Posttraumatic Stress Disorder: Acquisition, Recognition, Course, and Treatment
Abstract
Following exposure to trauma, a large number of survivors will develop acute symptoms of posttraumatic stress disorder (PTSD), which mostly dissipate within a short time. In a minority, however, these symptoms will evolve into chronic and persistent PTSD. A number of factors increase the likelihood of this occurring, including characteristic autonomic and hypothalamic-pituitary-adrenal axis responses. PTSD often presents with comorbid depression, or in the form of somatization, both of which significantly reduce the possibilities of a correct diagnosis and appropriate treatment. Mainstay treatments include exposure-based psychosocial therapy and selective serotonin reuptake inhibitors, such as paroxetine and sertraline, both of which have been found to be effective in PTSD. This paper looks at the course of PTSD, its disabling effect, its recognition and treatment, and considers possible new research directions.
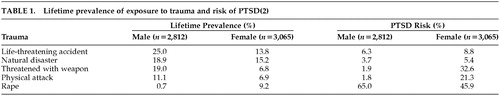
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder associated with a significant degree of morbidity. Lifetime prevalence rates in the community have been estimated at 1.3%–7.8%.1,2 However, as might be expected, a higher lifetime PTSD prevalence of around 30% has been reported for Vietnam veterans and female victims of rape in retrospective epidemiological studies.3,4 The risk of developing PTSD has been shown to vary according to the type of trauma. In common with many other psychiatric disorders, a higher prevalence of PTSD occurs in women than in men. The lifetime prevalence of exposure to trauma and the risk of developing PTSD are shown by gender and trauma in Table 1.2
Posttraumatic stress disorder has been recognized as a distinct psychiatric disorder since the introduction of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III)5 in 1980. According to this classification, a diagnosis of PTSD required exposure to a recognizable stressor that would evoke symptoms of distress in almost everyone. This definition was modified some years later6 to emphasize the requirement of avoidance phenomena, which consist of deliberate efforts to avoid thoughts, feelings, activities, and situations that aroused recollections of the trauma. The avoidance of recollection of the traumatic stressor only served to emphasize the fundamental importance in the development of PTSD of exposure to an extreme, life-threatening stressor.7 Changes to the definition of PTSD that were brought about in the DSM-IV8 included redefining the traumatic event. This had to involve actual or threatened death or serious injury or a threat to the physical integrity to self or others. In addition, the person experiencing the event had to respond with intense fear, helplessness, or horror. Clinically significant distress and impairment in social, occupational, or other important areas of functioning with a minimum duration of 1 month were further requirements.8 This definition of PTSD now encompasses the concept of “vicarious traumatization” (being confronted by serious injury to others), which has significance for emergency services and rescue workers.
Posttraumatic stress disorder frequently follows a chronic course and can be associated with recurrences related to exposure to multiple traumas. In addition, PTSD is frequently comorbid with other psychiatric conditions such as anxiety disorders, depression, and substance abuse.2
This paper will address 1) issues concerning the acquisition and course of PTSD, including physiologic and neuroendocrine factors; 2) recognition and impairment; 3) recent studies of psychotherapy and pharmacotherapy, especially with selective serotonin reuptake inhibitor (SSRI) drugs; and 4) suggest some directions for research.
THE COURSE OF PTSD
When faced with a traumatic experience, a large majority of the population will have a brief acute response to stress and will not develop any long-lasting pathologic sequelae, whereas a smaller proportion will experience persistent PTSD, either alone or in combination with major depression.9 Previous vulnerability to depressive or anxiety disorders increases the likelihood that PTSD will develop. Indeed, both vulnerability and protective factors will affect the individual in the transitional phase from the acute stress response. In this critical stage, the acute reaction will stabilize in some individuals, whereas there is progressive decompensation in others.
Pathological Development
Some of the symptoms of PTSD are considered to be reflections of the adaptive mental processes involved in the assimilation and integration of new information that results from exposure to trauma.7 This equates to PTSD symptoms representing part of a normal survival instinct in individuals exposed to trauma, and the pathologic development of PTSD only follows if the response that leads to resolution of the trauma is disrupted in some way. The main conclusion of biological research into PTSD is that exposure to one or more traumatic events triggers a chain of mental and biological events, which ultimately lead to prolonged PTSD.10
An examination of the longitudinal course of PTSD in a study of rape victims showed that 94% of rape survivors had all the clinical symptoms of PTSD 1 week after the traumatic event, thereby suggesting that such a clinical picture probably constitutes a normal reaction (Figure 1).11 At 3 and 9 months after the event, the proportion of rape victims expressing symptoms of PTSD declines to 15%–25%. Following this period, the curve remains horizontal, indicating that PTSD is an unremitting and chronic disorder that can last for any length of time. In the National Comorbidity Study (NCS), median duration of PTSD associated with worst lifetime trauma was 3 years for those receiving treatment and 5 years for those who did not receive treatment.2 However, this estimate does not take into account the real possibility that people may experience PTSD more than once in their lives. Indeed, a great many people report exposure to multiple traumas over a life course.12 Data from the NCS which used DSM-III-R criteria showed that 60.7% of men and 51.2% of women reported exposure to at least one lifetime traumatic event.2 DSM-IV criteria for PTSD expand the set of stressful experiences to include sudden death of a close friend, or loved one, or diagnosis of a life-threatening illness. Results from a community epidemiologic survey in the USA that used DSM-IV criteria reported that 90% of respondents had exposure to at least one lifetime traumatic event.13
Physiological Responses
It has been reported that nonphysically injured trauma survivors admitted to the emergency room, who subsequently went on to develop PTSD, had higher heart rates at the emergency department (mean=95.5 beats per minute, SD=13.9, versus mean=83.3 beats per minute, SD=10.9) (t=4.4, P<0.001) and 1 week later (mean=77.8 beats per minute, SD=11.9, versus mean=72.0 beats per minute, SD=9.5) (t=2.25, P<0.03), but not after 1 and 4 months.14 Similarly, in a study of trauma survivors, the physiologic response of heart rate, skin conductance and electromyography (frontalis) to mental imagery recorded a short time following the trauma has been shown to differentiate between those who go on to develop PTSD and those who do not (Figure 2).15 The importance of such physiologic responses is clear, since our clinical experience indicates that PTSD patients can re-access their trauma memories as often as 100 times a day and elicit these physiologic reactions each time. PTSD patients possibly continue to reinforce the initial impact of the trauma by reactivating it in this way. PTSD patients have also been reported to differentiate from normal survivors by poor habituation of skin conductance to a repetition of loud startling noises (Figure 3).16 Traumatized non-PTSD and anxious groups showed normal habituation by contrast. This may represent a primary defect of the central nervous system that continues to identify and classify the loud tones as threatening in people with PTSD. PTSD patients, therefore, continue to react, rather than rejecting the noises as redundant information and stopping the reaction to them. In a prospective study of 239 trauma survivors,17 the auditory startle response of all the trauma survivors is normal at 1 week. The response of those patients who go on to develop PTSD becomes abnormal between 1 and 4 months after the trauma, suggesting that this is the critical period during which the central nervous system adapts its response to ambiguous stimuli (such as loud noises) and determines whether PTSD develops.
