Cognitive Dysfunction and Depression During Treatment With Interferon-Alpha and Chemotherapy
Abstract
Trials with interferon-alpha (IFN-α) have provided contradictory findings regarding the presence of cognitive side effects. The development of depression in some patients also raises questions about whether cognitive dysfunction might be secondary to an organic, interferon-induced mood disorder. Thirty patients with chronic myelogenous leukemia were examined before and during treatment with IFN-α alone or IFN-α and chemotherapy. Increased depressive symptoms and declines in information processing and executive functions were observed, but depression alone could not account for cognitive dysfunction. There was some evidence suggesting that exposure to chemotherapy and higher cumulative IFN-α dose may contribute to cognitive impairment.
Clinical trials have identified several side effects of interferon-alpha (IFN-α) therapy, including flu-like symptoms and depression.1,2 Some investigators have described cognitive symptoms3 such as impairments in memory and executive functions, while others have found no evidence of cognitive decline.4 Differences in assessment techniques, treatment variables, and risk factors associated with different patient populations may have contributed to these discrepant findings.
Risk factors for cognitive dysfunction and depression during IFN-α treatment may include older age, higher doses, longer treatment duration, and the presence of a neurological or psychiatric disorder prior to starting IFN-α.5,6 For example, preexisting affective symptoms may be a risk factor for the development of depression during IFN-α therapy. One study7 found that pretreatment scores on the Montgomery-Åsberg Depression Rating Scale predicted the intensity of depressive symptoms after 4 weeks of IFN-α. Other studies have used the MMPI to identify patients at risk for the development of depression during IFN-α treatment for hepatitis C.8
The relationship between depressive and cognitive side effects is not well understood. Depression can produce impairments in attention and other cognitive skills,9 and an organic, interferon-induced depression may be responsible for the cognitive dysfunction. Another possibility is that depressive and cognitive symptoms may be independent and reflect separate physiological mechanisms. A study examining cognition and depression during IFN-α therapy may thus provide valuable information about the nature of the cognitive impairment.
The current study is one of only a few that utilizes a prospective research design to examine the cognitive and depressive side effects of IFN-α. The primary goal was to document any declines in cognitive function and examine the role of demographic and treatment variables that might act as risk factors. A second set of objectives addressed the depressive symptoms of IFN-α neurotoxicity, including their relationship to suspected risk factors, somatic symptoms, and cognitive impairment.
METHOD
Subjects
Thirty adults with chronic myelogenous leukemia were examined. These patients were enrolled in protocols utilizing IFN-α alone (n=13), IFN-α with low dose cytosine arabinoside (ARA-C) (n=15), or IFN-α with hydroxyurea (n=2). Throughout treatment all subjects had hemoglobin values that were 10 g/dl or greater.
Assessment
The institutional review board at M.D. Anderson Cancer Center approved the procedures used in this project. Participants were administered a neuropsychological battery consisting of standardized tests, which are described in a previous paper,3 at their pretreatment baseline and on-treatment assessments. Six neuropsychological measures were selected from the battery based on research3,10 showing that they were sensitive to the cognitive effects of IFN-α. These tests assessed the following abilities: 1) graphomotor speed—the Digit Symbol subtest from the Wechsler Adult Intelligence Scale—Revised,11 2) verbal learning and memory—consistent long-term retrieval (CLTR) and 30-minute delayed free recall from the Verbal Selective Reminding Test (VSRT),12 3) visual-motor and sequencing skills—the number of seconds to complete part A (TMT-A) and part B (TMT-B) of the Trail Making Test,13 and 4) verbal fluency—the Controlled Oral Word Association Test (COWAT) from the Multilingual Aphasia Examination.14 The COWAT has been reported to be sensitive to frontal lobe dysfunction.15
Often used as a screening instrument for depression, the MMPI16 is sensitive to both the depressive and somatic symptoms of IFN-α administration.3 The hypochondriasis (Hs), depression (D), and hysteria (Hy) scales of the MMPI were examined in 18 participants. Ten subjects received the Beck Depression Inventory (BDI).17 Although the original plan had been to administer the MMPI and BDI to all 30 subjects, only a portion of the sample completed these tests due to the inadvertent omission of these measures from the clinic's assessment battery.
