Mood Disorders and Their Treatment in Patients With Epilepsy
Abstract
Mood disorders in patients with epilepsy are not frequently diagnosed and not treated. Because of the high prevalence of depression and the resulting high suicide rate, precise diagnosis and effective therapy are very important. Frequently, the clinical pictures of depressive syndromes in epileptics do not correspond with those described in operationalized classification systems such as ICD-10 or DSM-IV. The incidence of depressive disorders in epileptics is estimated in the literature to be 30%–70%. Multifactorial pathogenetic models include the type of seizures, the location of the epileptic focus, and neurotransmitter dysfunctions, as well as hereditary and psychosocial influences, and negative psychotropic effects of antiepileptic drugs. Despite an insufficient number of available controlled studies, based on the current data, treatment with the newer serotonergic antidepressants can be recommended for patients with epilepsy.
The incidence of psychiatric diseases in epileptics is significantly higher than in the general population.1–4 Depressive and anxiety disorders are the most common psychiatric diseases in this patient group.1 Diagnosis and treatment of depressive disorders are very important because of the significantly higher suicide rate in epileptics.5 Although the suicide incidence reported in the literature varies significantly, it is four to five times higher than in the general population.6,7 In an overview of the causes of death in epileptics, Robertson reported a nine- to ten-fold higher suicide rate in this group compared with the general population. Risk factors for suicide include a history of self-injuries, a family history of suicide, emotional stress-causing events, psychiatric diseases such as depression or psychosis, and alcoholism.5,8 The available data show the urgent necessity of treating depressive disorders in epileptics. This article provides an overview of mood disorders in epileptics and their treatment.
EPIDEMIOLOGY AND CLASSIFICATION
According to most authors dealing with this subject, depression is the most common psychiatric disease in patients with epilepsy,5,9–11 and a significant cause of admission to a psychiatric clinic.12 Data on the prevalence vary, depending on the patient population. An average incidence of 30%–40% is, however, assumed. In general, depression in epileptics is more frequent and more severe than in patients with other neurological disorders or chronic organic diseases.3,13,14
Obtaining precise data on depression in epileptics is difficult because the groups investigated were very heterogeneous. In particular, data from hospitalized patients and patients in tertiary facilities are not representative of the majority of epileptics. Furthermore, the interpretation of data from the literature is difficult because some studies do not distinguish between depressive symptomatology and depressive disorder.5 However, in a Swedish study with patients with newly diagnosed epilepsy, the incidence of depressive disorders was seven times higher for these epileptics than for individuals in the comparison group. In the group of patients with focal epilepsy, a 17-fold increase of the incidence of depressive disorders was observed.15 Four other controlled studies16–19 showed a significantly increased incidence of depressive disorders in epileptics compared with a comparison group of subjects without epilepsy. Mania and disinhibition have been reported, but frequently have been quoted as being rare.20,21 To date, there are only case reports and retrospective reviews with small numbers of patients regarding mania and bipolar disorder; therefore no prevalence rate for these disorders is given in the literature.
Mood disorders include major depression, mania, bipolar disorder, and dysthymia, according to ICD-1022 and DSM-IV.23 The clinical picture of depressive disorders in epileptics does not, however, always correspond with the criteria listed in operationalized classification systems.11 When diagnosing a depressive disorder in epileptics, the chronological relation to the seizures must be taken into consideration. Therefore, the classification into peri-ictal, ictal, postictal, and interictal depression was established.
Peri-Ictal Depression
This depression is called preictal depression by some authors5 who relate it to the premonitory dysphoria. Since dysphoric symptoms are observed before and after the seizure, the term “peri-ictal depression” is now used in the literature.
Depressive syndromes frequently precede the seizure. They may last hours to days and are characterized by a depressive-anxious mood, or, sometimes, by dysphoria. With the occurrence of the seizure, the symptoms often come to an end.24 They may, however, continue for hours or days after the seizure. It is not yet known whether these premonitory depressive symptoms are a subclinical part of the seizure or if neurobiological processes, which are responsible for the depressive symptoms, induce a decrease of the convulsive threshold.25,26 In a recent study,27 one-third of the patients with focal seizures had peri-ictal depressive symptoms, whereas patients with generalized seizures did not show these symptoms.
