The Role of Norepinephrine in the Behavioral and Psychological Symptoms of Dementia
Abstract
The behavioral and psychological symptoms of dementia (BPSD) are common serious problems that are a major contributor to caregiver burden. Despite their significance, the underlying neurobiology of these disturbances is still unclear. This review examines the role of norepinephrine (NE) on BPSD, including depression, aggression, agitation and psychosis. A number of lines of evidence suggest that NE dysfunction leading to BPSD may result from increased NE activity and/or hypersensitive adrenoreceptors compensating for loss of NE neurons with progression of Alzheimer’s disease (AD). With greater appreciation of the underlying neurobiology of behavioral and psychological symptoms of dementia (BPSD) more effective, rational, targeted pharmacotherapy will hopefully emerge.
A notable source of morbidity and mortality, dementia accounts for an excess of health care costs.1 The total economic cost of the disorder, including direct and indirect medical costs, has been estimated to be $100 billion per year (in U.S. dollars).2 Alzheimer's disease (AD) is a progressive and incurable disease characterized by cognitive and behavioral abnormalities and is the most common cause of dementia in North America.1 The estimated prevalence of AD among individuals above 65 years of age in the United States is 10.3%.3
The behavioral abnormalities associated with AD frequently disrupt patient care and present a management problem to the caregiver. Behavioral and psychological symptoms of dementia (BPSD) that complicate AD include behaviors with psychotic features (e.g., delusions and hallucinations) as well as those without (e.g., depression, anxiety, agitation, wandering, hostility, and uncooperativeness).4 Disruptive agitated behaviors are common and occur in 40% to 90% of patients with AD at some point during the course of their illnesses.5,6 Evidence suggests that BPSD have a detrimental impact on both patients7 and caregivers.8 Aggression occurs in 18% to 65% of patients9–11 and is the behavioral disturbance that causes the greatest impact. Behaviors such as agitation and aggression complicate management12,13 and contribute to caregiver burden14 and the institutionalization of demented patients.15–18
The treatment of behavioral disorders in AD has emphasized the use of psychotropic medications.19–21 Studies in nursing homes,22–24 hospitals,25 and the community18 have shown that psychotropic medications are frequently used in elderly patients. Guidelines outlining the appropriate use of psychotropics in elderly institutionalized patients have been created in response to persistent psychotropic use.26 The Omnibus Budget Reconciliation Act of 1987 was enacted to curtail inappropriate and overuse of psychoactive medications in long-term care facilities, which often resulted in high incidence of adverse events.27,28 For example, antipsychotic medications, the most commonly used psychotropic for BPSD, can produce serious extrapyramidal side effects. Extrapyramidal symptoms such as rigidity, tremors, and parkinsonian gait29are common with antipsychotic drug administration and may limit treatment in the elderly demented population and lead to discontinuation of medication. Psychotropics have also been found to induce agitation, confusion, and cognitive impairment in elderly demented patients,30 further contributing to BPSD. Novel therapies targeting specific neurotransmitters are believed to underlie behavioral dysfunction in AD are being studied as alternatives to the modestly efficacious antipsychotics.31,32 The idea here is that we can rationalize treatment by studying the neurotransmitters involved. This has been demonstrated with serotonin, one of the best examined neurotransmitters.33,34
METHOD
Neuropathological and neurochemical disruptions in the central noradrenergic system have been established as important factors in the etiology of AD and BPSD. Evidence supporting these neurobiological alterations as well as the putative links between the noradrenergic system and behavior, including BPSD, are reviewed. Noradrenergic pharmacotherapies are also examined to assess the clinical significance they may have in AD and BPSD. Sources used to obtain articles included electronic databases (MEDLINE, EMBASE) and manual cross-references from bibliographies of relevant literature. The following keywords were used: norepinephrine, noradrenergic, Alzheimer's disease, dementia, behavior, behavioral disorders, aggression, agitation, psychosis, depression, and noncognitive.
NOREPINEPHRINE SYSTEM
The central noradrenergic system is known to have two different projections: 1) neurons originating from the ventrolateral tegmental noradrenergic cells—involved in sexual and feeding behaviors35—that flow to the forebrain, an area commonly involved in traumatic brain injury and associated with violent behavior and reduction in rage control,36 and 2) neurons originating from the locus coeruleus (LC), which line the bottom of the fourth ventricle in the rostral pons37 and are associated with cognitive functions.38 Noradrenergic neurons give rise to diffuse axonal projections that innervate large areas of the brain, including the cerebellum, thalamus, hypothalamus, and midbrain,35 and thus can be expected to influence many different functions.
Norepinephrine (NE) and epinephrine are catecholamines and are formed from the amino acid l-tyrosine which is actively transported from the blood. l-Tyrosine is converted to l-dihydroxyphenylacetic acid (l-dopa) through tyrosine hydroxylase, which is the rate limiting enzyme of the metabolic pathway. l-dihydroxyphenylacetic acid is then transformed to dopamine (DA), the immediate precursor of NE, by dopa-decarboxylase and transported into storage vesicles. Storage vesicles containing DA beta hydroxylase (DBH), found in NE-specific neurons, convert the stored DA to NE through β-hydroxylation. Dopamine producing neurons have storage vesicles without DBH.
Norepinephrine is the primary neurotransmitter of the sympathetic nervous system in the periphery but is also prevalent in the brain. Epinephrine is an analog of NE and is formed by N-methylation. It is released principally from the adrenal medulla; however, its function in the CNS has not been fully elucidated. Norepinephrine is released from the vesicles into the synaptic cleft at the occurrence of an action potential. Norepinephrine transporters on the synaptic terminals take up NE into storage vesicles. Monoamine oxidase (MAO) deaminates and, hence, deactivates free NE in the cytosol that has not been taken up into the vesicles, leading to the formation of the metabolite, 3,4-dihydroxyphenylglycol (DHPG). Dihydroxyphenylglycol is then converted to 3-methoxy-4-hydroxyphenylglycol (MHPG) by catechol O-methyltransferase (COMT). Residual NE in the synapse is first metabolized by COMT and then MAO to form MHPG and other metabolites.
Noradrenergic receptors are divided into α-adrenergic and β-adrenergic subtypes, each of which has further subtypes: α1, α2, β1, β2 and β3. The postsynaptic β-adrenergic receptors activate Gs type G proteins to stimulate adenylyl cyclase, leading to a physiological response. The α2 adrenergic receptors decrease adenylyl cyclase activity or activate specific K+ channels through coupling to Gi and/or Go,39 and are located both pre- and postsynaptically. The α1 adrenergic receptor stimulates the action of phospholipase C through Gx. and it is located on the postsynaptic terminals. Only α2 and β2 adrenoreceptors are located on both presynaptic and postsynaptic terminals. Norepinephrine and epinephrine stimulate both the α- and β-adrenergic receptors in the periphery and CNS with the effects, depending on the balance between the α- and β-receptors that are present and have been activated. Presynaptic α2 adrenoreceptors modulate the negative feedback of NE release and are pharmacologically different from postsynaptic α2 adrenergic receptors. Norepinephrine release can be enhanced by the stimulation of β2 adrenoreceptors. Epinephrine is more potent than norepinephrine on the β2 adrenoreceptor.
Norepinephrine mediates and is modulated by other neurochemicals in the brain, many known to affect behavior. A loss or alteration in any one neurotransmitter is likely to affect other neurotransmitters and potentially lead to deviations in behavior. The noradrenergic system interacts with acetylcholine,40–43 serotonin,44–46 DA47–49 and γ-aminobutyric acid.50 Other neurotrophic factors that interact with and modulate the noradrenergic system include a corticotropin releasing hormone,51 ,52 somatostatin,53 and neuropeptide Y.54 Many of these neurotrophic factors have been associated with behaviors such as aggression, anxiety, and depression in nondemented populations55 and thus alterations in particular neuropeptides may play a role in aggravating already disrupted neurotransmitter systems. Since multiple neurochemical systems are known to be compromised in AD,56 they may magnify behavioral disturbances.
