and its surrounding edema. Moreover, adult patients with malignant tumors requiring cranial RT generally have a poor prognosis, which precludes long-term outcome observations.5
Studies of patients with extracranial malignancies subject to incidental cerebral irradiation have offered important information concerning the effects of irradiation in the disease-free adult brain. One study of patient neuropsychological functions subsequent to the RT of skull base tumors reported stable memory and cognitive function up to 5 years after treatment.26 However, another study of patients with incidental brain irradiation reported impaired performance in memory and a wide range of cognitive functions. The neurocognitive symptoms appeared to be related to the total dose of radiation delivered.27 Apart from primary base-of-skull tumors, nasopharyngeal carcinoma (NPC) is one of the common extracranial malignancies that may involve incidental brain irradiation during its treatment. NPC is a highly prevalent malignant tumor affecting the Southern Chinese population. The annual incidence rate is 27.5 per 100,000 for males and 11.2 for females.28 Risk factors associated include childhood consumption of salted fish, dietary deficiency of vitamin-rich fresh vegetables, exposure to domestic smoke or chemical fumes, cigarette smoking, Epstein-Barr virus infection, and genetic predisposition.29 RT has been the mainstay treatment for NPC, with good curative rates at early stages. Owing to the frequent spread of the tumor to the skull base, the inferior parts of the temporal cortex are often included within the radiation portals to provide adequate oncological margins at the skull base.
The long-term survival of patients treated with RT provides an opportunity for the study of late cognitive effects after focused irradiation. Temporal lobe injury (TLI), a consequence of RT, has been reported in 1% to 3% of patients with NPC. The condition is usually diagnosed by the imaging evidence of hypodense or cystic lesions on computed tomography (CT), as well as hyperintense changes in T2-weighted images on magnetic resonance imaging (MRI). Clinical manifestations of TLI found in retrospective studies range from the asymptomatic to full cognitive impairment resembling dementia.30–37 Previous reports on the effects of RT for NPC have documented lower scores in intelligence assessment and memory tests.38–43 In this study we aimed to delineate the progress of memory functions in patients after RT for NPC from a longitudinal perspective. The potential applications of neuropsychological assessment as an early tool for the detection of late radiation-induced brain injury are discussed.
METHODS
Subjects
Two patient groups and a control group were studied. The patient groups comprised subjects who had completed curative-intent RT for NPC at least 2 years. These groups were recruited at the oncology follow-up clinic of the Prince of Wales Hospital in Hong Kong, and they also satisfied the following recruitment criteria: no intracranial extension of the tumor; no evidence of disease; no oncological treatment in progress at the time of referral; no chemotherapy administered within 6 months before assessment; no previous history of significant head injury or neurodegenerative disorder; no significant sensory deficits affecting communication; a stable physical condition for assessment; and consent for participation. To minimize the possibility of age-related memory impairment, only subjects under 60 years of age were included.
Two groups of NPC patients were recruited for comparison. The first group consisted of 40 patients with evidence of TLI on CT or MRI (the TLI-positive group). TLI was diagnosed by the detection of hypodense lesion on CT, or hyperintense lesion in T2-weighted images on MRI, in the temporal lobes. The lesion was diagnosed in most cases by MRI (83%). The reason for these patients' having undergone imaging was either that they presented with neuropsychiatric or cognitive complaints or that they were considered at risk for radiation-induced brain injury.35 The second group comprised 20 NPC patients with no imaging evidence of temporal lobe lesions (the TLI-negative group). They were recruited randomly from apparently relapse-free patients who consented to neurocognitive assessment. The negative TLI status was subsequently confirmed by imaging scan. If the subjects were found to have TLI, then they were reclassified as part of the TLI-positive group. Patients with imaging evidence of tumor recurrence, intracranial tumor extension, or cerebral metastases were excluded from entry into either group. The most recent imaging scan was taken at an average of 7.7±7.8 months from the date of neurocognitive assessment. On imaging, 17% of the patients had left-side lesions, 14% had right-side lesions, and 69% had bilateral lesions. Of the patients with bilateral lesions, 24% had more severe lesions on the right, 40% had more severe lesions on the left, and 36% had bilateral lesions of similar severity. A third group of healthy subjects, matched with the patients by age and educational level, was recruited as the comparison group. Healthy control subjects are recruited from volunteers who are free of major medical and neurological diseases. All subjects were Southern Chinese in ethnic origin. A follow-up assessment was arranged at an average of 28 months (range 11–42 months) after the initial assessment. An attempt was made to contact all patients or their relatives during the follow-up period, with the result that 10 TLI-positive patients and 7 TLI-negative patients were reassessed. Of those who were not reassessed, 9 TLI-positive patients and 1 TLI-negative patient had died. The study was approved by the Medical Ethics Committee of the Chinese University of Hong Kong.