Ictal Depression
Depressive symptoms may be a part of the seizure. Although anxiety is the most common affective symptom of a seizure, depressive symptoms of an aura have been observed in 1% of 2000 patients with simple focal seizures.28 Ictal depression seems to be more common in patients with temporal lobe epilepsy; in the literature, an incidence of 10% is reported.24,29 There was no association with the lateralization of the epileptic focus.24,28–30 Typically, ictal depression is characterized by a sudden onset of symptoms, with no relation of the symptoms to outside stimuli. In a few case reports, depressive-psychotic symptoms are described as symptoms of a nonconvulsive status epilepticus.31 In some cases, impulsive suicidality was observed during such an episode.32,33
Postictal Depression
This type of depressive disorder has been repeatedly described in the literature, but prevalence figures are still not available. In a recently published American study conducted in a tertiary epilepsy center, the authors observed postictal depressive symptoms with an average duration of 37 hours in 56 of 100 patients with difficult-to-control simple focal seizures.34 In a further investigation, the authors found, in many patients with postictal depression, unilateral frontal or temporal foci, without evidence of dominance in one hemisphere.1 After a seizure, depressive symptoms may last for up to 2 weeks.35,36 and may also lead to suicide.33,37–39 Blumer proposed the hypothesis that postictal depression is a consequence of the inhibitory mechanisms, which are responsible for the completion of the seizure.40 Psychiatric diseases and a seizure frequency of less than one seizure per month were significantly associated with postictal depressions.41
Interictal Depression
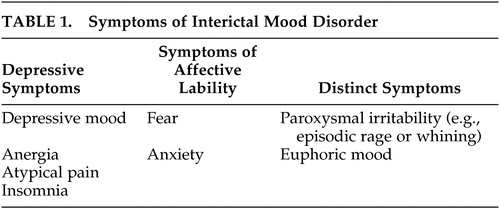
Interictal depression is the most common type of depression in epileptics.5,11,41 The clinical picture may be that of major depression, dysthymia, or bipolar affective disorder. The exact prevalence is not known. In the literature, it is estimated as 20%–70% depending on the patient group investigated.42 Bipolar affective symptoms are very rare, whereas episodic major depression and dysthymia are common. Most authors agree that interictal depression in epileptics cannot, in most cases, be classified according to the operationalized diagnostic systems.5,11,41 A chronic depressive course is observed in most cases. Some symptoms resemble those of dysthymia other symptoms are specific for interictal depression (Table 1). American authors43 suggested calling this affective disorder interictal mood disorder. Besides chronic depressive symptoms, pleomorphic symptoms are observed. These may include atypical pain and phases during which a euphoric or dysphoric affect, anxiety or phobias are predominant. Short symptom-free phases may also occur. In an investigation by Kanner, criteria for an interictal mood disorder were met in 70% of patients with interictal depression.41 Episodes of major depression may also occur in patients with interictal mood disorder. During a chronic depressive course, patients may get accustomed to the depressive state, which may be considered natural and may therefore not be reported to the physician. Since the symptoms are not as pronounced as those of major depression, the physician may not recognize them as depressive symptoms, and treatment is not initiated. In a recently published study,44 major depression was diagnosed in 30% of subjects, and interictal mood disorder in 25%. None of the patients received antidepressive therapy when symptoms were first reported to the physician.