There are two important questions for the clinician to address when trying to recognize the vulnerable patients that will develop PTSD: 1) Why does trauma lead to PTSD for them rather than some other psychiatric disorder or no disorder at all? 2) What are the risk factors for determining these patients? The acute stress response is universal and nonpredictive of PTSD. Moreover, as mentioned, patients who develop PTSD fail to show a remission of these acute symptoms and show abnormally increased heart rates several days after the trauma as well as other abnormal physiologic responses such as the increased startle response. It would therefore appear that PTSD might develop as a failure of the body to reverse the acute stress response.
Delayed and Chronic Forms of PTSD
The onset of PTSD can be delayed for years. In a large study by Solomon18 looking at individuals who presented for treatment within 6 years of the Lebanon war, 10% were considered delayed onset, 40% were delayed help-seeking, 33% were exacerbation of subclinical PTSD, 13% were reactivation of recovered PTSD, and the remaining 4% had other psychiatric disorders. This is confirmed in the study by Shalev et al.19 where 5.1% of patients were truly delayed onset PTSD and the rest were mainly PTSD patients who recovered and were then reactivated by another event.
Most cases of PTSD recover within 1 year, and after 6 years recovery without treatment is unlikely.2 However, up to 40% of patients with PTSD have a chronic condition. Chronic PTSD is prolonged and may be unremitting, and subject to reactivation upon exposure to stressors. In addition, it can be disabling and associated with substantial comorbidity. The risk of developing secondary comorbid disorders is related to a number of factors, including the severity of the trauma, gender, family history, past history, and the complexity of the PTSD reaction. Chronic PTSD is linked with abuse of alcohol, drugs, and medication. It is also associated with mortality from suicide.20 The frequency of comorbid disorders with PTSD is shown for male and females in Table 2.2 The percentages of individuals with PTSD who have at least one other lifetime disorder is 88.0% for men and 79.0% for women. The major comorbid disorder seen with PTSD is depression, occurring in 47.9% of men and 48.5% of women. Other comorbid disorders include dysthymia, specific phobia and generalized anxiety disorder.
Disability Associated With PTSD
The chronic form of PTSD is often debilitating. The disability associated with PTSD includes work impairment, change in life trajectories, impaired social relations, marital instability and perpetuation of violence. This not only represents a burden to the individual but to society as well.
In a study based on the analysis of the NCS data, which examined the effects of mental disorders on work impairment,21 work loss (defined as missing a full day of work), and work cut back (either missing part of a day or working less efficiently than usual) during the previous month was 0.8 days/month and 2.8 days/month, respectively. The amount of work impairment associated with PTSD was the same as that associated with major depression but less than that associated with panic disorder.21
In the NCS data, among those with PTSD, there is an increased risk of making suicide plans (odds ratio [OR]=2.4; 95% confidence interval [CI]=1.7–3.3) and an increased risk of attempting suicide (OR=6; 95% CI=3.4–10.7) for patients suffering from PTSD. In addition, marital instability, unemployment, and increased use of outpatient care contribute greatly to the burden to society.12 The NCS analyses showed that the most extreme adverse effects of traumatic events were associated with complex ongoing traumas that occurred in childhood, such as parental violence, alcoholism, or depression. Such experiences interfere with lifelong patterns of interpersonal relationships and the process of mastering basic educational skills.
In the study by Stein et al.,22 patients with PTSD reported significantly more functional impairment than patients without mental disorders. In addition, patients with PTSD made greater use of healthcare resources than non-mentally-ill patients and encountered considerable functional impairment.
A study of the quality of life with PTSD reported greater impairment at baseline for subjects with PTSD relative to those with major depression and obsessive-compulsive disorder on several domains of the 36-item Short-Form Health Survey.23 Similarly, in a study of PTSD among civilians, significant impairment was associated with PTSD as seen on the Sheehan Disability Scale, which measures the total work, family and social/leisure disability and the Vulnerability to the Effects of Stress Scale.24
RECOGNITION OF PATIENTS WITH PTSD
By definition, the patient with PTSD must have experienced a traumatic event before the onset of the disorder. The symptoms of PTSD revolve around reliving the traumatic experience, and include recurrent and intrusive thoughts and/or dreams of the trauma, difficulty in falling or staying asleep, irritability, hypervigilance, and avoidance of stimuli associated with the trauma.
Many patients, however, fail to seek medical help as they do not recognize that they have a problem. Certainly, the most commonly reported reason for not being in treatment among the 62% of PTSD cases in the NCS study was that they did not have a problem.12 Even those with quite severe impairment cited this reason. This failure to seek help is not only a question of lack of information or ability to perceive that they have a problem, but is representative of avoidance of trauma or addressing traumatic recollections. Those who recognized their need for help give a number of other reasons for being in treatment. Most commonly these were expense of treatment, uncertainty about where to go for help, thinking the problem will get better by itself, and wanting to solve the problem on one's own. Others reasons included the stigma, fear of forced hospitalization, language barriers, and dissatisfaction with the services.