Statistical Analysis
Published normative data were used to convert raw cognitive test scores to standardized t scores (mean=50, SD=10) in order to facilitate comparisons among the measures and to adjust for demographic factors (e.g., age). On the resulting scale higher values indicated better cognitive functioning. Minnesota Multiphasic Personality Inventory scores are traditionally reported as standardized t scores (mean=50, SD=10), with higher values reflecting greater symptom severity.18 Change scores were also calculated for the cognitive and MMPI measures by subtracting baseline standardized t scores from their matching on-treatment scores. These change scores were considered meaningful if they were equal to or greater than 15 points (i.e., 1.5 standard deviations within the measure's normative sample).
Each cognitive and MMPI variable was examined using a mixed design, repeated measures analysis of variance (ANOVA) with period (i.e., baseline assessment versus on-treatment assessment) as the repeated measure. Interferon-α is frequently administered with chemotherapy, and the possibility of synergistic effects was addressed by using protocol as a between-subjects factor. Patients receiving IFN-α with ARA-C or IFN-α with hydroxyurea were pooled into a single, combination therapy group to simplify data analysis. This combination therapy group was compared to subjects who received IFN-α alone. When the period-by-protocol interaction was significant, t tests were used to conduct planned comparisons between the baseline and on-treatment periods within each protocol group. Other group comparisons were made using t tests or the Wilcoxon Rank Sum test, depending upon whether the data met the assumptions for a parametric statistical analysis. Spearman's rho was used to examine bivariate correlations between variables of special interest, including cognitive measures, MMPI scales, and possible risk factors.
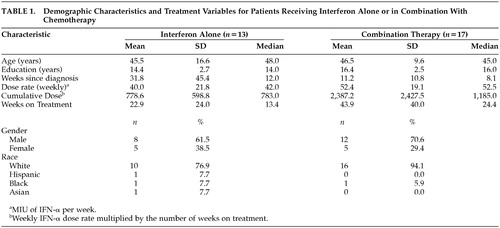
The interval from baseline assessment to follow-up is presented in Table 1 as the number of weeks on treatment. The IFN-α dose rate was the number of million international units (MIU) per week and the cumulative dose was the dose rate multiplied by the number of weeks on treatment.
RESULTS
Demographic and Treatment Characteristics
Demographic and treatment characteristics for the IFN-α alone and combination therapy groups are summarized in Table 1. Age, the number of weeks since diagnosis, and the dose rate did not differ between these groups. However, education level (t=−2.05, df=28, P<0.050), weeks on treatment (Wilcoxon Rank-Sum Test, P<0.015), and cumulative dose (Wilcoxon Rank-Sum Test, P<0.035) were greater among patients who received combination therapy.
Cognitive Function
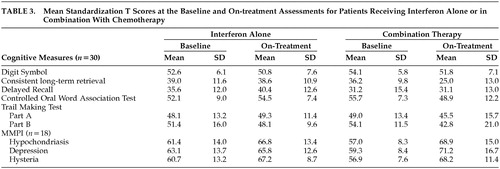
Table 2 presents the percentage of patients exhibiting adverse effects on the neuropsychological assessment. Sixteen patients (53.3%) exhibited a decline of at least 1.5 standard deviations on one or more of the cognitive tests. Mean scores from the baseline and on-treatment assessments are also presented (Table 2) and t tests indicated that the baseline scores did not differ between the protocol groups (Table 3). Scores from the Trail Making Test, which were log transformed prior to data analysis, were not normally distributed. Standardized t scores, however, are presented within the tables to facilitate comparisons.
Scores on the Digit Symbol subtest were lower during IFN-α therapy (F=5.44, df=1, 28, P<0.027). Although this decline was significant, the change in Digit Symbol performance was relatively small, and the average follow-up score of 51.3 was within the average range of the test's normative sample (see Table 2). The main effect for type of protocol (F=0.31, df=1, 28, P<0.582) and the period-by-protocol interaction were not significant for Digit Symbol (F=0.10, df=1, 28, P<0.756).