Mania
Mania has been reported in patients with orbitofrontal and basotemporal cortical lesions of the right hemisphere,9 but manic symptoms seem to be rare in patients with epilepsy.20,21 Epileptic patients with psychotic symptoms are more easily described in the literature as schizophrenic rather than as having an affective disorder.9 In the reported cases mania is mostly related to peri-ictal state, improved seizure control and with an epileptic focus in the nondominant hemisphere.20,21 To our knowledge, as mentioned above, no sufficient data about the prevalence of mania and bipolar disorder in epilepsy currently exists. There have been some reports about an incidence of 1.5% for mania in patients with temporal lobe epilepsy.9
PATHOGENESIS
At the present time, there is no uniform explanatory model for the pathogenesis of mood disorders in epileptic patients. A multifactorial genesis seems to be most likely. Epilepsy-specific variables such as seizure frequency, or duration of disease, are not associated with the occurrence of depression.5,11,41
On the other hand, there are indications of an increased incidence of depressive disorders in patients with temporal lobe epilepsy and complex partial seizures.45–47 The incidence of interictal depression was found to be increased, particularly when limbic structures were involved in the seizure occurrence (e.g., in mesial temporal lobe and frontal lobe epilepsy). In these cases, interictal depression was observed in 19%–65%.48–50 In this patient group, the incidence of depressive disorders is significantly higher than in patients with generalized seizures.8,48 Furthermore, in patients with an aura of mostly psychological symptoms, the incidence of depression is higher than in patients with other or no aura symptoms.11,41 In an Italian study, depressive disorders were significantly more common in patients with temporal lobe epilepsy as compared with patients with juvenile myoclonus epilepsy (Janz syndrome) or diabetes.51
The significance of laterality of an epileptic focus as a possible pathogenetic factor for the occurrence of depression is controversial. Some authors believe that a focus in the left hemisphere is a predisposing factor for the occurrence of interictal depression.2,46,52–54 In patients with complex focal seizures and interictal depression, images of single photon emission computed tomography (SPECT) and positron emission tomography showed hypoperfusion and decreased glucose metabolism in the left hemisphere as compared with the right hemisphere.50,55 Nevertheless, the significance of laterality for the occurrence of depression is still not sufficiently investigated. In another study,56 no evidence for a pronounced depressive syndrome was found in patients with left-sided foci. However, patients with left-sided foci and contralateral temporal and bilateral frontal hypoperfusion in the SPECT showed depressive symptoms that were more severe than those in patients without alterations of perfusion.
Abnormal neurotransmission has long been considered a significant factor in the pathogenesis of depressive disorders. In particular, the role of norepinephrine, serotonin, and dopamine deficiency in the etiopathogenesis of major depression is currently being discussed.57 In animal models for the investigation of epilepsy pathogenesis, it was demonstrated that norepinephrine deficiency caused an increased seizure frequency, whereas increased norepinephrine levels had an anticonvulsive effect.58–61 For serotonin, the opposite (high serotonin levels: increased seizure frequency, low serotonin levels: decreased seizure frequency) has been demonstrated in animal models.58,61 Although the role of neurotransmitters in the pathogenesis of epilepsy is not yet clear, there are indications that neurochemical processes are possibly involved in the comorbidity of epilepsy and depression. Jobe et al.62 provided a detailed overview of this interesting topic.
It has long been known that pharmacological antiepileptic treatment may induce negative psychotropic effects.63–65 Phenobarbital and phenytoin may induce depressive symptoms; an increased, sometimes impulsive, suicidality is sometimes observed under treatment with primidone.66 The risk of depression is increased with administration of newer antiepileptics such as tiagabine,67 vigabatrin,68 topiramate,69 and felbamate.70 Anticonvulsants may also have positive psychotropic effects. The mood-stabilizing effects of carbamazepine and the antimanic effects of valproate have been described in detail.57,71 Lamotrigine, gabapentin, and topiramate have been used successfully in the treatment of bipolar affective disorders.69,72,73 There are reports about the induction of mania with zonisamide74 and topiramate.75
Included in the multifactorial genesis are hereditary factors, which play an important role. A British investigation demonstrated that half of all patients with epilepsy and depression had a family history of affective diseases.76
After a surgical procedure for treatment of epilepsy, the risk of developing a depressive disorder is increased for a period of 3 to 6 months. Patients with a history of affective diseases are particularly at risk.5,43 Depression is most commonly associated with persistent seizures but may also develop as alternative symptoms in successfully treated patients.5,77 In patients with an improved quality of life due to a reduced seizure frequency after surgery, depression is significantly less common.78
Psychosocial factors play a significant role in the genesis of depressive symptoms as seizure prodromes. To suffer from a potentially chronic disease that requires pharmacological treatment for many years and is still associated with pronounced social stigmatization, is a considerable burden. In particular, the loss of autonomy (driving restrictions, occupational limitations, problems regarding insurance), the high unemployment rate of epileptics, family difficulties, and problems with partners due to drug-induced decrease in libido may lead to depression and dysphoria.32,79
In summary, mood disorders in epileptics have a multifactorial pathogenesis which includes neurobiological, pharmacological, and psychosocial factors.