RT Regimes
During the RT treatment that patients assessed in the present study had received, the nasopharynx and adjacent regions were irradiated to the equivalent of at least 66 Gy, in fraction sizes within the range of 1.6 Gy to 2.5 Gy. The tumor dose was 66 Gy to 71.2 Gy for patients subject to one course of RT. The standard radiation plan had a three-beam arrangement, comprising one left, one right, and one anterior 6-MV photon beam. The superior border of the beams was at 0 to 0.5 cm above the roof of the sphenoid sinus, but eye-shields in the anterior beam shielded the eyes and the corresponding parts of the temporal lobe. This shielded part of the temporal lobe lies between the roof of the sphenoid and floor of the pituitary fossa and receives about 70% of the tumor dose (being irradiated by the lateral beams but not the anterior beam). The inferiormost portion of the temporal lobes (the part lying below the floor of the pituitary fossa) remained unshielded and was subject to the tumor dose. In 16 patients, the eye-shield on one side was omitted to take account of tumor extension, and the parts of the temporal lobes that were usually shielded were also subject to the full tumor dose, whereas only the inferiormost portion was subject to the full dose in the standard plan.
For the purpose of analyzing the relationships between dose, volume, and outcome, patients were segregated by large differences in dose (one course versus two courses of RT) or large differences in the volume irradiated to tumor dose. Category 1 patients (n=38) were treated with routine shielding (the standard plan). Category 2 patients (n=15) were treated by a plan that omitted the eye-shield on one side (the large-volume plan). Category 3 patients (n=6) were treated by two courses of RT (the large-dose plan). One subject with TLI was treated in another center, and the RT dose details were not known.
Neurocognitive Assessment
Neurocognitive assessment comprised a mental status examination and a battery of neuropsychological tests. A checklist of psychiatric symptoms guided the mental status examination.44 The neuropsychological evaluation included the Chinese version of the Wechsler Adult Intelligence Scale–Revised (WAIS-R) verbal subtests,45 digit and visual spans,46 and Rey auditory and visual learning tests.47,48
Subjective memory problems and related complaints were recorded by using an interview with standard questions; subjects were asked about the presence of subjective memory problems, their duration, their nature (recent or remote memory), and their impact on daily life (missed appointments, need of written reminder).
Attention was assessed with the monotone test. The subject was presented with an audiotape excerpt of a series of pure tones and was asked to count their number. The test was completed with 12 trials and was a sensitive index for the assessment of attention.
Language function was estimated by using the Chinese version of the WAIS-R verbal tests (information, comprehension, and similarities) and the verbal fluency test. In the verbal fluency test, the subject was asked to generate exemplars under the categories of animals, vehicles, and food, each for one minute's duration. The total number of exemplars generated was taken as a score for verbal fluency and was compared with the scores of 50 independent healthy control subjects who had been matched to the patient groups by age and educational level.
Working memory was evaluated by using the digit and visual span tests. Episodic memory was evaluated with the Rey auditory and visual learning tests. In the verbal recall test, the subject was asked to register 15 words and to recall them immediately after registration. A total reminder of all items was given after the recall, and 5 learning trials were then performed. The subject was asked to retrieve the items after a 20-minute delay, and this was followed by a recognition task for which the subject was asked to recognize the 15 items out of 30 semantically and phonetically related items. In the visual recall test, 15 line drawings of geometrical figures were presented, and the subject was asked to draw the 15 figures after an exposure of 10 seconds for each. After the drawing trial, the 15 figures were presented to the subject again and the process was repeated to complete 5 learning trials. For visual recognition, the subject was asked to recognize the 15 figures out of 30 related figures after the recall trials. The number of items and figures recalled in each trial was recorded, and a learning curve was constructed.