Among the general population in the United States, it is estimated that 38% of people with PTSD are treated in any given year.25 The majority of these patients (28% of cases and 75% of those in treatment) are seen by the medical practitioners, while others are seen by the human services personnel or self-help groups. Only 22% of those with PTSD (38% of those in treatment) are seen by mental health professionals (psychiatrist, clinical psychologists, or other).
Patients with PTSD who present in primary care are likely to present with somatic symptoms such as pain associated with increased onset of arterial, lower gastrointestinal, dermatologic, and musculoskeletal disorders.26 Similarly, they also report numerous sleep disturbances. In a study of 1,832 subjects with PTSD from an urban general population, sleep disturbances also affected about 70% of the PTSD subjects.27 Violent or injurious behaviors during sleep, sleep paralysis, sleep talking, and hypnagogic and hypnopompic hallucinations were more frequently reported in respondents with PTSD.
In a study of patients with irritable bowel syndrome,28 18 (36%) of 50 patients were diagnosed with PTSD. Irritable bowel syndrome patients with a history of trauma were more likely to have other comorbid psychiatric diagnoses as well.
In a study to determine the relationship between a history of PTSD and somatization of symptoms, a history of PTSD was associated with significantly more symptoms in each of the somatic symptom groups, except pain.29 In addition, persons with PTSD were more likely to report each of the symptoms of somatization, compared to those with other psychiatric disorders. Prospectively, baseline history of PTSD signaled an increased risk of pain (OR=2.1) and conversion symptoms (OR=2.3) in the follow-up interval, relative to those with no disorder.
Chronic PTSD is linked with abuse of alcohol, drugs, and medication.12,30 In common with many other anxiety disorders, PTSD is often complicated by secondary depression (60%–80% of patients), particularly if the condition has not been treated. Patients will therefore present in either primary or secondary care with comorbid depression, which complicates the recognition of PTSD per se, and prevents the primary diagnosis from being made. Despite some of the symptoms of PTSD being shared with major depression, the clinician should be alerted by the presence of intrusive recollections and pervasive avoidance of a trauma. In addition, when PTSD is complicated by secondary depression, the symptom profile tends to differ from that of major depression, with less psychomotor retardation or agitation.20 In the study of PTSD in the primary care medical setting by Stein et al.,22 11.8% of primary care attendees met diagnostic criteria for either full or partial PTSD. Comorbidity with major depression (61% of cases of PTSD) and generalized anxiety disorder (39%) was common, but less so with social phobia (17%) and panic disorder (6%). Substance use disorder comorbidity (22%) was also fairly common.
Patients who suffer from the effects of chronic interpersonal violence are more likely to have chronic PTSD, and the symptom profile is likely to be more complex and often involves severe forms of dissociation not found in more typical cases of PTSD. The profile is so distinct it has been argued for the creation of a separate diagnosis to characterize this response known as “complex PTSD”31,32 or “disorders of extreme stress not otherwise specified.”33,34 Although this diagnosis is not included in DSM-IV due to the fact that the vast majority of patients with this symptom cluster also meet criteria for PTSD, it is nonetheless clear that a complex PTSD subtype exists. This subtype is more chronic and disabling than other cases of PTSD, and it is particularly common among patients who were exposed at an early age to chronic traumatic interpersonal violence.
Cortisol levels associated with PTSD
The neurobiology of the acute stress response is summarized in Figure 4. Sensory input from a traumatic event is transmitted to the amygdala, and following cortical input leads to activation of four simultaneous types of response. The first response is the activation of the startle response through the reticularis pontis caudalis. The second response, via the sympathetic nervous system, consists of a release of adrenaline to increase heart rate, blood pressure, blood flow, and increase of glucose to the muscles. This increase in adrenaline is also relevant for the formation of memory. Suppression of the parasympathetic nervous system, which occurs in order to shut down any responses that might compete with the sympathetic nervous system (e.g., digestion, tissue repair, ovulation), constitutes the third response and is independent of the sympathetic nervous system. Finally, in the fourth response, through the hypothalamic-pituitary-adrenal (HPA) axis, the hippocampus and amygdala activate the hypothalamus, which releases corticotrophin release factor (CRF), which in turn activates the pituitary gland to release adrenocorticotrophin hormone. This then stimulates the adrenal gland to release cortisol. The cortisol levels, which are proportional in magnitude to the levels of the stressor, inhibit the sympathetic nervous system, and via a negative feedback inhibition also attenuate the HPA axis.
In healthy individuals, circulating catecholamines and cortisol return to normal within hours. In the long term, exposure to a subsequent stressor (sensitization) can occur and create an exaggerated stress response, but baseline hormone levels will not indicate whether previous stressful events have occurred. However, in individuals with chronic PTSD, both baseline CRF and cortisol levels are decreased. There is also increased sympathetic nervous system activation to trauma, enhanced startle response to both neutral and trauma-related cues, and evidence of decreased parasympathetic nervous system activity. This has led to the view that PTSD may represent an extension of the normal stress response. One hypothesis proposed to explain this is that there are insufficient cortisol levels at the time of the trauma in some individuals, such that the other biologic reactions are not inhibited, and a prolonged activation of some of the stress responses occurs. In support of this, the cortisol levels found in the aftermath of a motor vehicle accident in patients who went on to develop PTSD were found to be lower than in those who remained well or in those who suffered from depression.35 A further study of cortisol levels taken in the emergency room from victims in the aftermath of a rape showed lower levels of cortisol in those who had suffered a previous assault than in those with no previous assault.36 Therefore, prior trauma is definitely a risk for developing PTSD, along with the more common risks such as exposure to trauma, avoidant personality, genetic or familial factors, and cognitive variables such as IQ and education. Studies to isolate which of these variables are particular risk factors for developing PTSD are difficult to design. However, one group of patients that have been shown to be a high-risk group for developing PTSD are the children of Holocaust survivors.37 Further study of this group showed that only the children of parents who developed PTSD during the Holocaust were at risk of PTSD themselves when faced with trauma. Children with PTSD whose parents had PTSD have low cortisol levels, while Holocaust children with no PTSD (and no parental PTSD) have normal cortisol levels. Interestingly, children with no trauma or PTSD but whose parents had PTSD also had low cortisol levels, indicating that the low cortisol may be related to factors that predate the trauma. In short, low cortisol levels may be related to risk for PTSD. The most likely explanation for this low cortisol before trauma is that the cortisol receptors in the pituitary are oversensitive, leading to a greater negative feedback inhibition, which results in lower cortisol levels. This increased receptor sensitivity in PTSD has been successfully demonstrated using a low-dose dexamethasone suppression test, which repeatedly showed an enhanced suppression of cortisol in PTSD patients (Figure 5).38–42 Therefore, under acute stress, this enhanced negative feedback could result in a premature shut down of the HPA axis, which would prevent the damping down of the sympathetic nervous system. This would lead to a cascade of consequences as detailed in Figure 6. If this cascade model for PTSD is correct, then possible areas for intervention that may help the patient with PTSD become apparent, such as: reduction of nonspecific arousal, reduction of memory-related distress, cognitive restructuring to avoid forming new associations and stimulus generalizations, cognitive restructuring to correct already formed negative associations, addressing any number of the cascade of alterations that have occurred since the trauma (sleep disruption, characterological issues, social avoidance).