TREATMENT
Despite the high prevalence of depressive disorders in epileptics, there are very few controlled studies on antidepressive treatment. There exists only one double-blind, placebo-controlled study on the effectiveness of two antidepressants in the treatment of major depression in patients with epilepsy.80 Preictal and ictal depression do not usually require antidepressant treatment; improvement in seizure frequency should reduce the occurrence of these forms of depression.5 Regarding post-ictal depression, Blumer40,43 suggested from clinical observations that proconvulsive antidepressant medication in lower doses than usual, along with optimal control of the epilepsy, would be the treatment of choice. Usually antidepressant therapy will be necessary in patients suffering from interictal depression or comorbid depressive disorders.5,11
If depression occurs, it should be determined whether the patient takes an antiepileptic drug with a known depression-inducing effect, or if treatment with an antiepileptic drug with mood-stabilizing effects was discontinued. In the first case, replacement by an antiepileptic drug with mood-stabilizing effects such as carbamazepine, valproate, lamotrigine, gabapentin or topiramate can be considered. In the latter case, the discontinued agent should be readministered.5,11,41
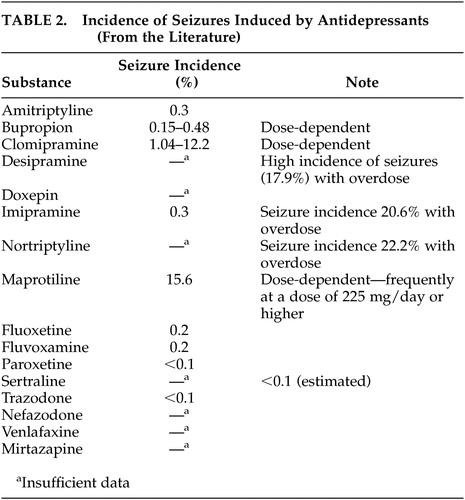
Since antidepressants have proconvulsive properties, physicians frequently have doubts about treatment with these agents. An increased seizure rate has been described in patients without a history of epilepsy with virtually all antidepressants; the seizure incidence is 0.1% to 0.5%.81 More frequent seizures were reported with higher doses of maprotiline, a tetracyclic antidepressant, and with clomipramine with a dose-dependent seizure incidence between 3.00% and 12.2%.9 According to recent reviews5,9,11 treatment with bupropion in patients with epilepsy can not be generally recommended, because of a seizure incidence of 0.15%–0.48%, especially with higher dosages. Monoamine oxidase inhibitors seem to have a lower epileptogenic potency, whereas older tricyclic substances induce seizures more frequently.82 In a systematic investigation, Jabbari et al.83 found a seizure incidence of 2.2% in patients without a history of seizures treated with different antidepressants. The seizures occurred after long-term treatment with high doses of tricyclic antidepressants or after rapid dosage increases (more than 25 mg/day). The seizure rate was higher in patients treated with noradrenergic antidepressants than in those receiving serotonergic agents. Under treatment with serotonin-selective antidepressants, seizures were only described in case reports.84,85 Clinical investigations showed that venlafaxine41,86 and nefazodone5 did not induce seizures, even in epileptics. Some authors87 believe that the epileptogenic effects of antidepressants do not normally present a problem if appropriate antiepileptic agents are administered and that the occurrence of seizures is related to dose and plasma level. Table 2 presents data from the literature on the incidence of seizures under treatment with individual antidepressants. The reported data were obtained from patients without a history of epilepsy. These data remind us again about the importance of controlled studies with epileptics to investigate the epileptogenic potency of the various antidepressant classes and develop pertinent regimens for the treatment of depressive disorders in epileptics. At the present time, recommendations for the pharmacological treatment of epileptics with depressive disorders, derived from the currently available data, include treatment with substances that rarely or never induced seizures in other patient groups. Based on this statement, treatment with selective serotonin reuptake inhibitors (SSRIs) can be recommended as first-line treatment.5,11,41 These substances are only slightly proconvulsive and are effective in the treatment of chronic dysthymic disorders. Citalopram and sertraline can be considered first-line SSRIs because of their minimal pharmacokinetic interactions with antiepileptic drugs.9,11 In agitated patients who need treatment with sedative preparations, mirtazapine can be considered a treatment option. Seizures under treatment with this agent were, according to the literature, only observed in a few patients.5,88 In general, dosages should be increased carefully and in small increments. Regular EEG recordings are recommended.