Results from the VSRT revealed a dissociation between delayed free recall and CLTR, a measure of verbal learning. Performance during delayed free recall did not differ with the assessment period (F=0.88, df=1, 28, P<0.375) or protocol type (F=2.57, df=1, 28, P<0.120). There was also no period-by-protocol interaction for delayed free recall (F=0.91, df=1, 28, P<0.349). In contrast, for CLTR the differences associated with assessment period (F=6.01, df=1, 28, P<0.021), protocol type (F=5.64, df=1, 28, P<0.025), and the period-by-protocol interaction (F=5.23, df=1, 28, P<0.030) were all significant. Planned comparisons indicated a significant decrease in CLTR scores among subjects who had received chemotherapy with IFN-α (t=−3.34, df=28, P<0.004) but not when IFN-α was administered alone (t=−0.12, df=28, P<0.904) (see Table 3).
A period-by-protocol interaction was also found for the COWAT (F=8.18, df=1, 28, P<0.008). Once more, planned comparisons revealed lower performance within the combination therapy group during treatment (t=−2.96, df=28, P<0.009), while scores within the IFN-α alone group did not differ between the baseline and on-treatment assessments (t=−1.14, df=28, P<0.278) (see Table 3). The main effects of assessment period (F=1.86, df=1, 28, P<0.183) and protocol (F=0.11, df=1, 28, P<0.742) were not significant for COWAT.
For TMT-A there were no significant effects for period (F=0.61, df=1, 28, P<0.442) or treatment protocol (F=0.01, df=1, 28, P<0.918), and there was no period-by-protocol interaction (F=0.71, df=1, 28, P<0.405). However, for TMT-B there was a decline while the patients were on IFN-α, as revealed by a main effect for period (F=7.87, df=1, 28, P<0.009). The main effect for protocol type (F=0.07, df=1, 28, P<0.792) and the period-by-protocol interaction (F=0.22, df=1, 28, P< 0.642) were not significant for TMT-B.
Risk Factors for Cognitive Impairment
The significant period-by-protocol interaction for COWAT and CLTR suggests that combination therapy may be associated with greater risk for cognitive decline. However, both treatment duration and cumulative IFN-α dose differed between the protocol groups. The change in COWA t scores was correlated with age (rho=−0.42, P<0.021), number of weeks on treatment (rho=−0.54, P<0.002), and cumulative IFN-α dose (rho=−0.58, P<0.001). The correlation between TMT-B and cumulative IFN-α dose approached significance (rho=−0.31, P<0.093). None of the other correlations between cognitive measures and possible risk factor variables were significant.
Emotional Function
Minnesota Multiphasic Personality Inventory scores were not normally distributed, and they were log transformed prior to data analysis. However, mean standardized t scores are reported in Table 2 and Table 3 and reveal increases on all three MMPI scales during IFN-α therapy. Table 3 also reveals similar MMPI scores at baseline for patients who received IFN-α alone or in combination with chemotherapy and t tests confirmed that there were no significant differences between these groups.
The main effect of assessment period was significant for the Hs scale (F=15.78, df=1, 16, P<0.001), but the main effect for protocol type (F=0.04, df=1, 16, P<0.840) and the period-by-protocol interaction were not significant (F=1.97, df=1, 16, P<0.180). Scores from the Hy scale were higher at the on-treatment assessment (F=22.47, df=1, 16, P<0.001), and there were no differences associated with the type of protocol (F=0.09, df=1, 16, P<0.765). The period-by-protocol interaction was not significant (F=1.05, df=1, 16, P<0.322) for the Hy scale. Higher D scale scores were noted during treatment (F=8.15, df=1, 16, P<0.012), and the main effect for protocol type (F=0.01, df=1, 16, P<0.915) and the period-by-protocol interaction (F=2.62, df=1, 16, P<0.125) were not significant. Six of these patients experienced an increase on the D scale of at least 15 points (i.e., 1.5 standard deviations) (Table 2). Scores on the D and Hs scales were not correlated, but the correlation between the D and Hy scales approached significance (rho=0.43, P<0.063).
An increase in depressive symptoms was also found on the BDI (t=3.38, df=9, P<0.008), as reflected by an elevation in the mean score from 6.8 (SD=5.03) to 11.9 (SD=7.52).