TREATMENT OF PTSD
Appropriate treatment of PTSD is essential to reduce symptoms and increase both the functioning and quality of life of the patient. Early intervention is particularly crucial in order to help prevent the development of secondary chronic morbidity.
There are five main treatment goals when treating PTSD: reducing the core symptoms, improving stress resilience, improving quality of life, reducing disability and reducing comorbidity.
A number of treatment outcome studies for PTSD have focused on cognitive-behavioral therapy programs, which include variants of exposure therapy, anxiety management and cognitive therapy. More recently, eye movement and desensitization therapy has been employed. Pharmacological treatments are being studied. Both kinds of treatment are effective.
Psychosocial treatment
Psychosocial treatment of PTSD has shown some promising results and, when effective, has been associated with low relapse rates. Of the existing treatments, exposure therapy has the strongest evidence of efficacy in different populations of trauma victims with PTSD. However, a minority of patients failed to show sufficient gains with this therapy.43 Anxiety management techniques have also been shown to be effective in the treatment of PTSD after rape,11 although not widely studied. Cognitive therapy has also been seen to be effective in rape victims.44 More recently, a study by Marks et al.,45 of different victim populations with a history of PTSD of at least 6 months' duration studied the treatment effect of prolonged exposure (imaginal and live) alone; cognitive restructuring alone; combined prolonged exposure and cognitive restructuring; or relaxation without prolonged exposure or cognitive restructuring. The study showed that both prolonged exposure and cognitive restructuring were each therapeutic on their own, were not mutually enhancing when combined, and were each superior to relaxation.
Interestingly, this lack of advantage for combining different types of psychosocial treatments was also found in a study by Foa et al.46 Studies attempting to examine augmentation of exposure therapy outcome with the addition of cognitive restructuring do not show any additional benefit over exposure therapy alone. No studies have examined the relative efficacy of pharmacotherapy and cognitive-behavioral therapy, and whether combination of the two will augment the efficacy of each.
Eye movement and desensitization reprocessing (EMDR) is a relatively new therapy for PTSD that consists of a form of exposure accompanied by saccadic eye movements.47 In EMDR, the therapist asks the patient to visualize images about the trauma, while inducing eye movements by asking the patient to track rapid side-to-side movement of the therapist's finger. A cognitive therapy component is also included by asking patients to replace negative thoughts with positive ones. There have been several studies of the efficacy of EMDR in the treatment of PTSD,46–49 although most have not been well controlled.43 A well-designed study49 found EMDR was inferior to prolonged exposure cognitive therapy.
Successful treatment of PTSD with regard to the patient will involve attitude to treatment, capacity to tolerate distress, availability of support, and extent of comorbidity.50 To determine the real benefit of psychosocial treatments for PTSD, further large, well-controlled studies that compare the benefits of specific techniques are required.
Pharmacotherapy
The principal goals of pharmacotherapy are reducing PTSD symptoms, improving resilience to stress and quality of life, and reducing disability and comorbidity. Reducing comorbidity is particularly important since patients rarely present with pure PTSD. There are three main classes of drugs that have demonstrated efficacy in the treatment of PTSD: tricyclic antidepressants (TCAs) such as amitriptyline and imipramine, monoamine oxidase inhibitors (MAOIs) such as phenelzine, and SSRIs such as sertraline, paroxetine, and fluoxetine. In addition, a variety of other drugs show promise including antiadrenergics, anticonvulsants and antidepressants (lamotrigine, nefazodone, clonidine).
There is considerable evidence from placebo-controlled trials that TCAs and MAOIs are effective in reducing symptoms of PTSD (Figure 7 and Figure 8).51–53 There is also preliminary evidence from open-label studies of carbamazepine, valproic acid, and from a small double-blind trial of lamotrigine, that anticonvulsants can produce benefit in PTSD.54–56
More recently, placebo-controlled studies have shown that the SSRIs sertraline57 and paroxetine58 are effective in decreasing symptoms of PTSD, and considerable interest has centered on these new data. Similarly, there have been positive placebo-controlled trials for fluoxetine, however results in USA combat veterans59,60 were not as positive as for the general PTSD population.24 A European study found benefit for fluoxetine in predominantly combat veterans, with relapse prevention effects in maintenance treatment.61,62
In the 12-week, double-blind study of sertraline in 187 psychiatric outpatients with DSM-III-R PTSD, sertraline treatment yielded a significantly greater improvement than placebo in mean change from baseline for Clinician-Administered PTSD Scale Part 2 (CAPS-2) total score (P=0.02).57 In addition, sertraline was significantly better than placebo for the symptom clusters avoidance/numbing (P=0.02) and arousal (P=0.03), but not on reexperiencing/intrusion (P=0.14). In this study, however, 73% of the population was female and 61.5% had suffered physical or sexual assault. Further studies of sertraline are required to demonstrate its efficacy in both genders and across all trauma types. A relapse-prevention study found the risk of relapse to be significantly less with sertraline than with placebo after 9 months of treatment.63
In a 5-week double-blind, randomized, placebo-controlled study of fluoxetine in 64 patients (22 women and 42 men) (31 veteran and 33 nonveterans), fluoxetine significantly reduced overall PTSD symptomatology as assessed by total CAPS score.59 However, the difference in PTSD symptom reduction occurred primarily in the subscales “numbing” (for the nonveteran group) and “arousal.” Fluoxetine was an effective antidepressant in the total sample as measured by the Hamilton Depression Rating Scale (HAM-D). However, these improvements in depression did not predict improvement in PTSD score. While there was substantial improvement in depression (HAM-D) in the veteran sample (P= 0.005), there were no meaningful changes in numbing symptoms (P=0.70). Conversely, the nonveteran sample had a modest improvement in depression (HAM-D, P=0.04), but there was a substantial improvement in numbing (P=0.002). In general, nonveteran patients responded much better than veteran patients, which is probably reflective of the higher level of symptomatology in the veterans. In another double-blind, randomized, placebo-controlled study in 53 civilians over a 12-week period, fluoxetine (up to 60 mg/day) significantly reduced overall PTSD symptomatology as assessed by the Duke Global Severity Rating for PTSD, the Structured Interview for PTSD, and the Davidson Trauma Scale (DTS).24 Vulnerability to the effects of stress also responded well to fluoxetine.