Besides proconvulsive properties, pharmacokinetic interactions of antidepressants with antiepileptic drugs must be considered. The metabolism of antidepressants may be significantly accelerated by concomitant administration of enzyme inducers such as carbamazepine, phenytoin, phenobarbital, or primidone. An insufficient antidepressant effectiveness may be due to antidepressant levels that are too low. On the other hand, some SSRIs are potent inhibitors of the cytochrome P-450 enzyme system and may significantly increase the serum levels of some antiepileptic drugs and therefore the risk of toxic side effects. Fluoxetine, fluvoxamine and paroxetine are known to have these effects. Sertraline and citalopram have, however, a lower potential for interactions.41,86 Basically, serum levels of antidepressants and antiepileptics should be determined regularly in patients receiving these medications.
Administration of lithium in epileptics has a secondary place because of its known association with encephalopathy especially when used in combination with carbamazepine.9 Lithium has been considered proconvulsant and induces EEG abnormalities5,11 but has been safely used in studies by Shukla et al.89 Considering their proven efficacy, antiepileptic drugs such as valproate, carbamazepine, lamotrigine, gabapentin and topiramate should be regarded as first choice drugs for the treatment of manic episodes or bipolar disorders in patients with epilepsy.5,11,41 Lithium can be a second-line alternative treatment and may prove useful when part of an augmentation strategy.9
Interestingly, the use of electroconvulsive therapy (ECT) is not contraindicated in epileptics90 and should be considered in patients with severe, treatment-resistant depression and sometimes can also be useful with manic episodes. The incidence of seizures after ECT is not increased in epileptics as compared to patients without a history of epilepsy.90 Several studies demonstrated that ECT increases the convulsive threshold by 50%–100% (overview in reference 91). There are also reports of successful treatment of seizures by ECT in epileptics who did not respond to various antiepileptic drugs.91,92 ECT is therefore a treatment option for epileptics with treatment-resistant depression.
In recent years, there have been increasing indications for successful treatment of major depression with repeated transcranial magnetic stimulation (rTMS).93–95 Only rTMS with a frequency rate of 5–25 Hz seems to be effective. Single pulse technique, frequently used in neurological diagnostics, is not effective.96 Data in the literature about the use of TMS in epileptics are not uniform.97 With low-frequency stimulation, there were indications of inhibition of the excitability of the motor cortex.98 On the other hand, epileptic seizures induced by rTMS were described in a few case reports. Dhuna et al.99 reported on the induction of two grand mal seizures in a patient with temporal lobe epilepsy. In this case, the hemisphere contralateral to the epileptic focus was stimulated. Two reports describe generalized seizures induced by rTMS in healthy volunteers.100,101 Based on these insufficient data, use of rTMS for treatment of depression in epileptics cannot be recommended.