Risk Factors for Depression
Spearman's rho was used to examine the relationship between change scores from the MMPI D scale and potential risk factors for increased depression during IFN-α therapy, including age, weeks since diagnosis, weeks on treatment, dose rate, and the cumulative dose. None of these correlations approached significance.
Findings from previous studies7,8 suggest that pretreatment depressive symptoms may be a risk factor for the development of greater depression during IFN-α therapy. Within the present sample the baseline MMPI D scale scores were not related to D scale changes during treatment (rho=−0.04, P<0.859). Scale scores of 70 or greater were interpreted as suggesting the presence of a clinically significant level of depression.18 Examination of individual scores indicated that five (27.8%) of 18 patients exceeded this cutoff at the time of the pretreatment assessment, and these scores were still elevated at the on-treatment evaluation. On average, D scale scores for these five depressed patients increased an additional 7.4 points during therapy, and this is similar to the mean change of 7.3 points that was observed within the entire sample. However, three subjects with normal baseline scores (i.e., D scale score < 70) developed new depression during treatment, and they all experienced D scale increases of at least 19 points and had on-treatment scores of 80 or greater.
Cognitive Function and Depression
Changes on the MMPI D scale were related to change scores from the TMT-B (rho=−0.56, P<0.017), but correlations between the D scale and other cognitive measures were not significant. Examination of raw data also revealed four patients with normal on-treatment D scale scores and clear evidence of dysfunction on the neuropsychological evaluation, as reflected by a decline of 1.5 standard deviations or more on at least one of the cognitive measures.
DISCUSSION
The present investigation utilized a prospective research design and obtained evidence for cognitive dysfunction and increased depressive symptoms during IFN-α therapy for chronic myelogenous leukemia. These observations are consistent with a number of previous investigations that identified similar side effects,3,10 but concerns have been raised about such studies because they did not obtain pretreatment data.4 Prospective neuropsychological studies often have disadvantages associated with repeated exposure to the same or similar test materials.19 Aside from the use of alternate test forms for the COWAT and VSRT, the present investigation did not control for repeated testing. However, practice effects would be expected to elevate scores at the on-treatment evaluation and, since test scores were lower at that assessment period, the results provide sound evidence for cognitive decline during IFN-α therapy. More than one-half of the patients in our study experienced a decrease (≥1.5 standard deviations) on one or more neuropsychological measures. These deficits were often of sufficient severity to present problems for the patients in their daily lives, including difficulty returning to work due to impaired cognitive efficiency.
On two of the cognitive measures there was some evidence for toxic interaction between IFN-α and chemotherapy. Significant performance decreases on the CLTR and the COWAT occurred only among subjects receiving combination therapy. It is important to note that patients on the combination therapy protocols also tended to have longer treatment intervals and a higher cumulative dose of IFN-α. However, there was no correlation between CLTR and either cumulative dose or weeks on treatment and, in the case of the COWAT, correlations with these potential risk factors were only of moderate strength. Both high cumulative IFN-α dose and chemotherapy may place patients at higher risk for cognitive dysfunction. Neurotoxicity can be more severe when IFN-α is combined with other treatments,1 ARA-C and hydroxyurea may have detrimental effects on cognitive functioning,20,21 and use of these agents in combination with IFN-α may have increased the level of cognitive impairment.
Depression can produce cognitive dysfunction, and the present study found increases on the BDI and the D scale of the MMPI. Changes in depressive symptoms were not highly related to changes on the Hs and Hy scales, both of which are sensitive to somatic complaints.18 Thus, physical side effects (e.g., fatigue) cannot fully account for the D scale elevations, and our results probably reflect the symptoms of a mood disorder. Thirty three percent of patients who completed the MMPI had D scale increases in excess of 15 points, and on-treatment scores were 80 or higher in three of these subjects. Increases in depressive symptoms were also observed with the BDI, and this level of symptom severity suggests the presence of a substance-induced mood disorder. Interferon-induced depression has serious implications for quality of life, and it would be helpful if clinicians could predict which patients are at risk for this side effect. However, contrary to the findings from two previous investigations,7,8 baseline depression scores were not correlated with increases in depressive symptoms during treatment in our study. This discrepancy may be due to differences in patient populations, assessment techniques, and IFN-α dose. For example, previous investigations studied populations with malignant melanoma7 or hepatitis C8 and utilized a different treatment schedule, including considerably higher doses of IFN-α for patients with melanoma. Other suspected risk factors also failed to demonstrate a relationship with depressive symptoms and, in the present study, there was no obvious characteristic that identified patients who developed new depression. These results support the monitoring of mood throughout IFN-α treatment, so the clinician can intervene as soon as symptoms develop.