In the 12-week, open-label study with paroxetine that was conducted in 19 civilians with DSM-III-R PTSD,64 patients showed significantly reduced mean PTSD symptom scores for both the DTS score (P<0.0001) (Figure 9) and the Impact of Event Scale score (P<0.0001). In addition, there were significant improvements in all symptom clusters for both scales. Interestingly, cumulative childhood trauma scores were significantly and negatively correlated with treatment response on the total DTS score (r=0.52, P<0.03). Since the study by Marshall, there has been a series of large clinical trials with paroxetine in PTSD. The clinical program involved 1180 patients with a DSM-IV diagnosis of PTSD, and demonstrated that paroxetine relieves the symptoms of PTSD, significantly reducing mean change from baseline in CAPS-2 total score and Clinical Global Impression global improvement scale. In addition, it significantly reduced the CAPS-2 score for reexperiencing/intrusion (P<0.001), avoidance/numbing (P<0.001), and arousal (P<0.001) compared with placebo. This new data, which was generated in 12-week, double-blind, randomized, fixed- or flexible-dose studies also confirmed that treatment benefit with paroxetine (20–50 mg/day) was observed across all trauma types and in both genders.65,66
In the fixed-dose study, which was conducted in the United States with 551 PTSD patients who had experienced traumas, including physical or sexual assault, witnessing an accident, experiencing a serious accident/fire/injury, being in war/combat or being involved in a natural disaster, paroxetine was given in doses of either 20 mg or 40 mg/day.58,66 Decreases from baseline in CAPS-2 total score were significantly greater (P<0.001) for both doses compared with placebo (Figure 10). In addition, decreases from baseline in the 8-Item Treatment Outcome PTSD Scale (TOP-8) total score and DTS total score were also significant for both doses (P<0.001). Similarly, in the U.S. flexible-dose study in which 307 patients received 20–50 mg/day of paroxetine, there were significant decreases from baseline for CAPS-2 total score (P<0.001) (Figure 10), TOP-8 total score (P<0.001), and DTS total score (P<0.001).58,65
Paroxetine was well tolerated with an adverse event profile similar to that in other anxiety indications.
CONCLUSIONS
Following exposure to trauma, almost all survivors develop some short-lasting symptoms of PTSD. A variety of factors determine the likelihood of such reactions becoming chronic, including physiological/autonomic and neuroendocrine (HPA axis) influences. Once developed, PTSD often becomes disabling and unremitting, being accompanied by a high incidence of comorbid depression as well as somatization, both of which may impede recognition of the condition. As with other anxiety disorders, PTSD is associated with substantial impairment. Prompt recognition and treatment of PTSD may help to prevent the development of secondary morbidity. Exposure therapy has shown promising results, and the utility of pharmacotherapy is now gaining increasing attention. Fluoxetine, sertraline, and paroxetine have all shown positive effects in PTSD, with the largest database existing for paroxetine. Other drugs (e.g., anticonvulsants) may have a useful place.
Mild PTSD may be successfully treated with psychotherapy but in moderate to severe cases combined pharmacotherapy with psychotherapy should be considered.
A number of interesting and important questions still remain to be answered, among which are the following:
What factors protect individuals from developing PTSD after trauma, and how might they be acquired? Do drugs, psychotherapy, exercise, or mindfulness-enhancing techniques help promote resilience?
How do drugs and psychosocial treatments compare? When, and how are they best combined?
Do the neuroendocrine and other biological alterations found in PTSD correct themselves with effective treatments? Can their persistence serve as markers of relapse risk?
Can high-risk individuals be identified early following trauma exposure, and then given preventive treatment? (e.g., Pitman et al.67).