NOREPINEPHRINE AND ALZHEIMER'S DISEASE
Alzheimer's disease is associated with multiple neurotransmitter system dysfunction. The most well studied neuronal system dysfunction is the cholinergic system. However, evidence supporting noradrenergic, serotonergic, dopaminergic, corticotropin-releasing factor, and somatostatin system dysfunction has also accumulated,56–58 and mounting evidence supports the presence of significant abnormalities in the noradrenergic system in AD.58–78
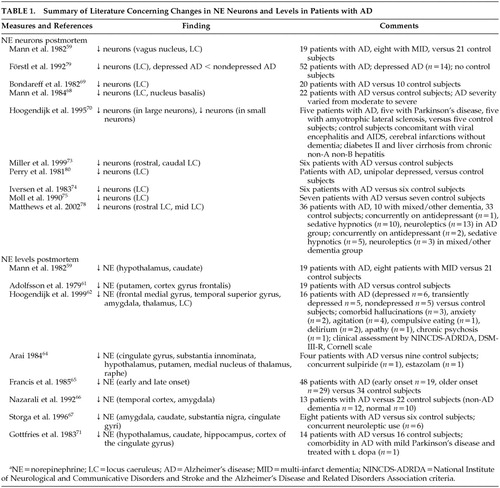
Norepinephrine Concentrations
Postmortem studies have consistently shown noradrenergic system involvement in the AD disease process with decreased NE levels being recognized in many brain areas.58,59,61,62,64–67,71,72,74–78 The majority of studies indicate significantly decreased cortical and subcortical NE levels in the frontal medial gyrus, temporal superior gyrus, cingulate gyrus, hippocampus, amygdala, thalamus, hypothalamus, caudate, putamen as well as the LC.58,59,62,64,66,67,71,75,76 However, some studies did not replicate these results due to possible confounders such as postmortem delay.
Loss of Noradrenergic Neurons
A loss of noradrenergic neurons in the LC, the major noradrenergic source in the brain, has been well established in patients with AD,59,68–70,73–75,79,80 and has not been found in other types of dementia such as vascular dementia.59 These changes in the LC may account for a deficiency in the noradrenergic system in the pathogenesis of AD.
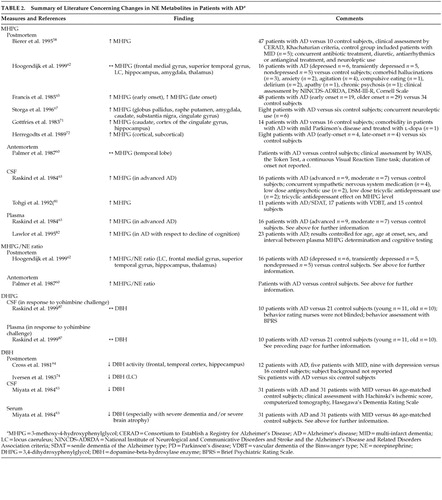
However, NE concentrations in tissue may not directly correlate with functional noradrenergic transmission. Rather, noradrenergic turnover and neuronal activity deduced through measurements of relative concentrations of NE and its predominant intraneuronal metabolite, MHPG, provide a more accurate representation of actual noradrenergic function. In postmortem studies, when both NE and MHPG were quantified together, although brain NE was often decreased, MHPG was found to be unchanged or high in AD patients compared to control subjects.58,62,65,67,71,72,81 These findings suggest that neuronal loss may lead to subsequent increased NE metabolism and hence increased noradrenergic activity, possibly as a compensatory mechanism for LC NE loss. Hoogendijk et al.62 demonstrated a significant inverse correlation between the MHPG/NE ratio and the number of remaining pigmented LC neurons in AD patients, inferring an overactivity or activation of the remaining LC neurons to counterbalance cerebral NE losses. It remains unclear whether this compensatory activity occurs in the noradrenergic neuron terminals in the projection areas, in the LC cell bodies or in both.62
Galanin (GAL), a major inhibitory modulator of cholinergic and noradrenergic transmission, has been shown to be hyperinnervated in the basal forebrain and cortex of AD patients. Galanin fibers are thought to originate in the LC. Miller et al.73 explored the possibility that GAL expression is increased in the LC of patients with AD. Although the authors observed a significant reduction in the amount of neuromelanin-pigmented, which identifies NE presence, neurons in the LC of AD patients, when investigated alongside age- and sex-matched control subjects, there was no significant difference found between the number of both the pigmented and nonpigmented GAL mRNA expressing neurons in either demented patients or control subjects. This resulted in a significant increase in the percentage of neuromelanin-pigmented GAL co-expressing cells in patients with AD compared to control subjects, hence, suggesting preservation or sparing of noradrenergic neurons co-expressing GAL mRNA in a brain area otherwise diminished of noradrenergic neurons. These results indicate a possible neuroprotective feature of GAL, safeguarding the GAL co-localized group of noradrenergic neurons from damage resulting from the disease process.73 The possibility that augmented GAL fiber innervation may be due to the enhanced expression of GAL within the remaining neurons in the LC region remains to be investigated further.
Link With Severity
There is a well-established link between AD severity and loss of noradrenergic neurons.69 Bondareff et al.69 compared total neuronal counts of 19 AD patients compared to 10 nondemented subjects and found the group of AD patients could be further divided into two distinct groups according to LC neuronal cell counts, which also correlated with severity of dementia. The authors reported an 81% decrease in nucleus LC neurons in the group of demented patients with more severe dementia, when compared to control subjects. The group of patients with less severe dementia showed a 20% decrease in LC neuronal loss. NE levels in the brain have also been found to have an inverse relationship with cognitive impairment.61,78 Lawlor et al.82 noted a significant positive relationship between basal plasma MHPG levels and cognitive impairment, further corroborating previous suppositions that increased MHPG from heightened NE turnover, as a result of losses of NE, is correlated with increased cognitive dysfunction.
Dopamine Beta Hydroxylase (DBH)
Studies of DBH may further substantiate evidence of noradrenergic dysfunction in AD. Both postmortem and antemortem studies have shown DBH, a noradrenergic biological marker, to be reduced in the hippocampus and frontal and temporal cortices of AD patients when compared to controls74,83,84 and patients with vascular dementia.83,84 However, these results must still be interpreted with caution as the extent to which DBH activity is associated with LC neuronal degeneration is still unclear.84
Cerebral Spinal Fluid (CSF)
Resting cerebral spinal fluid NE is thought to be generally increased with age, particularly in patients with advanced AD, and has been shown to be higher in AD patients when compared to control subjects.63,85 This is consistent with the proposed compensatory action of noradrenergic neurons. It is essential to note that the major method of NE clearance from the CSF is through NE reuptake by presynaptic noradrenergic terminals.86 Therefore, it is possible that AD related noradrenergic circuit degeneration may lead to impairment of NE reuptake resulting in increased CSF NE levels.87 Hence, measuring CSF NE concentrations may not allow a direct evaluation of CNS noradrenergic dynamics. It is more important to measure NE precursors and metabolites as well CSF NE levels for a comprehensive analysis of NE activity.88 Moreover, MHPG, unlike NE, crosses the blood-brain barrier freely, hence CSF MHPG can provide an indication of neuronal NE metabolism and dispersion of circulating MHPG into CSF. Therefore, it is imperative that plasma MHPG levels are accounted for in order to accurately interpret CNS noradrenergic activity.63 Raskind et al.87 compared levels of NE, its metabolite DHPG, and precursor dopa in both CSF and plasma following administration of yohimbine (a selective α2 adrenergic receptor blocker), clonidine (a selective α2 agonist), and placebo in older subjects with AD, older subjects without AD and younger subjects. The results suggested that the increase in CSF NE subsequent to yohimbine administration in both older subjects and AD subjects is not likely due to a reduction in NE clearance but rather mechanisms that compensate for NE neuron loss through augmented NE biosynthesis.
Cerebral spinal fluid NE response to manipulation of the α2 adrenergic receptor by the administration of yohimbine and clonidine has been studied to evaluate noradrenergic tone, yet a very limited amount of evidence is available regarding noradrenergic dysfunction. A general increase in CSF NE has been seen in response to yohimbine in both AD and older control subjects (with a greater increase in the AD subjects), when compared to younger control subjects.87,89 Results of studies of the CSF NE response to clonidine have been inconsistent, with insignificant results predominating. Further research into this area is needed to elucidate the value and capacity of CSF catechols as an index of neuronal neurotransmission function.
Compensating for lost neurons results in the tonic activation of the noradrenergic system leading to an increased noradrenergic response. Mechanisms that may account for this include the following: 1) Neuronal plasticity38,89—resulting in the hyperinnervation of certain brain regions. This may be a result of regenerating neurons and has been observed in rats.38,89 2) Increased NE synthetic capacity and firing rate in damaged LC neurons.38,89 3) Decreased ability of presynaptic α2 receptors to inhibit noradrenergic activity.38,89 Studies have indicated a loss of α2 receptor inhibition in AD, leading to the tonically activated state of the noradrenergic system and increased CSF NE levels.87,89 This increase in compensatory activity is usually accompanied by compensatory activity in other neurotransmitter systems as well.