New depressive symptoms did not appear to be responsible for the cognitive dysfunction observed in this study. Changes on the MMPI D scale were related to decreased performance on the TMT-B, but correlations with other cognitive measures were not significant and several patients with normal D scale scores exhibited declines on the neuropsychological assessment. Much of the cognitive impairment could not be attributed to an organic, interferon-induced depressive disorder, and this finding raises the possibility of different underlying mechanisms for the depressive and cognitive symptoms of interferon neurotoxicity.
Several physiological mechanisms have been proposed for the neurotoxic effects of IFN-α, including actions mediated through neuroendocrine, neurotransmitter, and cytokine pathways.6 Interferon-α has structural and functional similarities to adrenocorticotropic hormone,22 and alterations in endocrine function may contribute to the somatic and mood symptoms of IFN-α toxicity.6 There is some evidence for actions on β-endorphin and dopamine23,24 as well, and the cognitive and behavioral symptoms of chronic IFN-α administration have been compared to Parkinson's disease.6
Patients with IFN-α neurotoxicity have been reported to exhibit mild to moderate symptoms of frontal-subcortical brain dysfunction,3,10 including cognitive and behavioral slowing, apathy, impaired executive functions, and decreased memory. The current study obtained similar findings, including evidence on neuropsychological assessment for impairments in information processing and frontal lobe executive functions. Although performance on a word learning task (i.e., CLTR) decreased during treatment, there was no decline in delayed recall of the type expected with hippocampal dysfunction.25 It has been said that the frontal lobes have a directive, controlling role in the acquisition of new information,26 and prefrontal dysfunction may have been responsible for the decline on CLTR. The hypothesis that frontal lobe dysfunction is involved in some IFN-α neurotoxic effects is also consistent with physiological data showing decreased prefrontal metabolism during low-dose IFN-α therapy.27
In conclusion, the current study found evidence of neurotoxicity during IFN-α therapy that included alterations in both cognition and mood. These findings support the monitoring of cognitive and emotional function during IFN-α therapy, and when indicated, prompt treatment for symptoms of depression. However, mood changes were not responsible for much of the cognitive decline, and different strategies may be required to manage the cognitive side effects. Opiate agonists could have some potential as a treatment for the physiologic and cognitive symptoms.28 Additional research to examine risk factors and physiological mechanisms of neurotoxicity may be helpful for developing methods to reduce these adverse effects of IFN-α therapy.
ACKNOWLEDGMENTS
A portion of these findings was presented at the 30th annual meeting of the International Neuropsychological Society, Toronto, Canada, February 13–16, 2002.