During the follow-up assessment, each subject performed the digit and visual span and the Rey auditory and visual memory tests.
Statistical Analyses
Differences in memory performance between the patient and control groups were analyzed. Categorical data were evaluated with the chi-square test. Continuous variables were analyzed with one-way analysis of variance (ANOVA). Progress of memory functions was evaluated with paired t-tests. The level for statistical significance was set at P<0.05. The analysis was carried out with the Statistical Package for Social Sciences, version 9.0.49
RESULTS
Sample Characteristics
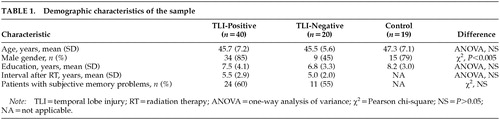
Forty TLI-positive patients and 20 TLI-negative NPC patients were assessed. As a comparison, 19 healthy control subjects who had been matched to the patients by age and educational level were also assessed. The three groups did not differ significantly in age, years of education, or post-RT interval. Demographic characteristics of the sample are displayed in Table 1.
There was no difference in age, educational level, or post-RT interval between patients who had and those who had not attended follow-up assessment (t-tests, P>0.05). The mean duration of subjective memory problems was 1.1±1.0 years in the TLI-positive and 1.4±1.1 in the TLI-negative groups (t-test, P>0.05). One TLI-positive subject qualified for a DSM-IV diagnosis of major depressive episode. Another suffered from panic disorder. One TLI-negative subject had adjustment disorder with depressed mood.50
Correlates of Cognitive Function
Subjective Memory Problems:
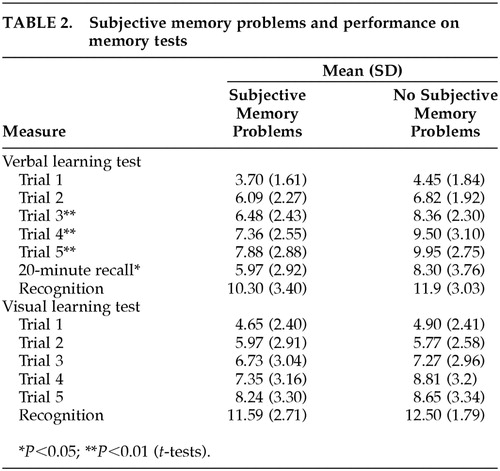
Twenty-four subjects (60%) in the TLI-positive group had subjective memory problems, compared with 11 subjects (55%) in the TLI-negative group (Pearson chi-square, P>0.05). Patients with subjective memory problems, when compared with those having no subjective memory difficulties, performed worse on the auditory verbal learning test. A similar difference was not found in performance on the visual learning test (t-test, P>0.05; Table 2).
TLI Status:
The patient groups had lower scores on the WAIS-R information and comprehension subtests when compared with healthy control subjects (one-way ANOVA, information: F=5.46, df=2,70, P<0.01; comprehension: F=4.4, df=2,70, P<0.05; similarities: F=2.65, df=2,70, P>0.05). After a post hoc Bonferroni test, differences between scores for the TLI-positive and TLI-negative groups were not significant.
On working memory assessment with digit and visual spans, the TLI-positive group scored lower on forward visual span than healthy control subjects (one-way ANOVA, F=3.39, df=2,67, P<0.05). On episodic memory assessment, the patient groups recalled fewer items across all trials in the Rey auditory test. The difference in recall became significant from the third trial onward (one-way ANOVA, P<0.05). Differences in 20-minute delay recall and recognition scores were not statistically significant, but the patient groups recalled and recognized fewer items (one-way ANOVA, P>0.05). In the Rey visual learning test, the patient groups also had lower scores across most trials (one-way ANOVA, P<0.05). All groups performed similarly on the recognition tasks (one-way ANOVA, P>0.05). The performance of all groups is shown in Figure 1 and Figure 2.