 |
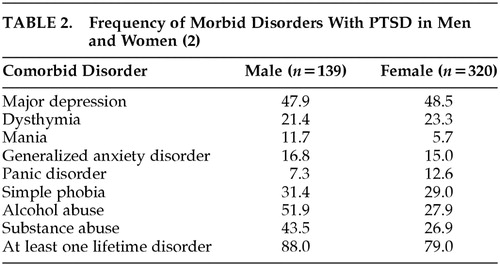 |
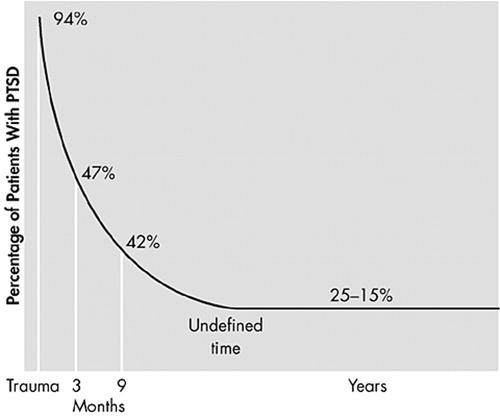
FIGURE 1. Rate of PTSD Symptoms in Rape Victims
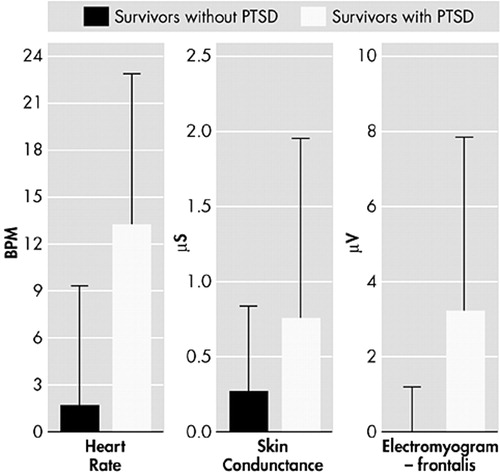
FIGURE 2. Physiological Responses to Trauma-Related Mental Imaging
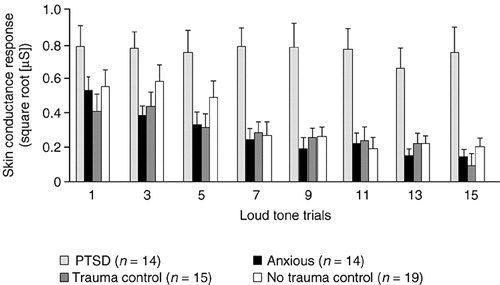
FIGURE 3. Habituation of Skin Conductance Responses
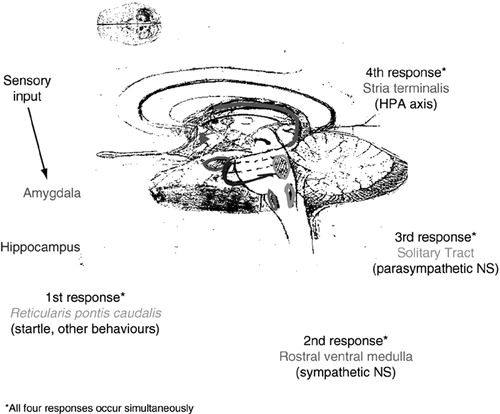
FIGURE 4. Neurobiology of the Acute Stress Responses
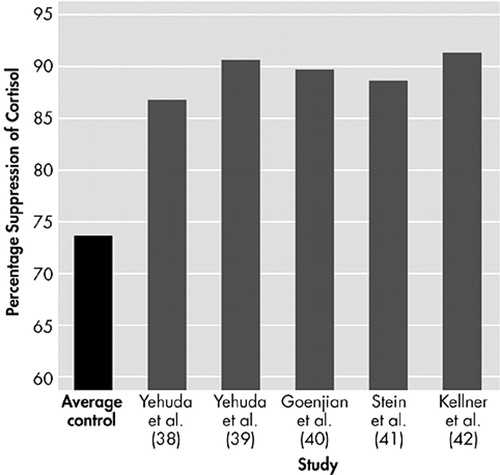
FIGURE 5. Enhanced Suppression of Cortisol Following Low-Dose Dexamethasone
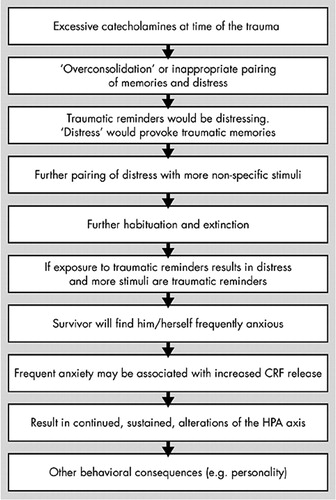
FIGURE 6. Cascade Model of PTSD
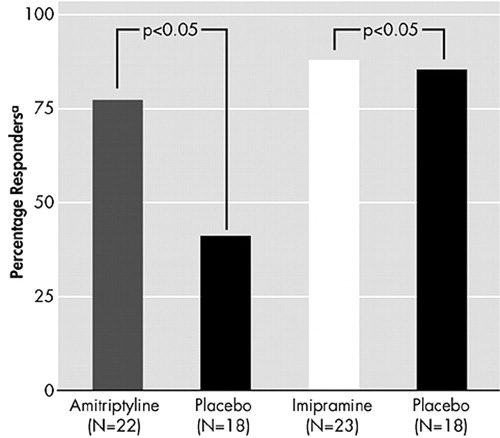
FIGURE 7. Response to Tricyclic Antidepressants in Combat-Related PTSD
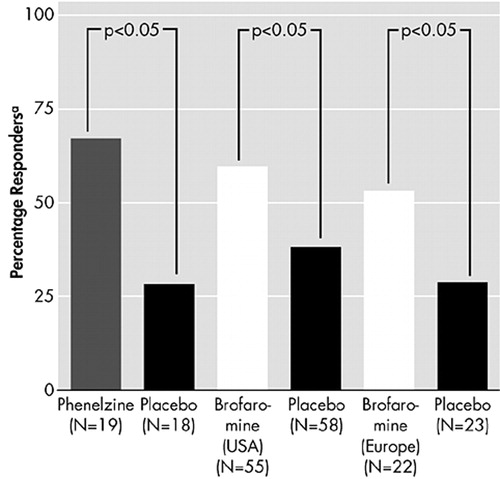
FIGURE 8. Response to MAOI Drugs in PTSD
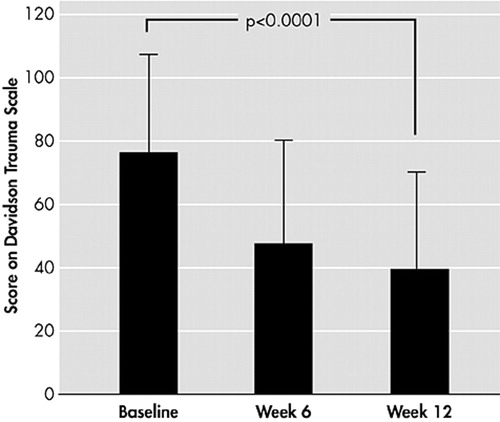
FIGURE 9. Effects of Open-Label Paroxetine in PTSD
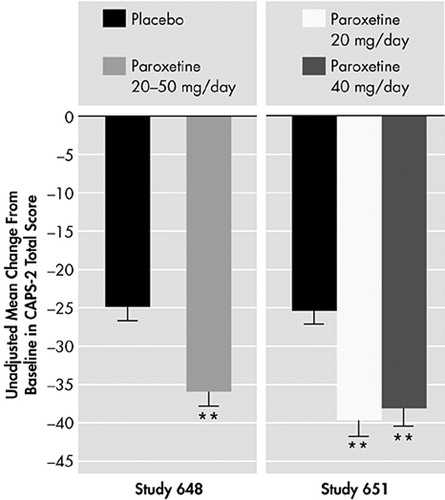
FIGURE 10. Response to Paroxetine in PTSD: Multicenter Trials
1 Davidson JRT, Hughes D, Blazer DG, et al: Post-traumatic stress disorder in the community: an epidemiological study. Psychol Med 1991; 21:713–721Crossref, Medline, Google Scholar
2 Kessler RC, Sonnega A, Bromet E, et al: Posttraumatic stress disorder in the National Comorbidity Study. Arch Gen Psychiatry 1995; 52:1048–1060Crossref, Medline, Google Scholar
3 Kulka RA, Fairbank JA, Jordan BK, et al: Trauma and the Vietnam War Generation. New York, Brunner/Mazel, 1990Google Scholar
4 Resnick HS, Kilpatrick DG, Dansky BS, et al: Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol 1993; 61:984–991Crossref, Medline, Google Scholar
5 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd ed (DSM-III). Washington, DC, APA, 1980Google Scholar
6 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd ed, revised (DSM-III-R). Washington, DC, APA, 1987Google Scholar
7 Turnbull GJ: A review of post-traumatic stress disorder, part I: historical development and classification. Injury 1998; 29:87–91Crossref, Medline, Google Scholar
8 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV). Washington, DC, APA, 1994Google Scholar
9 Shalev AY, Freedman S, Peri T, et al: Prospective study of post-traumatic stress disorder and depression following trauma. Am J Psychiatry 1988; 155:630–637Crossref, Google Scholar
10 Yehuda R, McFarlane AC: Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. Am J Psychiatry 1995; 152:1705–1713Crossref, Medline, Google Scholar
11 Foa EB, Rothbaum BO, Riggs D, et al: Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive behavioral procedures and counseling. J Consult Clin Psychol 1991; 59:715–723Crossref, Medline, Google Scholar
12 Kessler RC: Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry 2000; 61(suppl 5):4–12Google Scholar
13 Breslau N, Kessler RC, Chilcoat HD, et al: Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry 1998; 55:626–632Crossref, Medline, Google Scholar
14 Shalev AY, Sahar T, Freedman S, et al: A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch Gen Psychiatry 1988; 55:553–559Crossref, Google Scholar
15 Shalev AY, Orr SP, Pitman RK: Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with post-traumatic stress disorder. Am J Psychiatry 1993; 150:620–624Crossref, Medline, Google Scholar
16 Shalev AY, Orr SP, Peri T, et al: Physiological responses to loud tones of Israeli post-traumatic stress disorder patients. Arch Gen Psychiatry 1992; 49:870–875Crossref, Medline, Google Scholar
17 Shalev AY, Pitman RK, Orr SP, et al: Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. Am J Psychiatry 2000; 157:255–261Crossref, Medline, Google Scholar
18 Solomon Z, Kostler M, Shalev A, et al: Delayed post-traumatic stress disorders. Psychiatry 1989; 52:428–436Crossref, Medline, Google Scholar
19 Shalev AY, Peri T, Canetti L, et al: Predictors of PTSD in injured trauma survivors: a prospective study. Am J Psychiatry 1996; 153:219–225Crossref, Medline, Google Scholar
20 Ballenger JC, Davidson JR, Lecrubier Y, et al: Consensus statement on posttraumatic stress disorder from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry 2000; 61(suppl 5):60–66Google Scholar