Both postmortem and antemortem evidence supports a consistent link between noradrenergic dysfunction and AD (Table 1 and Table 2). Postmortem studies provide the strongest evidence of noradrenergic degeneration in AD. Although postmortem evidence may provide a descriptive representation of the loss of noradrenergic neurons, postmortem results may not directly reflect the antemortem state. Nonetheless, much of the evidence reviewed reveals decreased levels of both NE and noradrenergic neurons, while MHPG levels have been found to be increased, suggesting increased NE metabolism and, hence, activity. The fact that this correlates positively with increased AD severity, further validates these findings.
NOREPINEPHRINE AND BEHAVIOR
The LC is sensitive to both variations in the body's internal homeostasis and external environmental stimuli, which can activate not only the central noradrenergic system and sympathoadrenal system but may result in behavioral manifestations as well. The LC is involved in regulation of levels of arousal, flight-and-fight responses,89,90 agitation, anxiety,89 sleep-wake cycle, and levels of vigilance and emotion70,91,92 as well as aggressive behaviors.93–95 Noradrenergic projections from the LC also modulate sympathetic nervous system response, including blood pressure, pulse rate and danger signals. Hence, the noradrenergic system, directly or indirectly, has the potential to modify a variety of behaviors through actions in both the cortical and subcortical regions. A disruption of such an integrated system could potentially lead to abnormal behavioral responses to otherwise ordinary stimuli. LC damage has been determined in several neurologic disorders that are characterized by altered behavior including Parkinson's disease, schizophrenia and depression.70,92
The link between NE disruption and behavior has been studied in a variety of psychiatric disturbances including depression, anxiety, agitation and aggression. In depression there is evidence of α2 receptor dysfunction including postsynaptic α2 receptor downregulation,96–98 increased presynaptic α2 receptor sensitivity,99 and increased α2 receptor density in the LC.100,101 Decreased noradrenergic receptor sensitivity and increased noradrenergic turnover have been noted in patients with anxiety,102 generalized anxiety disorder,103 and posttraumatic stress disorder.104 High levels of NE release have been associated with aggression in animals, healthy adults93,105,106 and patients with depression and mania.107,108 In animal models, NE enhances aggressive behaviors106,109 and β-adrenoreceptor blockers have been shown to decrease aggression.106 Finally NE and NE metabolite levels are positively correlated with aggressive behaviors in patients with personality disorders.110–112 In summary, there is significant evidence of noradrenergic system involvement in a variety of psychiatric and behavioral disorders from animals and nondemented human studies.
NOREPINEPHRINE AND BEHAVIOR IN DEMENTIA (BPSD)
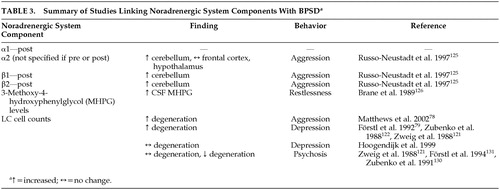
As previously mentioned, behavioral problems, particularly agitation and aggression are prevalent in AD. This has been consistently shown in various studies.113–116 A prospective, 10-year, longitudinal study on the change in aggressive behavior throughout the course of dementia by Keene et al.117 measured physical aggression, aggressive resistance, physical threats, verbal aggression, refusing to speak, destructive behavior and general irritability. The authors reported 96% of the 99 subjects with AD/vascular dementia showed profound or continuing aggressive behavior of at least one type during the dementia disease process, with verbal aggression as the most common behavioral disturbance. As the noradrenergic system has been implicated in the disorder of behavior in animals as well as other psychiatric ailments, it has been suggested that NE may likely play a role in the noncognitive behavioral disturbances associated with AD.118 Agitation, particularly aggression and akathisia, has been linked with increased norepinephrine activity.119 Noradrenergic system components have been associated with behaviors associated with dementia (Table 3). The role of norepinephrine in the facilitation of BPSD in AD is reviewed below.
Depression
Depression may complicate AD and has been found to be most common among people with mild severity of dementia.120 Both AD and depression share similar signs and symptoms, including loss of interest and appetite, apathy, and sleep disturbances.4 Studies have found that AD patients suffering from comorbid depression have significantly more LC neuron degeneration when compared to nondepressed AD patients.79,121,122 However, contradictory evidence exists. Hoogendijk et al.123 also compared LC neuronal cell counts between depressed and nondepressed AD patients but found no significant differences in LC degeneration between depressed and nondepressed AD patients. This may be due to methodological differences between the studies such as confounding by level of severity of both dementia121 and depression,121,122 LC sample section size and location of analysis.79,122 A reduction in NE has also been observed in cortex of depressed AD patients124 further supporting the role altered noradrenergic function may play in influencing depressive symptoms. Thus, the literature currently supports an association between NE and depression in AD.
Aggression and Agitation
Russo-Neustadt and Cotman125 observed a small but significant increase in total β-adrenoreceptor concentration in the cerebella of aggressive AD patients when compared to nonaggressive AD patients and healthy control subjects. This finding suggests a possible link between aggression/agitation and β-adrenergic receptor binding.
Since presynaptic α2 receptors regulate the negative feedback of NE and postsynaptic receptors are thought to regulate postsynaptic neuronal function, the differential location of the α2 adrenoreceptor may play a role in modulating aggression.106 Activation of the α2 adrenoreceptor by an agonist may lead to differing results depending on whether the post- or presynaptic receptors were stimulated or, possibly, depending on the ratio of the activated receptors. A postmortem study125 of the frontal cortex, hypothalamus and cerebellum (areas innervated by LC neurons) of agitated AD patients, nonagitated AD subjects and healthy elderly control subjects revealed a significantly elevated level of α2 receptors in the cerebellum of aggressive subjects, 70% higher than the nonagitated AD subjects. The levels of α2 receptors found in the nonagitated AD patients were slightly but not significantly higher than the levels found in the healthy elderly control subjects. Measurements of the α2 receptors in the frontal cortex and hypothalamus were not found to vary significantly.
There is some evidence that supports an association between motor restlessness and increased MHPG levels. MHPG levels have been found to be correlated with restlessness in AD patients.126
In vivo studies have also been performed to address the link between BPSD and NE. Yohimbine has been reported to lead to increased release of NE and successive activation of the postsynaptic α1 adrenoreceptors.127 It has been shown that AD patients are more sensitive to administration of yohimbine, compared to other elderly patients and younger subjects,87,128 and exhibit more agitated behaviors. This may be due to increased postsynaptic noradrenergic sensitivity in AD.87,89 This is plausible, as postsynaptic β-receptors have been reported to be up-regulated in the prefrontal cortex and hippocampus of AD patients,89 consistent with the postulated increase in sensitivity to noradrenergic stimulation.87 As a result of noradrenergic overactivation, AD patients would no longer be able to focus attention and their mechanisms of coping with stressful stimuli would be compromised. Even in the absence of stressful stimuli, the noradrenergic system would be active. This may account for the aggression displayed in many AD patients.38,62 These findings further consolidate the probability of an adrenergic dysfunction contributing to the pathophysiology of agitated behaviors in AD.
Psychosis
Psychosis is a cluster of clinically disruptive behaviors often observed in AD patients. Psychotic behaviors involve delusions and hallucinations and can aggravate aggression.129 Both evidence supporting the association between psychotic behaviors and noradrenergic preservation,130 and evidence showing no association between psychosis and NE121,131 have been published. Zubenko and associates130 found higher NE concentrations in the substantia nigra of psychotic AD patients when compared to nonpsychotic AD patients. Förstl et al.131 found that AD patients with auditory hallucinations or delusions had significantly higher neuron counts in the parahippocampal gyrus and a trend towards lower dorsal raphe nucleus neuron counts compared to AD patients without psychosis; however, this was not observed in the LC. The link between the noradrenergic system and psychotic behaviors is still unclear and further investigation is necessary.