RT Regimes:
Subjects treated with the large-volume RT plan had lower scores in the WAIS-R subtests of comprehension (t=2.21, df=48, P<0.05) and similarities (t=2.06, df=48, P<0.05) when compared to those treated with standard plan. Performance on the memory tests was similar between RT regimes (t-test, P>0.05). Subjects treated with the large-volume plan showed no difference in cognitive function according to the side on which shielding was omitted (t-test, P>0.05). Subjects treated with the high-dose plan did not differ in performance on neurocognitive testing when compared to those treated with the standard plan (t-test, P>0.05).
Progress of Memory Functions
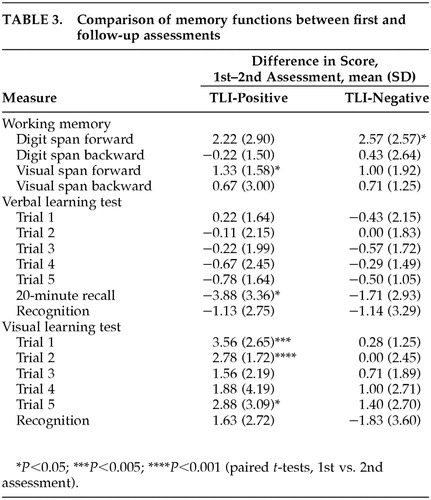
At follow-up testing, the scores of the TLI-positive and TLI-negative subjects did not differ significantly from each other on most verbal and visual memory tests, except than TLI-negative subjects recalled fewer items than TLI-positive subjects in the 20-minute delayed recall (t=2.65, df=14, P<0.05). In both TLI-positive and TLI-negative subjects who were reassessed, performance on digit and visual spans was stable. There was a trend toward improvement in the verbal memory tests at follow-up, but the difference was not significant (paired t-tests, P>0.05). An opposite trend for deterioration of visual memory was found at follow-up. The two patient groups showed similar directions of change, but the difference was more marked in the TLI-positive group (Table 3).
Comparison of initial memory performance between those who were and were not reassessed was done for each patient group. For TLI-positive subjects, there was a trend of worse initial memory performance for subjects not reassessed. The difference in verbal memory score was not significantly different, but the difference in visual memory performance was more marked (t=3.07, df=37, P<0.05). Conversely, there was a trend for better memory performance in the TLI-negative group for those who declined reassessment. The differences were significant at 20-minute delayed verbal recall and recognition of visual memory (t=–2.3, df=19, P<0.05).
DISCUSSION
The late cognitive effects of incidental irradiation to the temporal lobes were studied in NPC patients treated with RT. The patient groups had scores comparable to those of the healthy control subjects on tests of auditory working memory. However, they performed less well in other episodic memory assessments. In both verbal and visual learning tests, the difference between the patient groups and the control group became more significant as the number of learning trials increased. It is likely that the number of items recalled in the first two trials was more dependent on working memory, whereas performance in subsequent trials was dependent on episodic memory. Although the patients retrieved fewer items during the spontaneous recall, the differences in recognition after a 20-minute delay were not statistically significant. These findings suggest that the memory deficits after RT may be more related to retrieval problems than to encoding strategies and the rate of forgetting, which are less affected.
The patient groups also had lower scores than the control group in tests related to verbal abilities. Although matched by control subjects with similar age and educational levels, both the TLI-positive and TLI-negative groups scored at a lower level in WAIS-R tests of verbal intelligence. This result suggests that even when there are no obvious aphasic problems, subtle language deficits may occur after RT. The irradiation most commonly affected the inferior portions of the temporal cortex, and thus the deficits in recall may reflect an underlying disturbance in episodic memory caused by damage to the mesial temporal structures. The relatively poor performance in verbal tests suggests a functional impairment in language. This functional impairment may reflect underlying semantic memory deficits, which are closely associated with lesions in the temporal cortex.51,52
Our study provided additional information about the progress of memory function with time. At follow-up assessment, verbal memory was found to be better preserved than visual memory. This finding is in accordance with the usual notion that language function is more resilient. Firm interpretation was limited by the small sample size during reassessment.
Contrary to what might have been expected on the basis of common belief, the TLI-positive group did not perform significantly worse than the TLI-negative group. Several factors may have contributed to this result.