21 Kessler RC, Frank RG: The impact of psychiatric disorders on work loss days. Psychol Med 1997; 27:861–873Crossref, Medline, Google Scholar
22 Stein MB, McQuaid JR, Pedrelli P, et al: Post-traumatic stress disorder in the primary care medical setting. Gen Hosp Psychiatry 2000; 22:261–269Crossref, Medline, Google Scholar
23 Malik ML, Connor KM, Sutherland SM, et al: Quality of life and posttraumatic stress disorder: a pilot study assessing changes in SF-36 scores before and after treatment in a placebo-controlled trial of fluoxetine. J Trauma Stress 1999; 12:387–393Crossref, Medline, Google Scholar
24 Connor KM, Sutherland SM, Tupler LA, et al: Fluoxetine in post-traumatic stress disorder: randomised, double-blind study. Br J Psychiatry 1999; 175:17–22Crossref, Medline, Google Scholar
25 Kessler RC, Zhao S, Katz SJ, et al: Past-year use of outpatient services for psychiatric problems in the National Comorbidity Survey. Am J Psychiatry 1999; 156:115–123Crossref, Medline, Google Scholar
26 Schnurr PP, Spiro A III, Paris AH: Physician-diagnosed medical disorders in relation to PTSD symptoms in older male military veterans. Health Psychol 2000; 19:91–97Crossref, Medline, Google Scholar
27 Ohayon MM, Shapiro CM: Sleep disturbances and psychiatric disorders associated with post-traumatic stress disorder in the general population. Compr Psychiatry 2000; 41:469–478Crossref, Medline, Google Scholar
28 Irwin C, Falsetti SA, Lydiard RB, et al: Comorbidity of posttraumatic stress disorder and irritable bowel syndrome. J Clin Psychiatry 1996; 57:576–578Crossref, Medline, Google Scholar
29 Andreski P, Chilcoat H, Breslau N: Post-traumatic stress disorder and somatization symptoms: a prospective study. Psychiatry Res 1998; 79:131–138Crossref, Medline, Google Scholar
30 Chilcoat HD, Breslau N: Posttraumatic stress disorder and drug disorders: testing causal pathways. Arch Gen Psychiatry 1998; 55:913–917Crossref, Medline, Google Scholar
31 Herman JL: Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress 1992; 5:377–391Crossref, Google Scholar
32 Zlotnick C, Zakriski AL, Shea MT, et al: The long-term sequelae of sexual abuse: support for a complex posttraumatic stress disorder. J Trauma Stress 1996; 9:195–205Crossref, Medline, Google Scholar
33 van der Kolk BA, Pelcovitz D, Roth S, et al: Dissociation, somatization, and affect dysregulation: the complexity of adaptation to trauma. Am J Psychiatry 1996; 153(July suppl):83–93Google Scholar
34 Pelcovitz D, van der Kolk B, Roth S, et al: Development of a criteria set and a Structured Interview for Disorders of Extreme Stress (SIDES). J Trauma Stress 1997; 10:3–15Medline, Google Scholar
35 McFarlane AC, Atchison M, Yehuda R: The acute stress response following motor vehicle accidents and its relation to PTSD. Ann NY Acad Sci 1997; 821:437–441Crossref, Medline, Google Scholar
36 Resnick HS, Yehuda R, Pitman RK, et al: Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry 1995; 152:1675–1677Crossref, Medline, Google Scholar
37 Yehuda R, Bierer LM, Schneider J, et al: Low cortisol and risk of PTSD in adult offspring of Holocaust survivors. Am J Psychiatry 2000; 157:1252–1259Crossref, Medline, Google Scholar
38 Yehuda R, Southwick SM, Krystal JH, et al: Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 1993; 150:83–86Crossref, Medline, Google Scholar
39 Yehuda R, Boisoneau D, Lowy MT, et al: Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry 1995; 52:583–593Crossref, Medline, Google Scholar
40 Goenjian AK, Yehuda R, Pynoos RS, et al: Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry 1996; 153:929–934Crossref, Medline, Google Scholar
41 Stein MB, Yehuda R, Koverola C, et al: Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry 1997; 42:680–686Crossref, Medline, Google Scholar
42 Kellner M, Baker DG, Yehuda R: Salivary cortisol and PTSD symptoms in Persian Gulf War combatants. Ann NY Acad Sci 1997; 821:442–443Crossref, Medline, Google Scholar