In summary, evidence from both animal and human studies supports a link between noradrenergic dysfunction and behavior, particularly aggressive behavior. Depressed patients, patients suffering from anxiety disorders and AD patients all show blunted growth hormone (GH) responses to clonidine, strongly suggesting a disruption in noradrenergic control. Intensified noradrenergic activity and/or hypersensitive adrenoreceptors are thought to mediate this NE dysfunction in aggression associated both with and without AD as well as anxiety and depression. It is believed that compensation for the loss of the noradrenergic neurons that occurs during the progression of AD leads to an increase in the number of noradrenergic receptors and/or increased activity of remaining noradrenergic neurons, the potential source of aggressive behavior. This is credible as β1, β2 and α2 receptor upregulation has been shown in aggressive AD patients when compared to nonaggressive AD patients. The clinical expression of aggression likely is also dependent on other neurotransmitters. In summary, it has been shown that both α and β-adrenoreceptors are involved in the modulation of aggression. However, the exact role each receptor plays is still uncertain and further research is needed to clearly assess the link with NE and behavior, particularly BPSD.
NORADRENERGIC PHARMACOTHERAPY
The probability that the noradrenergic system contributes to the pathophysiology of BPSD associated with AD has led to potential therapeutic interventions. Pharmacological manipulation of the CNS noradrenergic system through the blockade of the beta adrenergic receptors has been studied as a treatment for a variety of neuropsychiatric disorders.132 As it is believed norepinephrine is involved in the modulation of behavior, noradrenergic agents have much pharmacological potential for the amelioration of behavioral disturbances. Studying the effects of noradrenergic agents on the various behavioral disturbances associated with dementia can also lead to a better understanding of their neurochemical etiology in the way neuroleptic response led to an understanding of the role of DA in schizophrenia.
Beta Blockers
Previous studies have indicated propanolol, a long-acting β-blocker, to be an effective treatment for aggression and agitation in patients with dementia who have been unsuccessfully treated with conventional therapies.133–137 In a randomized double-blind crossover placebo-controlled study, Greendyke et al.133 evaluated the efficacy of propanolol for an 11-week period at a maintenance dosage of 520 mg/day, in assaultive patients with organic brain impairment. The authors reported significantly fewer assaults and attempted assaults by the nine patients who completed the study, during the active treatment phase when compared to the placebo phase. Five patients showed marked improvement, two showed moderate improvement and two showed little or no improvement of violent behavior. A similar study was repeated by Greendyke and Kanter138 using pindolol, another β1 blocking agent, rather than propanolol. Pindolol wields a partial agonist effect thereby exerting similar therapeutic benefits as propanolol but with less cardiovascular side effects such as hypotension, bradycardia and decreased cardiac output. Patients were maintained on an individualized optimum dosage between 40–60 mg/day for a minimum of 10 days. Significant reductions in assaultiveness, hostility, lack of communication, uncooperativeness and repetitive behaviors were noted. Shankle et al.135 observed a 71% response rate in patients with either AD or vascular dementia (VaD) with both agitated and aggressive behaviors who were treated with propanolol. The 12 patients received 20 mg propanolol per day which was increased to an efficacious maximum that ranged between 30 and 80 mg per day. One patient suffered an adverse event (bradycardia), which was resolved by lowering the dose. A case series by Weiler et al.137 also demonstrated success in relieving treatment refractory disruptive behavior in 4 patients with AD and 2 patients with VaD. The propanolol doses ranged from 80 mg/day to 520 mg/day and no adverse events were noted. These studies indicate that the modulation of the β-adrenoreceptors through the use of β-blockers has been useful in the reduction of aggressive behaviors. However, many of these studies involved patients with varying dementia etiologies. A randomized controlled trial with AD patients treated for BPSD using β-blockers is necessary before one can accurately judge effectiveness. Important clinical considerations must be recognized when using β-blockers in elderly patients. β-Blockers may exacerbate various concomitant illnesses such as chronic obstructive pulmonary disease, diabetes mellitus and peripheral vascular disease. Additionally, beta-blockers have been shown to increase plasma concentrations of some antipsychotics, including chlorpromazine and thioridazine.139
Antidepressants
Imipramine, a tricyclic antidepressant, inhibits the reuptake of noradrenaline and has been shown to prevent upregulation of β-adrenoreceptors induced by stress140 and may hinder the induction of tyrosine hydroxylase in the LC following cold stress,51 suggesting a lessening of stress-induced norepinephrine release. Reifler et al.141 compared the use of imipramine with placebo in 61 AD patients, of those, 28 had a diagnosis of depression and 33 did not. The authors found that the depressed patients scored significantly higher than the nondepressed patients on the Hamilton Depression Rating Scale and though all patients improved significantly during the study, the depressed patients improved significantly more than the patients without depression. Although clinical recommendations have been made about avoiding the use of tertiary amines in the elderly demented population,142 imipramine was found to be extremely well tolerated in Reifler's study, as no significant differences in adverse events were noted in the imipramine-treated group compared to the placebo-treated group.
Mirtazapine is a noradrenergic and specific serotonergic antidepressant that directly blocks the pre- and postsynaptic α2 receptors and antagonizes the 5-hydroxytryptamine (5-HT)2 and 5-HT3 receptors with high affinity and 5-HT1 receptors with low affinity.143 Mirtazapine has been used with favorable results in a case series by Raji and Brady144 in AD patients suffering from depression as well as anxiety, weight loss and insomnia. Three patients were given a trial of mirtazapine. Two patients were started at 7.5 mg and titrated up over 2 weeks to 15 mg. One of those patients was further titrated up over 2 more weeks to 30 mg. The third patient was started at 15 mg and was increased to 30 mg after 2 weeks. No changes were noted in cognition; however, improvement in appetite and sleep was documented within 2–4 weeks and cessation of anxiety and depression was noted at 2 months. None of the patients suffered any adverse effects. This case series suggests that mirtazapine may be an effective therapy for depressed AD patients with comorbid insomnia, anxiety and appetite loss. However, there is a lack of randomized controlled trials using mirtazapine, and therefore the extent of the usefulness of mirtazapine pharmacotherapy is still unknown and remains to be explored.
Other antidepressants with putative effects on the NE system include venlafaxine, bupropion, and reboxetine, though none of these agents have been tested for BPSD.
Psychostimulants
Psychostimulants, such as methylphenidate (MPD), have been used in the past to treat affective disorders, particularly depression. MPD is believed to block both norepinephrine and DA uptake and promote catecholamine release.145 Few controlled studies have evaluated the efficacy of psychostimulants in the treatment BPSD.
A case series by Maletta and Winegarden145 examined three severely demented patients, suffering from apathy and anorexia resulting in weight loss, using methylphenidate therapy. Two patients were started on 5 mg bid and one patient was started on 5 mg once daily. Doses were increased up to the maintenance dose of 10 mg bid. Appetite was found to increase within 3–7 days of initiating therapy, and complete resolution of anorexia, leading to weight gain, was observed within a few weeks. Improvement in social interaction and hence a decrease in apathy was also noted by the nurses. The patients were maintained on methylphenidate for 9, 16 and 24 months, respectively, and were eventually tapered off successfully. No adverse effects were noted and no other psychotropic was used during the period of treatment. Kaufmann et al.146 also found favorable results with one dementia patient who was treated successfully for depressive symptoms including appetite loss and apathy, using methylphenidate.
Branconnier and Cole147 also conducted a placebo-controlled study in demented, apathetic patients using 20–30 mg MPD and found an improvement in apathy that correlated with a lessening of depressive symptoms. Galynker et al.148 treated 12 AD patients and 15 VaD patients using methylphenidate and found a significant improvement in negative symptoms.
Although the benefits of methylphenidate therapy in the elderly have been reported for the last 50 years, the results are limited by poor designs and lack of standardized assessments for behavior and even diagnoses. Methylphenidate appears to be useful in alleviating depressive symptoms including appetite loss and apathy; however, more research is needed to explore the value of; MPD in the pharmacotherapy of other behavioral disturbances associated with AD. Furthermore, as noted previously, psychostimulants have effects on other neurotransmitters, and it is not clear whether their beneficial effects for BPSD are due to their actions on NE or other systems.
It is difficult to treat BPSD pharmacologically as some patients may be very sensitive to first-line therapy with antipsychotic medications. Treatment emergent side effects may be intolerable resulting in the discontinuation of the drug. Hence, prescribing medication for BPSD often involves trying a series of medications on each patient. The use of β adrenergic antagonists for BPSD such as agitation and aggression may be clinically advantageous due to the absence of the extrapyramidal symptoms and sedation commonly seen with antipsychotics. Other adrenergic agents have been studied for the alleviation of other behavioral symptoms frequently associated with dementia, however, the vast majority of noradrenergic agents prescribed are for depression. Although the pharmacological management of depression with noradrenergic drugs has been shown to be successful, there is a great need for further research to elucidate the role of noradrenergic pharmacotherapy in the treatment of depression associated with AD. Given the modest efficacy of antipsychotics and the variable responses to antidepressants, randomized controlled trials of noradrenergic agents for BPSD are urgently needed.