According to studies published up to the present, vagus nerve stimulation seems to be another very promising procedure in the treatment of therapy-resistant depression.102,103 There were indications of an antidepressive effectiveness in clinical observations of improved mood and cognition among epileptics in controlled studies on vagus nerve stimulation,104,105 as well as in direct investigations of the effects of vagus nerve stimulation on affective symptoms.106 The neurotransmitters norepinephrine and serotonin play a significant role in the pathogenesis of depression, and increased noradrenergic and serotonergic neurotransmission caused by the stimulation of the locus caeruleus is assumed to be the antidepressive mechanism of action.103 It was demonstrated in animal experiments that vagus nerve stimulation activates the locus caeruleus, the nucleus in the brain with the highest density of noradrenergic neurons.107 In the cerebrospinal fluid of epileptics treated with vagus nerve stimulation, 5-hydroxyindoleacetic acid, a metabolic product of serotonin, was significantly increased.108 The antidepressive effectiveness of vagus nerve stimulation in epileptics may have been due to a reduced seizure frequency. Mood-improving effects were, however, also observed in patients with seizure rates that were not reduced.106 The first controlled study included patients with treatment-resistant major depression and showed an antidepressive effect of vagus nerve stimulation, even in psychiatric patients without epilepsy. Further studies are needed before a general treatment recommendation can be given. Nevertheless, based on the reported literature, vagus nerve stimulation can be considered a potential treatment option in epileptics who suffer from depression and treatment-resistant seizures.
At the present time, data supporting a benefit of psychotherapy are not sufficient. However, supporting psychotherapy should be included in the basic therapy of epileptics with depression. With cognitive-behavioral treatment, dealing with the disease may become easier, and appropriate coping strategies may be developed.
DISCUSSION
Diagnosis and treatment of depressive disorders in epileptics are important because of the high incidence and the increased suicide rate. Diagnoses of depressive disorders that take neurobiological and psychosocial causes into consideration are essential in initiating causal therapy. Because of insufficient data on pharmacological antidepressive therapy in epileptics, controlled studies are urgently needed. For many patients, epilepsy, per se, significantly limits the quality of life. A depression that is unrecognized and untreated worsens the quality of life for these patients. Consequently, therapy for depression is absolutely needed to improve the quality of life of these patients.
 |
 |
1 Kanner AM, Soto A: Ictal recordings in postictal psychosis and postictal depression (abstract). Neurology 1998; 50:A397Google Scholar
2 Mendez MF, Cummings JL, Benson DF: Depression in epilepsy: significance and phenomenology. Arch Neurol 1986; 43:766–770Crossref, Medline, Google Scholar
3 Mendez MF, Doss RC, Taylor JL, et al: Depression in epilepsy: relationship to seizures and anticonvulsant therapy. J Nerv Ment Dis 1993; 181:444–447Crossref, Medline, Google Scholar
4 Robertson MM: Ictal and interictal depression in patients with epilepsy, in Aspects of Epilepsy and Psychiatry. Edited by Trimble MR. New York, John Wiley & Sons, 1986, pp 213–333Google Scholar
5 Lambert MV, Robertson MM: Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999; 40(suppl 10):S21-S47Google Scholar
6 Barraclough B: Suicide and epilepsy, in Epilepsy and Psychiatry. Edited by Reynolds FH, Trimble MR. Edinburgh, Churchill-Livingstone, 1981, pp 72–76Google Scholar
7 Harris EC, Barraclough B: Suicide as an outcome for mental disorders: a meta-analysis. Br J Psychiatry 1997; 170:205–228Crossref, Medline, Google Scholar
8 Robertson MM, Trimble MR, Townsend HR: Phenomenology of depression in epilepsy. Epilepsia 1987; 28:364–372Crossref, Medline, Google Scholar
