Huntington’s Disease Patients Show Impaired Perception of Disgust in the Gustatory and Olfactory Modalities
Abstract
Patients with Huntington’s disease show deficits in recognizing disgust in the facial expressions and vocal intonations of others. In this study, the authors demonstrate that these disgust-related deficits extend to foul-smelling olfactory stimuli and inappropriate combinations of taste stimuli.
Huntington’s disease is a genetically transmitted, progressive neurodegenerative disease, the pathology of which is initially concentrated within the striatum. The disease is characterized by abnormal movements and dementia, although psychiatric problems are common. Patients with Huntington’s disease may also show abnormal social behavior, including changes in personal hygiene and, occasionally, sexual misconduct.1
Patient’s with Huntington’s disease show an inability to recognize the facial expression of disgust in others while their ability to correctly perceive other types of facial expressions remains relatively unaffected.2 This deficit in the perception of disgust, which is present in clinically presymptomatic patients, extends to deficits in the perception of the vocal intonation of disgust.2,3 Deficits may also be detected in patients using self-assessment disgust questionnaires.2 The hypothesis that patients with Huntington’s disease would also show deficits in reactions to disgusting smells and tastes was examined in this study.
METHODS
Eight patients with Huntington’s disease from the Queen Elizabeth Psychiatric Hospital, Birmingham, United Kingdom (all tested positive for the Huntington’s disease mutation and showed early clinical signs of the disease) and eight comparison subjects, who were free of any neurological or psychiatric disorder, participated. All subjects gave informed consent, and local ethical approval was obtained (South Birmingham Ethics Committee).
Subjects were initially screened to determine whether they had olfactory deficits, as impairments in olfactory identification and threshold sensitivity are often observed in Huntington’s disease patients.4–6 Olfactory function was tested using the combined olfactory test of Robson et al.,7 which has been validated as a test of olfactory threshold and identification in clinical populations. Olfactory identification was tested using nine test substances (coffee, baby powder, peanut butter, chocolate, peppermint, Marmite, motor oil, menthol vapor rub and vinegar) which were presented individually in covered plastic containers. Subjects were required to state which of 4 listed odorous substances had actually been presented. Olfactory threshold was determined using serial dilutions of 1-butanol (4%–0.00061%). Two patients were excluded from the olfactory disgust part of the experiment on the basis of this screening. Two comparison subjects (age- and sex-matched) were similarly excluded from this part of the study.
A variety of substances, whose smells had been reliably rated during extensive pretesting with normal subjects as either very pleasant or extremely disgusting, were used. The five disgusting smelling substances were sour milk, ammonium polysulfide, valeric acid (vomit-like), 3-methylindole (cow dung-like) and stale cauliflower water, while the five substances which smelt very pleasant were peach water, coconut bath milk, strawberry juice, orange shower gel and banana essence. Each sample was presented blind, for a maximum of 5 seconds at a distance of 2 cm from the participant’s nose, with an interval of 20 seconds between each presentation. Subjects were required to indicate their reaction to each smell on 10-cm anchored line rating scales which ranged from very pleasant to very disgusting.
Potential deficits in the perception of disgust in the gustatory modality were determined by recording patients’ reactions, using line rating scales, to six food substances that were presented individually. The food substances were bourbon biscuits, salt and vinegar crisps, raspberry yogurt, sherbet, ketchup, and salt. Each substance was re-presented in either appropriate combinations (which in extensive pretesting with normal subjects were reliably labeled as pleasant) or inappropriate combinations (which had previously been rated as having disgusting tastes). The four stimulus combinations that formed the pleasant combinations were bourbon biscuits with sherbet or yogurt, and crisps with salt or ketchup. The four pairings that formed the unpleasant combinations were bourbon biscuits with salt or ketchup and crisps with sherbet or yogurt. Responses to the stimuli were recorded using line rating scales as described above.
RESULTS
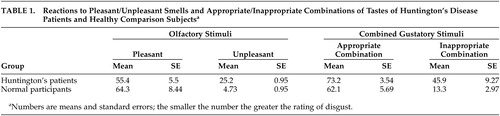
The olfactory disgust data (Table 1) were analyzed using a two-way mixed design analysis of variance (ANOVA), with participant group as the between subjects factor and smell type as the repeated measure. A significant smell/group interaction was observed (F=6.89, df=1, 10, p=0.027). A significant difference in the ratings of the degree of disgust of the two types of smells was found (F=62.56, df=1, 10, p<0.0001), while no significant difference across the two groups of subjects on the olfactory stimuli was found (F=1.50, df=1, 10, p=0.25). These results demonstrate that the Huntington’s disease patients were less disgusted than the comparison group by the unpleasant olfactory stimuli.