CONCLUSIONS
The bulk of neuropathological studies in AD show noradrenergic neuron degeneration, decreased NE levels and increased NE metabolites. This evidence strongly and consistently suggests enhanced NE turnover. The vast majority of studies; however, have been done postmortem, and consequently caution is necessary in their interpretation. As altered behavior may be a result of “state” rather than “trait” phenomenon, it is important that the behavior being examined is manifested at the time of death, so as to accurately measure the neurological changes believed to be responsible. Postmortem delay is a confounding variable in neurophysiological analysis as postmortem results may not correctly represent the antemortem state. The consequences of delay in freezing brain tissue after death and prolonged preservation of brain tissue prior to catecholamine analysis have not yet been fully accounted for and may limit the usefulness of postmortem studies.
Norepinephrine is involved in the sympathetic “flight-or-fight” response and thus is sensitive to environmental challenges and can modulate behavior accordingly. The noradrenergic system has been shown to mediate behavior, particularly aggression, in animals as well as in psychiatric illnesses. Although there is substantial evidence correlating NE with aggression, a direct link between NE function and aggression associated with dementia needs to be examined further. More research into identifying the role of the noradrenergic receptors and their subtypes in controlling behavior in AD is necessary to elucidate the extent behavior dysregulation may be accounted for by noradrenergic dysfunction. A limitation of the current knowledge of the role of NE in BPSD is lack of information on other behaviors such as apathy, wandering, and sexually disinhibited behaviors. The current literature search revealed studies that addressed depression, agitation, aggression and psychosis only.
Drugs that target NE have been successfully used in treatment of depression, which is commonly found in AD as well. However, there is little research, especially randomized controlled trials, into the use of noradrenergic treatments for BPSD. Studies on beta blocker treatment for aggression and psychostimulant use for affective disorders appear to be the better studied pharmacologic interventions in AD; however, they too suffer from lack of standardization in behavioral assessments, heterogeneity in dementia diagnoses and small sample sizes. Further comprehensive research is necessary to explore the potential clinical utility of noradrenergic agents for the pharmacological management of disruptive behaviors associated with dementia.
The study of the neurobiology of BPSD in AD is fraught with methodological difficulties. While much has been learned over the past two decades, the goal of a unitary etiological hypothesis may never be reached given the heterogeneity of AD and its variable effects on behavior. Providing putative links between discrete neurotransmitter dysfunction and behavior; however, will allow for more targeted pharmacological interventions. Only then will treatment of BPSD be truly “rational.”
ACKNOWLEDGMENTS
This study was funded in part by a grant from the Physicians' Services Incorporated Foundation, grant number 98-39.
The authors thank Luis Lewis, Jr. and Florance Chan for their assistance.
 |
 |
 |
1 Fratiglioni L, De Ronchi D, Aguero-Torres H: Worldwide prevalence and incidence of dementia. Drugs Aging 1999; 15:365–375Crossref, Medline, Google Scholar
2 Fillit HM: The pharmacoeconomics of Alzheimer's disease. Am J Manag Care 2000; 6(22 suppl):S1139-S1144; discussion S1145-S1138Google Scholar
3 Evans DA, Funkenstein HH, Albert MS, et al: Prevalence of Alzheimer's disease in a community population of older persons: higher than previously reported. JAMA 1989; 262:2551–2556Crossref, Medline, Google Scholar
4 Raskind MA: Geriatric psychopharmacology: management of late-life depression and the noncognitive behavioral disturbances of Alzheimer's disease. Psychiatr Clin North Am 1993; 16:815–827Crossref, Medline, Google Scholar
5 Teri L, Rabins P, Whitehouse P, et al: Management of behavior disturbance in Alzheimer disease: current knowledge and future directions. Alzheimer Dis Assoc Disord 1992; 6:77–88Crossref, Medline, Google Scholar
6 Reisberg B, Borenstein J, Salob SP, et al: Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry 1987; 48(May suppl):9–15Google Scholar
7 Gilley DW, Whalen ME, Wilson RS, et al: Hallucinations and associated factors in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 1991; 3:371–376Link, Google Scholar
8 Rabins PV, Mace NL, Lucas MJ: The impact of dementia on the family. JAMA 1982; 248:333–335Crossref, Medline, Google Scholar
9 Nagaratnam N, Lewis-Jones M, Scott D, et al: Behavioral and psychiatric manifestations in dementia patients in a community: caregiver burden and outcome. Alzheimer Dis Assoc Disord 1998; 12:330–334Crossref, Medline, Google Scholar
10 Stoppe G, Brandt CA, Staedt JH: Behavioural problems associated with dementia: the role of newer antipsychotics. Drugs Aging 1999; 14:41–54Crossref, Medline, Google Scholar
11 Mega MS, Cummings JL, Fiorello T, et al: The spectrum of behavioral changes in Alzheimer's disease. Neurology 1996; 46:130–135Crossref, Medline, Google Scholar
12 Petrie W, Lawson E, Hollender M: Violence in geriatric patients. JAMA 1982; 248:443–444Crossref, Medline, Google Scholar
13 Gaspar D: Hollymoor Hospital Dementia Service: analysis of outcome of 230 consecutive referrals to a psychiatric-hospital dementia service. Lancet 1980; 1:1401–1405Medline, Google Scholar
14 Hebert R, Dubois MF, Wolfson C, et al: Factors associated with long-term institutionalization of older people with dementia: data from the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci 2001; 56:M693-M699Google Scholar
15 Kunik ME, Yudofsky SC, Silver JM, et al: Pharmacologic approach to management of agitation associated with dementia. J Clin Psychiatry 1994; 55(Feb suppl):13–17Google Scholar
16 Cohen-Mansfield J, Billig N: Agitated behaviors in the elderly, I: a conceptual review. J Am Geriatr Soc 1986; 34:711–721Crossref, Medline, Google Scholar
17 Gleason RP, Schneider LS: Carbamazepine treatment of agitation in Alzheimer's outpatients refractory to neuroleptics. J Clin Psychiatry 1990; 51:115–118Medline, Google Scholar
18 Ryden MB: Aggressive behavior in persons with dementia who live in the community. Alzheimer Dis Assoc Disord 1988; 2:342–355Crossref, Medline, Google Scholar
19 Herrmann N: Recommendations for the management of behavioral and psychological symptoms of dementia. Can J Neurol Sci 2001; 28(suppl 1):S96-S107Google Scholar
20 Yeager BF, Farnett LE, Ruzicka SA: Management of the behavioral manifestations of dementia. Arch Intern Med 1995; 155:250–260Crossref, Medline, Google Scholar
21 Treatment of agitation in older persons with dementia: the expert consensus panel for agitation in dementia. Postgrad Med Special Issue 1998, pp 1–88Google Scholar
22 Lantz MS, Louis A, Lowenstein G, et al: A longitudinal study of psychotropic prescriptions in a teaching nursing home. Am J Psychiatry 1990; 147:1637–1639Crossref, Medline, Google Scholar
23 Avorn J, Dreyer P, Connelly K, et al: Use of psychoactive medication and the quality of care in rest homes: findings and policy implications of a statewide study. N Engl J Med 1989; 320:227–232Crossref, Medline, Google Scholar
24 Gurwitz JH, Soumerai SB, Avorn J: Improving medication prescribing and utilization in the nursing home. J Am Geriatr Soc 1990; 38:542–552Crossref, Medline, Google Scholar
25 Hesse KA, Driscoll A, Jacobson S: Neuroleptic prescriptions for acutely ill geriatric patients. Arch Intern Med 1993; 153:2581–2587Crossref, Medline, Google Scholar