First, the subjects with TLI might have been selected with biases toward those whose functioning was better. Subjects with severe global cognitive impairment (delirium or dementia) would certainly not have been able to comprehend the tests and were therefore excluded. Subtle differences in cognitive abilities between the two groups could not be detected. However, it is also very important to note that cognitive deficits identified through the conventional neuropsychological approach are not entirely dependent on the presence of lesions defined by neuroimaging techniques. Findings of TLI might not be directly translatable into severe cognitive or memory impairment. Cognitive impairment also occurred in the absence of imaging lesions. These considerations suggest a need for independent cognitive assessment to detect radiation brain injury in otherwise clinically asymptomatic and imaging-negative patients.
Second, the better initial performance of TLI-positive subjects who attended reassessment suggested that those who had not been reassessed might have had a poorer prognosis. This would be in accordance with the higher mortality in the TLI-positive group. On the other hand, the poorer initial performance of TLI-negative subjects who accepted reassessment suggested that there might be bias in the direction of the well-maintained ones declining reassessment. This possibility suggests a need for longitudinal follow-up of TLI-negative subjects with suboptimal baseline memory performance.
The selection of patients for imaging, who received subsequent diagnosis of TLI, was based on two main sources: longitudinal assessment of high-risk patients as reported in the literature, and the presence of either neuropsychiatric or cognitive complaints. It is possible that other, asymptomatic TLI-positive subjects who were not assessed might perform differently in memory assessments. It is interesting to note that patients with subjective memory problems scored more poorly in verbal learning than those without such complaints. The same trend was noted in both TLI-positive and TLI-negative subjects. This trend suggested that there was a genuine association between subjective complaints and objective assessments. Such a finding offers practical hints to the clinician about the validity of memory questionnaires for the early detection and screening of cognitive dysfunction. It is also possible that depression in NPC patients might have affected the memory performance and their complaints about memory problems. During the mental status examination, depressive symptoms were comprehensively inquired about. Although most patients had negative feelings toward the diagnosis of cancer and side effects of RT, clinical depression was not prevalent. It was thus unlikely that the results of neurocognitive assessments were significantly affected by mood state.
Subjects irradiated under a large-volume plan had lower scores on the WAIS-R verbal tests. This finding may also be related to semantic memory deficits because a greater volume of the temporal cortex was irradiated to the tumor dose level and was therefore susceptible to neurotoxicity. Further systematic evaluation of semantic memory performance is needed to clarify this question. No significant difference in cognitive function was found in subjects irradiated with the high dose when compared with subjects treated with the standard dose. Taking the threshold hypothesis into account, if neurotoxicity occurred with the standard dose, then further irradiation would have little additive cognitive effects on the already damaged neural tissue. However, because only 6 subjects who had been treated with large doses were assessed, interpretation of the results must be very cautious.
This study has provided a systematic evaluation of the memory profile of patients with NPC after RT. Memory deficits were also identified in patients without imaging changes typical of TLI. It can be argued that the subjects performed poorly as a general effect of malignancy and chronic ill health, and that cognitive impairment was not directly related to cranial RT.53,54 Theoretically, the recruitment of another group of patients with other cancer and/or NPC patients who had not received RT would best ascertain the effects of RT on cognition. However, because RT is the best-documented and most effective treatment for NPC, it would be impossible to recruit NPC subjects who were treated solely with other therapies. The recruitment of non-NPC cancer patients also poses problems. Systemic effects of different cancers are difficult to control and compare. It should be noted that in the NPC model, patients who were subject to curative-intent therapy did not have distant metastatic disease. The treatment was a local one involving only the head and neck region, of a limited duration of 1.5 to 2 months. It is therefore less likely that the general condition was compromised to such an extent that cognitive functions were impaired.
Although conventional objective criteria for radiation-induced brain injury usually rely on imaging, the role of neurocognitive assessment should not be undervalued. A comprehensive multidimensional assessment including neuropsychological assessment, imaging investigation, and memory questionnaires is important for the early detection of radiation-induced brain injury.