43 Foa EB, Meadows EA: Psychosocial treatments for post-traumatic stress disorder: a critical review. Annu Rev Psychol 1997; 48:449–480Crossref, Medline, Google Scholar
44 Resick PA, Schnicke MK: Cognitive processing therapy for sexual assault victims. J Consult Clin Psychol 1992; 60:748–756Crossref, Medline, Google Scholar
45 Marks I, Lovell K, Noshirvani H, et al: Treatment of post-traumatic stress disorder by exposure and/or cognitive restructuring: a controlled study. Arch Gen Psychiatry 1988; 55:317–325Google Scholar
46 Foa EB, Dancu CV, Hembree EA, et al: A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol 1999; 67:194–200Crossref, Medline, Google Scholar
47 Shapiro F: Eye Movement Desensitization and Reprocessing: Basic Principles, Protocols, and Procedures. New York, Guilford, 1995Google Scholar
48 Rothbaum BO: A controlled study of eye movement desensitization and reprocessing in the treatment of posttraumatic stress disordered sexual assault victims. Bull Menninger Clin 1997; 61:317–334Medline, Google Scholar
49 Devilly GJ, Spence SH: The relative efficacy and treatment distress of EMDR and a cognitive-behavior trauma treatment protocol in the amelioration of posttraumatic stress disorder. J Anxiety Disord 1999; 13:131–157Crossref, Medline, Google Scholar
50 Foa EB: Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry 2000; 61(suppl 5):43–51Google Scholar
51 Davidson J, Kudler H, Smith R, et al: Treatment of posttraumatic stress disorder with amitriptyline and placebo. Arch Gen Psychiatry 1990; 47:259–266Crossref, Medline, Google Scholar
52 Kosten TR, Frank JB, Dan E, et al: Pharmacotherapy for posttraumatic stress disorder using phenelzine or imipramine. J Nerv Ment Dis 1991; 179:366–370Crossref, Medline, Google Scholar
53 Davidson JR: Biological therapies for posttraumatic stress disorder: an overview. J Clin Psychiatry 1997; 58(suppl 9):29–32Google Scholar
54 Lipper S, Davidson JR, Grady TA, et al: Preliminary study of carbamazepine in post-traumatic stress disorder. Psychosomatics 1986; 27:849–854Crossref, Medline, Google Scholar
55 Fesler FA: Valproate in combat-related posttraumatic stress disorder. J Clin Psychiatry 1991; 52:361–364Medline, Google Scholar
56 Hertzberg MA, Butterfield MI, Feldman ME, et al: A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry 1999; 45:1226–1229Crossref, Medline, Google Scholar
57 Brady K, Pearlstein T, Asnis GM, et al: Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA 2000; 283:1837–1844Crossref, Medline, Google Scholar
58 Stein DJ, Hewett K, Oldham M, et al: Paroxetine in the treatment of PTSD. Presented at the 7th World Congress of Biological Psychiatry, Berlin, Germany, 2001Google Scholar
59 van der Kolk BA, Dreyfuss D, Michaels M, et al: Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry 1994; 55:517–522Medline, Google Scholar
60 Hertzberg MA, Feldman ME, Beckham JC, et al: Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry 2000; 12:101–105Crossref, Medline, Google Scholar
61 Martenyi F, Brown EB, Zhang H, et al: Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry 2002; 63:199–206Crossref, Medline, Google Scholar
62 Martenyi F, Brown EB, Zhang H, et al: Fluoxetine v placebo in prevention of relapse in post-traumatic stress disorder. Br J Psychiatry 2002; 181:315–320Crossref, Medline, Google Scholar
63 Davidson J, Pearlstein T, Londborg P, et al: Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry 2001; 158:1974–1981Crossref, Medline, Google Scholar
64 Marshall RD, Schneier FR, Fallon BA, et al: An open trial of paroxetine in patients with noncombat-related, chronic posttraumatic stress disorder. J Clin Psychopharmacol 1998; 18:10–11Crossref, Medline, Google Scholar
65 Tucker P, Zaninelli R, Yehuda R, et al: Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry 2001; 62:860–868Crossref, Medline, Google Scholar
66 Marshall RD, Beebe KL, Oldham M, et al: Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 2001; 158:1982–1988Crossref, Medline, Google Scholar
67 Pitman RK, Sanders KM, Zusman RM, et al: Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002; 51:189–192Crossref, Medline, Google Scholar