26 Beers MH, Ouslander JG, Rollingher I, et al: Explicit criteria for determining inappropriate medication use in nursing home residents: UCLA Division of Geriatric Medicine. Arch Intern Med 1991; 151:1825–1832Crossref, Medline, Google Scholar
27 Semla TP, Palla K, Poddig B, et al: Effect of the Omnibus Reconciliation Act 1987 on antipsychotic prescribing in nursing home residents. J Am Geriatr Soc 1994; 42:648–652Crossref, Medline, Google Scholar
28 Dodds MJ: Pre- and post-OBRA '87 psychoactive drug use in long-term care facilities. J Long Term Care Adm 1996; 24:17–22Medline, Google Scholar
29 Zayas EM, Grossberg GT: Treating the agitated Alzheimer patient. J Clin Psychiatry 1996; 57(suppl 7):46–51; discussion 52–44Google Scholar
30 Lanctôt KL, Bowles SK, Herrmann N, et al: Drugs mimicking dementia: dementia symptoms associated with psychotropic drugs in institutionalised cognitively impaired patients. CNS Drugs 2000; 14:381–390Crossref, Google Scholar
31 Lanctôt KL, Best TS, Mittmann N, et al: Efficacy and safety of neuroleptics in behavioral disorders associated with dementia. J Clin Psychiatry 1998; 59:550–561; quiz 562–553Crossref, Medline, Google Scholar
32 Schneider LS, Pollock VE, Lyness SA: A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990; 38:553–563Crossref, Medline, Google Scholar
33 Herrmann N, Lanctôt KL: The management of behavioral disturbances in dementia: the role of serotonergic therapies. IDrugs 1998; 1:214–220Medline, Google Scholar
34 Lanctôt KL, Herrmann N, Mazzotta P: Role of serotonin in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci 2001; 13:5–21Link, Google Scholar
35 Coull JT: Pharmacological manipulations of the alpha 2-noradrenergic system: effects on cognition. Drugs Aging 1994; 5:116–126Crossref, Medline, Google Scholar
36 Yudofsky SC, Silver JM, Hales RE: Treatment of agitation and aggression, in Textbook of Psychopharmacology. Edited by Schatzberg AF, Nemeroff CB. Washington, DC, American Psychiatric Press, 1998, pp 881–900Google Scholar
37 Redmond D: Studies of the nucleus locus ceruleus in monkeys and hypothesis for neuropsychopharmacology, in Psychopharmacology: The Third Generation of Progress. Edited by Meltzer H. New York, Raven Press, 1987, pp 967–975Google Scholar
38 Friedman JI, Adler DN, Davis KL: The role of norepinephrine in the pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer's disease. Biol Psychiatry 1999; 46:1243–1252Crossref, Medline, Google Scholar
39 Nestler EJ, Alreja M, Aghajanian GK: Molecular control of locus coeruleus neurotransmission. Biol Psychiatry 1999; 46:1131–1139Crossref, Medline, Google Scholar
40 Beani L, Bianchi C, Giacomelli A, et al: Noradrenaline inhibition of acetylcholine release from guinea-pig brain. Eur J Pharmacol 1978; 48:179–193Crossref, Medline, Google Scholar
41 Vizi ES: Presynaptic modulation of neurochemical transmission. Prog Neurobiol 1979; 12:181–290Crossref, Medline, Google Scholar
42 Vizi ES, Pasztor E: Release of acetylcholine from isolated human cortical slices: inhibitory effect of norepinephrine and phenytoin. Exp Neurol 1981; 73:144–153Crossref, Medline, Google Scholar
43 Bianchi C, Spidalieri G, Guandalini P, et al: Inhibition of acetylcholine outflow from guinea-pig cerebral cortex following locus coeruleus stimulation. Neurosci Lett 1979; 14:97–100Crossref, Medline, Google Scholar
44 Jaim-Etcheverry G, Zieher L: Coexistence of monoamines in peripheral adrenergic neurones, in Co-Transmission. Edited by Cuello A. London, Macmillan, 1982, pp 189–207Google Scholar
45 Murphy DL, Andrews AM, Wichems CH, et al: Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry 1998; 59(suppl 15):4–12Google Scholar
46 Gillespie DD, Manier DH, Sanders-Bush E, et al: The serotonin/ norepinephrine-link in brain, II: role of serotonin in the regulation of beta adrenoceptors in the low agonist affinity conformation. J Pharmacol Exp Ther 1988; 244:154–159Medline, Google Scholar
47 Soderpalm B, Andersson L, Carlsson M, et al: Serotonergic influence on the growth hormone response to clonidine in rat. J Neural Transm 1987; 69:105–114Crossref, Medline, Google Scholar
48 Yamamoto BK, Novotney S: Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 1998; 71:274–280Crossref, Medline, Google Scholar
49 Gresch PJ, Sved AF, Zigmond MJ, et al: Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem 1995; 65:111–116Crossref, Medline, Google Scholar
50 Ferraro L, Tanganelli S, Calo G, et al: Noradrenergic modulation of gamma-aminobutyric acid outflow from the human cerebral cortex. Brain Res 1993; 629:103–108Crossref, Medline, Google Scholar
51 Melia KR, Duman RS: Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci USA 1991; 88:8382–8386Crossref, Medline, Google Scholar
52 Veith RC, Lewis N, Langohr JI, et al: Effect of desipramine on cerebrospinal fluid concentrations of corticotropin-releasing factor in human subjects. Psychiatry Res 1993; 46:1–8Crossref, Medline, Google Scholar
53 Gerner RH, Yamada T: Altered neuropeptide concentrations in cerebrospinal fluid of psychiatric patients. Brain Res 1982; 238:298–302Crossref, Medline, Google Scholar
54 Minthon L, Edvinsson L, Gustafson L: Correlation between clinical characteristics and cerebrospinal fluid neuropeptide Y levels in dementia of the Alzheimer type and frontotemporal dementia. Alzheimer Dis Assoc Disord 1996; 10:197–203Crossref, Medline, Google Scholar
55 de Wied D, van Ree JM: Neuropeptides: animal behaviour and human psychopathology. Eur Arch Psychiatry Neurol Sci 1989; 238:323–331Crossref, Medline, Google Scholar
56 Herrmann N, Lanctôt KL: From transmitters to treatment: the pharmacotherapy of behavioural disturbances in dementia. Can J Psychiatry 1997; 42(suppl 1):51S-64SGoogle Scholar
57 Lawlor BA, Sunderland T, Mellow AM, et al: Hyperresponsivity to the serotonin agonist m-chlorophenylpiperazine in Alzheimer's disease: a controlled study. Arch Gen Psychiatry 1989; 46:542–549Crossref, Medline, Google Scholar
58 Bierer LM, Haroutunian V, Gabriel S, et al: Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of the cholinergic deficits. J Neurochem 1995; 64:749–760Crossref, Medline, Google Scholar
59 Mann DM, Yates PO, Hawkes J: The noradrenergic system in Alzheimer and multi-infarct dementias. J Neurol Neurosurg Psychiatry 1982; 45:113–119Crossref, Medline, Google Scholar
60 Palmer AM, Francis PT, Bowen DM, et al: Catecholaminergic neurones assessed ante-mortem in Alzheimer's disease. Brain Res 1987; 414:365–375Crossref, Medline, Google Scholar
61 Adolfsson R, Gottfries CG, Roos BE, et al: Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry 1979; 135:216–223Crossref, Medline, Google Scholar
62 Hoogendijk WJ, Feenstra MG, Botterblom MH, et al: Increased activity of surviving locus ceruleus neurons in Alzheimer's disease. Ann Neurol 1999; 45:82–91Crossref, Medline, Google Scholar
63 Raskind MA, Peskind ER, Halter JB, et al: Norepinephrine and MHPG levels in CSF and plasma in Alzheimer's disease. Arch Gen Psychiatry 1984; 41:343–346Crossref, Medline, Google Scholar
64 Arai H, Kosaka K, Iizuka R: Changes of biogenic amines and their metabolites in postmortem brains from patients with Alzheimer-type dementia. J Neurochem 1984; 43:388–393Crossref, Medline, Google Scholar
65 Francis PT, Palmer AM, Sims NR, et al: Neurochemical studies of early-onset Alzheimer's disease: possible influence on treatment. N Engl J Med 1985; 313:7–11Crossref, Medline, Google Scholar
66 Nazarali AJ, Reynolds GP: Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer's disease: a postmortem study. Cell Mol Neurobiol 1992; 12:581–587Crossref, Medline, Google Scholar
67 Storga D, Vrecko K, Birkmayer JG, et al: Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett 1996; 203:29–32Crossref, Medline, Google Scholar