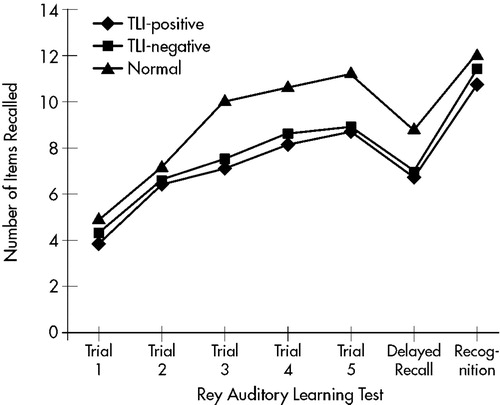
FIGURE 1. Verbal memory performance in patient and control groups.
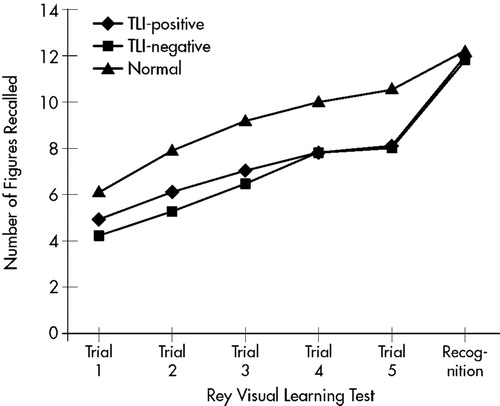
FIGURE 2. Visual memory performance in patient and control groups.
 |
 |
 |
1 Filley CM: Toxic leukoencephalopathy. Clin Neuropharmacol 1999; 22:249-260Medline, Google Scholar
2 Keime-Guibert F, Napolitano M, Delattre JY: Neurological complications of radiotherapy and chemotherapy. J Neurol 1998; 245:695-708Crossref, Medline, Google Scholar
3 Williams JM, Davis KS: Central nervous system prophylactic treatment for childhood leukemia: neuropsychological outcome studies. Cancer Treat Rev 1986; 13:113-127Crossref, Medline, Google Scholar
4 Cousens P, Waters B, Said J, et al: Cognitive effects of cranial irradiation in leukemia: a survey and meta-analysis. J Child Psychol Psychiatry 1988; 29:839-852Crossref, Medline, Google Scholar
5 Roman D, Sperduto P: Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys 1995; 31:983-998Crossref, Medline, Google Scholar
6 Fossen A, Abrahamsen TG, Storm-Mathisen I: Psychological outcome in children treated for brain tumor. Pediatr Hematol Oncol 1998; 15:479-488Crossref, Medline, Google Scholar
7 Copeland DR, de Moor C, Moore BD 3rd, et al: Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J Clin Oncol 1999; 17:3476-3486Crossref, Medline, Google Scholar
8 Kramer JH, Crowe AB, Larson DA, et al: Neuropsychological sequelae of medulloblastoma in adults. Int J Radiat Oncol Biol Phys 1997; 38:21-26Crossref, Medline, Google Scholar
9 Mulhern RK, Reddick WE, Palmer SL, et al: Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann Neurol 1999; 46:834-841Crossref, Medline, Google Scholar
10 Smibert E, Anderson V, Godber T, et al: Risk factors for intellectual and educational sequelae of cranial irradiation in childhood acute lymphoblastic leukaemia. Br J Cancer 1996; 73:825-830Crossref, Medline, Google Scholar
11 Hill JM, Kornblith AB, Jones D, et al: A comparative study of the long-term psychosocial functioning of childhood acute lymphoblastic leukemia survivors treated by intrathecal methotrexate with or without cranial radiation. Cancer 1998; 82:208-218Crossref, Medline, Google Scholar
12 Rodgers J, Horrocks J, Britton PG, et al: Attentional ability among survivors of leukaemia. Arch Dis Child 1999; 80:318-323Crossref, Medline, Google Scholar
13 Cetingul N, Aydinok Y, Kantar M, et al: Neuropsychologic sequelae in the long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol 1999; 16:213-220Crossref, Medline, Google Scholar
14 Christie D, Leiper AD, Chessells JM, et al: Intellectual performance after presymptomatic cranial radiotherapy for leukaemia: effects of age and sex. Arch Dis Child 1995; 73:136-140Crossref, Medline, Google Scholar
15 Waber DP, Tarbell NJ: Toxicity of CNS prophylaxis for childhood leukemia. Oncology 1997; 22:259-264Google Scholar
16 Mulhern RK, Kepner JL, Thomas PR, et al: Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol 1998; 16:1723-1728Crossref, Medline, Google Scholar
17 Grill J, Renaux VK, Bulteau C, et al: Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys 1999; 45:137-145Crossref, Medline, Google Scholar
18 Anderson VA, Godber T, Smibert E, et al: Cognitive and academic outcome following cranial irradiation and chemotherapy in children: a longitudinal study. Br J Cancer 2000; 82:255-262Crossref, Medline, Google Scholar