 |
 |
 |
1 Meyers CA, Valentine AD: Neurological and psychiatric adverse effects of immunological therapy. CNS Drugs 1995; 3:56–68Crossref, Google Scholar
2 Capuron L, Gumnick JF, Musselman DL, et al: Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002; 26:643–652Crossref, Medline, Google Scholar
3 Pavol MA, Meyers CA, Rexer JL, et al: Pattern of neurobehavioral deficits associated with interferon alfa therapy for leukemia. Neurology 1995; 45:947–950Crossref, Medline, Google Scholar
4 Mapou RL, Law WA, Wagner K, et al: Neuropsychological effects of interferon alfa-n3 treatment in asymptomatic human immunodeficiency virus-1-infected individuals. J Neuropsychiatry Clin Neurosci 1996; 8:74–81Link, Google Scholar
5 Merimsky O, Chaitchik S: Neurotoxicity of interferon-α. Anticancer Drugs 1992; 3:567–570Crossref, Medline, Google Scholar
6 Valentine AD, Meyers CA, Kling MA, et al: Mood and cognitive side effects of interferon-α therapy. Semin Oncol 1998; 25(1, suppl 1):39–47Google Scholar
7 Capuron L, Ravaud A: Prediction of the depressive effects of interferon alfa therapy by the patient's initial affective state (letter). N Engl J Med 1999; 340:1370Crossref, Medline, Google Scholar
8 Scalori A, Apale P, Roffi L: Psychological screening before interferon therapy (letter). Hepatology 2001; 33:480Crossref, Medline, Google Scholar
9 Tarbuck AF, Paykel ES: Effects of major depression on the cognitive function of younger and older patients. Psychol Med 1995; 25:285–296Crossref, Medline, Google Scholar
10 Meyers CA, Scheibel RS, Forman AD: Persistent neurotoxicity of systemically administered interferon-alpha. Neurology 1991; 41:672–676Crossref, Medline, Google Scholar
11 Wechsler D: Wechsler Adult Intelligence Scale—Revised. San Antonio, Tex, Psychological Corp, 1981Google Scholar
12 Hannay HJ, Levin HS: Selective reminding test: an examination of the equivalence of four forms. J Clin Exp Neuropsychol 1985; 7:251–263Crossref, Medline, Google Scholar
13 Reitan RM, Davison LA (eds): Clinical Neuropsychology: Current Status and Applications. Washington, DC, VH Winston & Sons, 1974Google Scholar
14 Benton AL, Hamsher K: Multilingual Aphasia Examination. Iowa City, Iowa, AJA Associates, 1983Google Scholar
15 Miller E: Verbal fluency as a function of a measure of verbal intelligence and in relation to different types of cerebral pathology. Br J Clin Psychol 1984; 23:53–57Crossref, Medline, Google Scholar
16 Hathaway SR, McKinley JC: Minnesota Multiphasic Personality Inventory. Minneapolis, National Computer Systems, 1970Google Scholar
17 Beck AT, Ward CH, Mendelson M, et al: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
18 Lachar D: The MMPI: Clinical Assessment and Automated Interpretation. Los Angeles, Western Psychological Services, 1974Google Scholar
19 McCaffrey RJ, Ortega A, Orsillo SM, et al: Practice effects in repeated neuropsychological assessments. Clin Neuropsychol 1992; 6:32–42Crossref, Google Scholar
20 Baker WJ, Royer GL Jr, Weiss RB: Cytarabine and neurologic toxicity. J Clin Oncol 1991; 9:679–693Crossref, Medline, Google Scholar
21 Barry M, Clarke S, Mulcahy F, Back D: Hydroxyurea-induced neurotoxicity in HIV disease (letter). AIDS 1999; 13:1592–1594Crossref, Medline, Google Scholar
22 Blalock JE, Smith EM: Human leukocyte interferon: structural and biological relatedness to adrenocorticotrophic hormone and endorphins. Proc Natl Acad Sci USA 1980; 77:5972–5974Crossref, Medline, Google Scholar
23 Birmanns B, Saphier D, Abramsky O: α-Interferon modifies cortical EEG activity: dose-dependence and antagonism by naloxone. J Neurol Sci 1990; 100:22–26Crossref, Medline, Google Scholar
24 Ho BT, Huo YY, Lu JG, et al: Opioid-dopaminergic mechanisms in the potentiation of d-amphetamine discrimination by interferon-alpha. Pharmacol Biochem Behav 1992; 42:57–60Crossref, Medline, Google Scholar
25 Squire LR: Two forms of human amnesia: an analysis of forgetting. J Neurosci 1981; 1:635–640Crossref, Medline, Google Scholar
26 Fletcher PC, Henson RNA: Frontal lobes and human memory: insights from functional brain imaging. Brain 2001; 124:849–881Crossref, Medline, Google Scholar
27 Juengling FD, Ebert D, Gut O, et al: Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000; 152:383–389Crossref, Medline, Google Scholar
28 Valentine AD, Meyers CA, Talpaz M: Treatment of neurotoxic side effects of interferon-alpha with naltrexone. Cancer Invest 1995; 13:561–566Crossref, Medline, Google Scholar