68 Mann DM, Yates PO, Marcyniuk B: A comparison of changes in the nucleus basalis and locus caeruleus in Alzheimer's disease. J Neurol Neurosurg Psychiatry 1984; 47:201–203Crossref, Medline, Google Scholar
69 Bondareff W, Mountjoy CQ, Roth M: Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 1982; 32:164–168Crossref, Medline, Google Scholar
70 Hoogendijk WJ, Pool CW, Troost D, et al: Image analyser-assisted morphometry of the locus coeruleus in Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis. Brain 1995; 118(part 1):131–143Google Scholar
71 Gottfries CG, Adolfsson R, Aquilonius SM, et al: Biochemical changes in dementia disorders of Alzheimer type (AD/SDAT). Neurobiol Aging 1983; 4:261–271Crossref, Medline, Google Scholar
72 Herregodts P, Bruyland M, De Keyser J, et al: Monoaminergic neurotransmitters in Alzheimer's disease: an HPLC study comparing presenile familial and sporadic senile cases. J Neurol Sci 1989; 92:101–116Crossref, Medline, Google Scholar
73 Miller MA, Kolb PE, Leverenz JB, et al: Preservation of noradrenergic neurons in the locus ceruleus that coexpress galanin mRNA in Alzheimer's disease. J Neurochem 1999; 73:2028–2036Medline, Google Scholar
74 Iversen LL, Rossor MN, Reynolds GP, et al: Loss of pigmented dopamine-beta-hydroxylase positive cells from locus coeruleus in senile dementia of Alzheimer's type. Neurosci Lett 1983; 39:95–100Crossref, Medline, Google Scholar
75 Moll G, Gsell W, Wichart I, et al: Cholinergic and monoaminergic neuromediator systems in DAT: neuropathological and neurochemical findings, in Alzheimer's Disease: Epidemiology, Neuropathy, Neurochemistry, and Clinics. Edited by Maurer K, Riederer P, Beckmann H. New York, Springer Verlag, 1990, pp 235–243Google Scholar
76 Nyberg P, Adolfsson R, Hardy JA, et al: Catecholamine topochemistry in human basal ganglia: comparison between normal and Alzheimer brains. Brain Res 1985; 333:139–142Crossref, Medline, Google Scholar
77 Yates CM, Simpson J, Gordon A, et al: Catecholamines and cholinergic enzymes in pre-senile and senile Alzheimer-type dementia and Down's syndrome. Brain Res 1983; 280:119–126Crossref, Medline, Google Scholar
78 Matthews KL, Chen CP, Esiri MM, et al: Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry 2002; 51:407–416Crossref, Medline, Google Scholar
79 Förstl H, Burns A, Luthert P, et al: Clinical and neuropathological correlates of depression in Alzheimer's disease. Psychol Med 1992; 22:877–884Crossref, Medline, Google Scholar
80 Perry EK, Tomlinson BE, Blessed G, et al: Neuropathological and biochemical observations on the noradrenergic system in Alzheimer's disease. J Neurol Sci 1981; 51:279–287Crossref, Medline, Google Scholar
81 Tohgi H, Ueno M, Abe T, et al: Concentrations of monoamines and their metabolites in the cerebrospinal fluid from patients with senile dementia of the Alzheimer type and vascular dementia of the Binswanger type. J Neural Transm Park Dis Dement Sect 1992; 4:69–77Crossref, Medline, Google Scholar
82 Lawlor BA, Bierer LM, Ryan TM, et al: Plasma 3-methoxy-4-hydroxyphenylglycol (MHPG) and clinical symptoms in Alzheimer's disease. Biol Psychiatry 1995; 38:185–188Crossref, Medline, Google Scholar
83 Miyata S, Nagata H, Yamao S, et al: Dopamine-beta-hydroxylase activities in serum and cerebrospinal fluid of aged and demented patients. J Neurol Sci 1984; 63:403–409Crossref, Medline, Google Scholar
84 Cross AJ, Crow TJ, Perry EK, et al: Reduced dopamine-beta-hydroxylase activity in Alzheimer's disease. Br Med J (Clin Res Ed) 1981; 282:93–94Crossref, Medline, Google Scholar
85 Elrod R, Peskind ER, DiGiacomo L, et al: Effects of Alzheimer's disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry 1997; 154:25–30Crossref, Medline, Google Scholar
86 Li PP, Warsh JJ, Godse DD: Rat brain norepinephrine metabolism: substantial clearance through 3,4-dihydroxyphenylethyleneglycol formation. J Neurochem 1983; 41:1065–1071Crossref, Medline, Google Scholar
87 Raskind MA, Peskind ER, Holmes C, et al: Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer's disease. Biol Psychiatry 1999; 46:756–765Crossref, Medline, Google Scholar
88 Holmes C, Eisenhofer G, Goldstein DS: Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl 1994; 653:131–138Crossref, Medline, Google Scholar
89 Peskind ER, Wingerson D, Murray S, et al: Effects of Alzheimer's disease and normal aging on cerebrospinal fluid norepinephrine responses to yohimbine and clonidine. Arch Gen Psychiatry 1995; 52:774–782Crossref, Medline, Google Scholar
90 Anand A, Charney DS: Norepinephrine dysfunction in depression. J Clin Psychiatry 2000; 61(suppl 10):16–24Google Scholar
91 Arnsten AF, Steere JC, Hunt RD: The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function: potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1996; 53:448–455Crossref, Medline, Google Scholar
92 Chan-Palay V, Asan E: Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson's disease with and without dementia and depression. J Comp Neurol 1989; 287:373–392Crossref, Medline, Google Scholar
93 Torda C: Effects of catecholamines on behavior. J Neurosci Res 1976; 2:193–202Crossref, Medline, Google Scholar
94 Thoa NB, Eichelman B, Richardson JS, et al: 6-Hydroxydopa depletion of brain norepinephrine and the function of aggressive behavior. Science 1972; 178:75–77Crossref, Medline, Google Scholar
95 Geyer MA, Segal DS: Shock-induced aggression: opposite effects of intraventricularly infused dopamine and norepinephrine. Behav Biol 1974; 10:99–104Crossref, Medline, Google Scholar
96 Charney DS, Heninger GR, Sternberg DE, et al: Adrenergic receptor sensitivity in depression: effects of clonidine in depressed patients and healthy subjects. Arch Gen Psychiatry 1982; 39:290–294Crossref, Medline, Google Scholar
97 Gilles C, Ryckaert P, De Mol J, et al: Clonidine-induced growth hormone secretion in elderly patients with senile dementia of the Alzheimer type and major depressive disorder. Psychiatry Res 1989; 27:277–286Crossref, Medline, Google Scholar
98 Checkly S, Corn T, Glass I, et al: Responsiveness of central alpha-2 adrenoceptors in depression, in The Biology of Depression. Edited by Deakin J. London, Gaskell, 1986, pp 100–120Google Scholar
99 Leckman JF, Maas JW, Redmond DE Jr, et al: Effects of oral clonidine on plasma 3-methoxy-4-hydroxyphenethyleneglycol (MHPG) in man: preliminary report. Life Sci 1980; 26:2179–2185Crossref, Medline, Google Scholar
100 Ordway GA, Widdowson PS, Smith KS, et al: Agonist binding to alpha 2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem 1994; 63:617–624Crossref, Medline, Google Scholar
101 Ressler KJ, Nemeroff CB: Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry 1999; 46:1219–1233Crossref, Medline, Google Scholar
102 Abelson JL, Glitz D, Cameron OG, et al: Blunted growth hormone response to clonidine in patients with generalized anxiety disorder. Arch Gen Psychiatry 1991; 48:157–162Crossref, Medline, Google Scholar
103 Nutt DJ: Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry 2001; 62(suppl 11):22–27; discussion 28Google Scholar
104 Geracioti TD Jr, Baker DG, Ekhator NN, et al: CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 2001; 158:1227–1230Crossref, Medline, Google Scholar
105 Gerra G, Zaimovic A, Avanzini P, et al: Neurotransmitter-neuroendocrine responses to experimentally induced aggression in humans: influence of personality variable. Psychiatry Res 1997; 66:33–43Crossref, Medline, Google Scholar
106 Haller J, Makara GB, Kruk MR: Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci Biobehav Rev 1998; 22:85–97Crossref, Medline, Google Scholar