19 Jason GW, Pajurkova EM, Taenzer PA, et al: Acute effects on neuropsychological function and quality of life by high-dose multiple daily fractionated radiotherapy for malignant astrocytomas: assessing the tolerability of a new radiotherapy regimen. Psychooncology 1997; 6:151-157Crossref, Medline, Google Scholar
20 Wenz F, Steinvorth S, Lohr F, et al: Acute central nervous system (CNS) toxicity of total body irradiation (TBI) measured using neuropsychological testing of attention functions. Int J Radiat Oncol Biol Phys 1999; 44:891-894Crossref, Medline, Google Scholar
21 Gregor A, Cull A, Traynor E, et al: Neuropsychometric evaluation of long-term survivors of adult brain tumors: relationship with tumor and treatment parameters. Radiother Oncol 1996; 41:55-59Crossref, Medline, Google Scholar
22 Vigliani MC, Sichez N, Poisson M, et al: A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4-year results. Int J Radiat Oncol Biol Phys 1996; 35:527-533Crossref, Medline, Google Scholar
23 Taylor BV, Buckner JC, Cascino TL, et al: Effects of radiation and chemotherapy on cognitive function in patients with high-grade glioma. J Clin Oncol 1998; 16:2195-2201Crossref, Medline, Google Scholar
24 Peper M, Steinvorth S, Schraube P, et al: Neurobehavioral toxicity of total body irradiation: a follow-up in long-term survivors. Int J Radiat Oncol Biol Phys 2000; 46:303-311Crossref, Medline, Google Scholar
25 Weitzner MA, Meyers CA: Cognitive functioning and quality of life in malignant glioma patients: a review of the literature. Psychooncology 1997; 6:169-177Crossref, Medline, Google Scholar
26 Glosser G, McManus P, Munzenrider J, et al: Neuropsychological function in adults after high dose fractionated radiation therapy of skull base tumors. Int J Radiat Oncol Biol Phys 1997; 38:231-139Crossref, Medline, Google Scholar
27 Meyers CA, Geara F, Wong PF, et al: Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys 2000; 46:51-55Crossref, Medline, Google Scholar
28 Hong Kong Department of Medical and Health Services, 1987. Annual Departmental Report.Google Scholar
29 Yuen JM, Wang XL, Xiang YB, et al: Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer 2000; 85:364-369Crossref, Medline, Google Scholar
30 Ho JHC: Nasopharyngeal carcinoma. West J Med 1985; 143:70-73Medline, Google Scholar
31 Ho JHC, Lau WH, Fong M, et al: Treatment of nasopharyngeal carcinoma (NPC), in Cancer Campaign, vol 5, Nasopharyngeal Carcinoma, edited by Grundmann E. Stuttgart, Gustav Fischer, 1981, pp 279-285Google Scholar
32 Woo E, Lam K, Yu YL, et al: Temporal lobe and hypothalamic-pituitary dysfunctions after radiotherapy for nasopharyngeal carcinoma: a clinical syndrome. J Neurol Neurosurg Psychiatry 1988; 51:1302-1307Crossref, Medline, Google Scholar
33 Lee AWM, Law SCK, Ng SH, et al: Retrospective analysis of nasopharyngeal carcinoma treated during 1976-1985: late complications following megavoltage irradiation. Br J Radiol 1992; 65:918-928Crossref, Medline, Google Scholar
34 Lee AW, Ng SH, Ho JH, et al: Clinical diagnosis of late temporal lobe necrosis following radiation therapy for nasopharyngeal carcinoma. Cancer 1987; 61:1535-1542Crossref, Google Scholar
35 Woo E, Lam K, Yu YL, et al: Cerebral radionecrosis: is surgery necessary? J Neurol Neurosurg Psychiatry 1987; 50:1407-1414Crossref, Medline, Google Scholar