The mean ratings of pleasantness for the gustatory substances (biscuits, crisps, yogurt, sherbet, ketchup and salt), when presented individually, were similar for both the patient (mean=58.1, SE=7.3) and comparison (mean=68.8, SE=4.8) groups. A two-way mixed design ANOVA, with the participant groups as the between subjects factor and pleasantness of taste as the repeated measure, showed no significant interaction between food type or between the patient and comparison groups (F=1.33, df=5, 70, p=0.26), and no significant difference across the two participant groups (F=1.86, df=5, 70, p=0.195) was observed. A significant difference was found for the type of food eaten (F=5.93, df=5, 70, p<0.0001). This suggests that there were no significant differences across the two groups in their reaction to the gustatory stimuli, although both the patient and comparison groups found some of the test foods to be more appealing than others.
The mean score of the Huntington’s disease patients on the Pleasantness Rating Scale was markedly higher than that of the comparison group for the inappropriate food combinations, although the two groups made similar responses to the appropriate food combinations (Table 1). A two-way mixed design ANOVA was performed with appropriateness of food combination as the repeated measure and the participant groups as the between subjects factor. A significant difference was found for appropriateness of food combination (F=60.2, df=1, 14, p<0.0001) and for the interaction between appropriateness of food combination and the patient and comparison groups (F=19.8, df=1, 14, p=0.001) but not for the patient and comparison group factor (F=2.52, df=1, 14, p=0.16). This shows that the Huntington’s disease patients were less disgusted than the comparison group by the inappropriate food combinations.
DISCUSSION
The failure of patients with Huntington’s disease to respond normally to disgusting smells and tastes may be due to a simple deficiency in olfactory or gustatory perception. This type of deficiency has been reported in patients with Huntington’s as well as other neurological diseases, including Alzheimer’s disease and Down’s disease.4–6 A deficiency in olfactory or gustatory perception is, however, unlikely since the performance of the Huntington’s disease patients used in this study was similar to that of the comparison group on the validated test of olfactory identification and threshold.7 Furthermore, the responses for both groups were similar for pleasant olfactory stimuli, individually presented gustatory stimuli, and pleasant combined gustatory stimuli.
The results of this study suggest the problems Huntington’s disease patients experience in recognizing facial expressions of disgust may be connected with or analogous to deficits in responses to disgusting smells and tastes. This implies that the experience of disgust across a range of modalities is encoded by a common central mechanism that most likely involves the striatum due to its pivotal role in Huntington’s disease pathology, and the anterior insula, which plays a role in the responses associated with offensive tastes and is activated by facial expressions of disgust.8
 |
1 Nance MA, Sanders G: Characteristics of individuals with Huntington’s disease in long term care. Mov Disord 1996; 11:542–548Crossref, Medline, Google Scholar
2 Sprengelmeyer R, Young AW, Calder AJ, et al: Loss of disgust: perception of faces and emotions in Huntington’s disease. Brain 1996; 119(part 5):1647–1665Google Scholar
3 Gray JM, Young AW, Barker WA, et al: Impaired recognition of disgust in Huntington’s disease gene carriers. Brain 1997; 120(part 11):2029–2038Google Scholar
4 Murphy C: Loss of olfactory function in dementing disease. Physiol Behav 1999; 66:177–182Crossref, Medline, Google Scholar
5 Moberg PJ, Doty RL: Olfactory function in Huntington’s disease patients and at-risk offspring. Int J Neurosci 1997; 89:133–139Crossref, Medline, Google Scholar
6 Bylsma FW, Moberg PJ, Doty RL, et al: Odor identification in Huntington’s disease patients and asymptomatic gene carriers. J Neuropsychiatry Clin Neurosci 1997; 9:598–600Link, Google Scholar
7 Robson AK, Woollons AC, Ryan J, et al: Validation of the combined olfactory test. Clin Otolaryngol 1996; 21:512–518Crossref, Medline, Google Scholar
8 Phillips ML, Young AW, Senior C, et al: A specific neural substrate for perceiving facial expressions of disgust. Nature 1997; 389:495–498Crossref, Medline, Google Scholar