107 Redmond DE Jr, Katz MM, Maas JW, et al: Cerebrospinal fluid amine metabolites: relationships with behavioral measurements in depressed, manic, and healthy control subjects. Arch Gen Psychiatry 1986; 43:938–947Crossref, Medline, Google Scholar
108 Swann AC, Secunda SK, Koslow SH, et al: Mania: sympathoadrenal function and clinical state. Psychiatry Res 1991; 37:195–205Crossref, Medline, Google Scholar
109 Eichelman B: Neurochemical and psychopharmacologic aspects of aggressive behavior, in Psychopharmacology: The Third Generation of Progress. Edited by Meltzer H. New York, Raven Press, 1987, pp 697–704Google Scholar
110 Brown GL, Goodwin FK, Ballenger JC, et al: Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1979; 1:131–139Crossref, Medline, Google Scholar
111 Gerra G, Avanzini P, Zaimovic A, et al: Neurotransmitter and endocrine modulation of aggressive behavior and its components in normal humans. Behav Brain Res 1996; 81:19–24Crossref, Medline, Google Scholar
112 Coccaro EF, Lawrence T, Trestman R, et al: Growth hormone responses to intravenous clonidine challenge correlate with behavioral irritability in psychiatric patients and healthy volunteers. Psychiatry Res 1991; 39:129–139Crossref, Medline, Google Scholar
113 Tsai SJ, Hwang JP, Yang CH, et al: Physical aggression and associated factors in probable Alzheimer disease. Alzheimer Dis Assoc Disord 1996; 10:82–85Crossref, Medline, Google Scholar
114 Ware CJG, Fairburn CG, Hope RA: A community-based study of aggressive behaviour in dementia. Int J Geriatr Psychiatry 1990; 5:337–342Crossref, Google Scholar
115 Lyketsos CG, Steinberg M, Tschanz JT, et al: Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 2000; 157:708–714Crossref, Medline, Google Scholar
116 Lee MM, Strauss ME, Dawson DV: Changes in emotional and behavioral symptoms of Alzheimer's disease. Am J Alzheimer Dis Other Demen 2000; 15:176–179Crossref, Google Scholar
117 Keene J, Hope T, Fairburn CG, et al: Natural history of aggressive behaviour in dementia. Int J Geriatr Psychiatry 1999; 14:541–548Crossref, Medline, Google Scholar
118 Raskind MA, Peskind ER: Neurobiologic bases of noncognitive behavioral problems in Alzheimer disease. Alzheimer Dis Assoc Disord 1994; 8(suppl 3):54–60Google Scholar
119 Lindenmayer JP: The pathophysiology of agitation. J Clin Psychiatry 2000; 61(suppl 14):5–10Google Scholar
120 Margallo-Lana M, Swann A, O'Brien J, et al: Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatry 2001; 16:39–44Crossref, Medline, Google Scholar
121 Zweig RM, Ross CA, Hedreen JC, et al: The neuropathology of aminergic nuclei in Alzheimer's disease. Ann Neurol 1988; 24: 233–242Google Scholar
122 Zubenko GS, Moossy J: Major depression in primary dementia: clinical and neuropathologic correlates. Arch Neurol 1988; 45:1182–1186Google Scholar
123 Hoogendijk WJ, Sommer IE, Pool CW, et al: Lack of association between depression and loss of neurons in the locus coeruleus in Alzheimer disease. Arch Gen Psychiatry 1999; 56:45–51Crossref, Medline, Google Scholar
124 Zubenko GS, Moossy J, Kopp U: Neurochemical correlates of major depression in primary dementia. Arch Neurol 1990; 47: 209–214Google Scholar
125 Russo-Neustadt A, Cotman CW: Adrenergic receptors in Alzheimer's disease brain: selective increases in the cerebella of aggressive patients. J Neurosci 1997; 17:5573–5580Crossref, Medline, Google Scholar
126 Brane G, Gottfries CG, Blennow K, et al: Monoamine metabolites in cerebrospinal fluid and behavioral ratings in patients with early and late onset of Alzheimer dementia. Alzheimer Dis Assoc Disord 1989; 3:148–156Crossref, Medline, Google Scholar
127 Anden NE, Pauksens K, Svensson K: Selective blockade of brain alpha 2-autoreceptors by yohimbine: effects on motor activity and on turnover of noradrenaline and dopamine. J Neural Transm 1982; 55:111–120Crossref, Medline, Google Scholar
128 Peskind ER, Elrod R, Dobie DJ, et al: Cerebrospinal fluid epinephrine in Alzheimer's disease and normal aging. Neuropsychopharmacology 1998; 19:465–471Crossref, Medline, Google Scholar
129 Aarsland D, Cummings JL, Yenner G, et al: Relationship of aggressive behavior to other neuropsychiatric symptoms in patients with Alzheimer's disease. Am J Psychiatry 1996; 153:243–247Crossref, Medline, Google Scholar
130 Zubenko GS, Moossy J, Martinez AJ, et al: Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol 1991; 48:619–624Crossref, Medline, Google Scholar
131 Förstl H, Burns A, Levy R, et al: Neuropathological correlates of psychotic phenomena in confirmed Alzheimer's disease. Br J Psychiatry 1994; 165:53–59Crossref, Medline, Google Scholar
132 Yudofsky S, Silver J, Schneider S: Pharmacologic treatment of aggression. Psychiatr Annals 1987; 27:397–407Crossref, Google Scholar
133 Greendyke RM, Kanter DR, Schuster DB, et al: Propranolol treatment of assaultive patients with organic brain disease: a double-blind crossover, placebo-controlled study. J Nerv Ment Dis 1986; 174:290–294Crossref, Medline, Google Scholar
134 Petrie WM, Ban TA: Propranolol in organic agitation (letter). Lancet 1981; 1:324Crossref, Medline, Google Scholar
135 Shankle WR, Nielson KA, Cotman CW: Low-dose propranolol reduces aggression and agitation resembling that associated with orbitofrontal dysfunction in elderly demented patients. Alzheimer Dis Assoc Disord 1995; 9:233–237Crossref, Medline, Google Scholar
136 Yudofsky S, Williams D, Gorman J: Propranolol in the treatment of rage and violent behavior in patients with chronic brain syndromes. Am J Psychiatry 1981; 138:218–220Crossref, Medline, Google Scholar
137 Weiler PG, Mungas D, Bernick C: Propranolol for the control of disruptive behavior in senile dementia. J Geriatr Psychiatry Neurol 1988; 1:226–230Crossref, Medline, Google Scholar
138 Greendyke RM, Kanter DR: Therapeutic effects of pindolol on behavioral disturbances associated with organic brain disease: a double-blind study. J Clin Psychiatry 1986; 47:423–426Medline, Google Scholar
139 Silver JM, Yudofsky SC, Kogan M, et al: Elevation of thioridazine plasma levels by propranolol. Am J Psychiatry 1986; 143:1290–1292Crossref, Medline, Google Scholar
140 Stanford SC: Central noradrenergic neurones and stress. Pharmacol Ther 1995; 68:297–242Crossref, Medline, Google Scholar
141 Reifler BV, Teri L, Raskind M, et al: Double-blind trial of imipramine in Alzheimer's disease patients with and without depression. Am J Psychiatry 1989; 146:45–49Crossref, Medline, Google Scholar
142 Peisah C, Brodaty H: Practical guidelines for the treatment of behavioural complications of dementia. Med J Aust 1994; 161:558–563Crossref, Medline, Google Scholar
143 de Boer T: The pharmacologic profile of mirtazapine. J Clin Psychiatry 1996; 57(suppl 4):19–25Google Scholar
144 Raji MA, Brady SR: Mirtazapine for treatment of depression and comorbidities in Alzheimer disease. Ann Pharmacother 2001; 35:1024–1027Crossref, Medline, Google Scholar
145 Maletta GJ, Winegarden T: Reversal of anorexia by methylphenidate in apathetic, severely demented nursing home patients. Am J Geriatr Psychiatry 1993; 1:234–243Crossref, Medline, Google Scholar
146 Kaufmann MW, Cassem NH, Murray GB, et al: Use of psychostimulants in medically ill patients with neurological disease and major depression. Can J Psychiatry 1984; 29:46–49Crossref, Medline, Google Scholar
147 Branconnier RJ, Cole JO: The therapeutic role of methylphenidate in senile organic brain syndrome. Proc Annu Meet Am Psychopathol Assoc 1980; 69:183–196Medline, Google Scholar
148 Galynker I, Ieronimo C, Miner C, et al: Methylphenidate treatment of negative symptoms in patients with dementia. J Neuropsychiatry Clin Neurosci 1997; 9:231–239Link, Google Scholar
149 D'Amato RJ, Zweig RM, Whitehouse PJ, et al: Aminergic systems in Alzheimer's disease and Parkinson's disease. Ann Neurol 1987; 22:229–236Crossref, Medline, Google Scholar