36 Glass JP, Hwang TL, Leavens ME, et al: Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer 1984; 54:1966-1972Crossref, Medline, Google Scholar
37 Leung SF, Kreel L, Tsao SY: Asymptomatic temporal lobe injury after radiotherapy for nasopharyngeal carcinoma: incidence and determinants. Br J Radiol 1992; 65:710-714Crossref, Medline, Google Scholar
38 Lee PWH, Hung BKM, Woo EKW, et al: Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry 1989; 52:488-492Crossref, Medline, Google Scholar
39 Hua MS, Chen ST, Tang LM, et al: Neuropsychological function in patients with nasopharyngeal carcinoma after radiotherapy. J Clin Exp Neuropsychol 1998; 20:684-693Crossref, Medline, Google Scholar
40 Bederson JB, Harsh GR 4th, Walker JA, et al: Radiation-induced bilateral cystic temporal lobe necrosis: reversal of memory deficit after fenestration and internal shunting. case report. J Neurosurg 1990; 72:503-505Crossref, Medline, Google Scholar
41 Parkin AJ, Hunkin NM: Memory loss following radiotherapy for nasopharyngeal carcinoma: an unusual presentation of amnesia. B J Clin Psychol 1991; 30:349-357Crossref, Medline, Google Scholar
42 Chu CC, Huang CC, Chen HJ: Klüver-Bucy syndrome: report of a case with nasopharyngeal cancer after irradiation and chemotherapy. Chin Med J (Taipei) 1994; 54:432-435Google Scholar
43 Cheung MC, Chan AS, Law SC, et al: Cognitive function of patients with nasopharyngeal carcinoma with and without temporal lobe radionecrosis. Arch Neurology 2000; 57:1347-1352Medline, Google Scholar
44 Janca A, Hiller W: ICD-10 checklists: a tool for clinicians' use of the ICD-10 classification of mental and behavioral disorders. Compr Psychiatry 1996; 37:180-187Crossref, Medline, Google Scholar
45 The Hong Kong Psychological Society (Division of Clinical Psychology): The Wechsler Adult Intelligence Scale. Revised Cantonese version. Hong Kong, The Hong Kong Psychological Society, 1989Google Scholar
46 Wechsler D: Wechsler Memory Scale-Revised. San Antonio, TX, The Psychological Corporation, 1987Google Scholar
47 Spreen O, Strauss E: Memory, in A Compendium of Neuropsychological Tests, 2nd edition. New York, Oxford University Press, 1998, pp 326-340Google Scholar
48 Lezak MD: Memory I: tests, in Neuropsychological Assessment, 3rd edition. New York, Oxford University Press, 1995, pp 486-487Google Scholar
49 Statistical Package for Social Sciences 9.0. Chicago, SPSS Inc., 1999Google Scholar
50 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Press, 1994Google Scholar
51 Squire S: Memory and the Brain. New York, Oxford University Press, 1987Google Scholar
52 Tranel D, Damasio AR: Neurobiological Foundations of Human Memory, in Handbook of Memory Disorders. Edited by Baddeley AD, Wilson BA, Watts FN. West Sussex, UK, Wiley, 1995, pp 27-52Google Scholar
53 Johnson BE, Patronas N, Hayes W, et al: Neurologic, computed cranial tomographic, and magnetic resonance imaging abnormalities in patients with small-cell lung cancer: further follow-up of 6- to 13-year survivors. J Clin Oncol 1990; 8:48-56Crossref, Medline, Google Scholar
54 Johnson BE, Becker B, Goff WB 2d, et al: Neurologic, neuropsychologic, and computer cranial tomography scan abnormalities in 2- to 10-year survivors of small-cell lung cancer. J Clin Oncol 1985; 3:1659-1667Crossref, Medline, Google Scholar