9 Barry JJ, Lembke A, Huynh N: Affective disorders in epilepsy, in Psychiatric Issues in Epilepsy: A Practical Guide to Diagnosis and Treatment. Edited by Ettinger AB, Kanner AM. Philadelphia, Lippincott Williams & Wilkins, 2001, pp 45–71Google Scholar
10 Hauser WA, Hesdorffer DC: Psychosis, depression, and epilepsy: epidemiologic considerations. Ibid, pp 7–17Google Scholar
11 Kanner AM, Nieto JC: Depressive disorders in epilepsy. Neurology 1999; 53(5 suppl 2):S26-S32Google Scholar
12 Betts T: Depression, anxiety, and epilepsy, in Epilepsy and Psychiatry. Edited by Reynolds FH, Trimble MR. Edinburgh, Churchill-Livingstone, 1981, pp 72–76Google Scholar
13 Kogeorgos J, Fonagy P, Scott DF: Psychiatric symptom patterns of chronic epileptics attending a neurological clinic: a controlled investigation. Br J Psychiatry 1982; 140:236–243Crossref, Medline, Google Scholar
14 Standage KF, Fenton GW: Psychiatric symptom profiles of patients with epilepsy: a controlled investigation. Psychol Med 1975; 5:152–160Crossref, Medline, Google Scholar
15 Forsgren L, Sidenvall R, Blomquist HK, et al: An incident case-referent study of febrile convulsions in children: genetical and social aspects. Neuropediatrics 1990; 21:153–159Crossref, Medline, Google Scholar
16 Bear D, Fedio P: Quantitative analysis of interictal behavior in temporal lobe epilepsy. Arch Neurol 1977; 34:454–467Crossref, Medline, Google Scholar
17 Dodrill CB, Batzel W: Interictal behavioral features of patients with epilepsy. Epilepsia 1986; 27:564–576Crossref, Google Scholar
18 Master DR, Toone BK, Scott DF: Interictal behavior in temporal lobe epilepsy, in Advances in Epileptology: XVth Epilepsy International Symposium. Edited by Porter RJ, Mattson RH, Ward AA Jr, Dam M. New York, Raven Press, 1984, pp 557–565Google Scholar
19 Trimble MR, Perez MR: Quantification of psychopathology in adult patients with epilepsy, in Epilepsy and Behavior. Edited by Kulig BM, Meinard H, Stores G. Lisse, Netherlands, Swets & Zeitlinger, 1980, pp 118–126Google Scholar
20 Barczak P: Hypomania following complex partial seizures. Br J Psychiatry 1988; 152:137–139Crossref, Medline, Google Scholar
21 Wolf P: Manic episodes in epilepsy, in Advances in Epileptology: XIIIth Epilepsy International Symposium. Edited by Akimoto H, Kazamatsuri H, Seino M, Ward AA Jr. New York, Raven Press, 1982, pp 237–240Google Scholar
22 World Health Organization: The ICD 10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva, WHO, 1993Google Scholar
23 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV). Washington, DC, APA, 1994Google Scholar
24 Devinsky O, Bear DM: Varieties of depression in epilepsy. Neuropsychiatry Neuropsychol Behav Neurol 1991; 4:49–61Google Scholar
25 Blanchet P, Frommer GP: Mood change preceding epileptic seizures. J Nerv Ment Dis, 1986; 174:471–476Crossref, Medline, Google Scholar
26 Sailer U, Bohr K, Bauer G: Epileptische Prodromi und episodische Verstimmungen: unspezifische Beschwerdebilder oder status epilepticus non convulsivus. Nervenarzt 1991; 62:240–243Medline, Google Scholar
27 Hughes J, Devinsky O, Feldmann E, et al: Premonitory symptoms in epilepsy. Seizure 1993; 2:201–203Crossref, Medline, Google Scholar
28 Williams D: The structure of emotions reflected in epileptic experiences. Brain 1956; 79:29–67Crossref, Medline, Google Scholar
29 Weil AA: Ictal emotions occurring in temporal lobe dysfunction. Arch Neurol 1959; 1:87–97Crossref, Medline, Google Scholar
30 Devinsky O, Feldmann E, Bromfield E, et al: Structured interview for simple partial seizures: clinical phenomenology and diagnosis. J Epilepsy 1991; 4:107–116Crossref, Google Scholar
31 Lim J, Yagnik P, Schraeder P, et al: Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry 1986; 49:833–836Crossref, Medline, Google Scholar
32 Betts T: Neuropsychiatry, in A Textbook of Epilepsy, 4th ed. Edited by Laidlaw J, Richens A, Chadwick D. Edinburgh, Churchill-Livingstone, 1993, pp 397–458Google Scholar
33 Mendez MF, Doss RC: Ictal and psychiatric aspects of suicide in epileptic patients. Int J Psychiatry Med 1992; 22:231–237Crossref, Medline, Google Scholar
34 Soto A, Kanner AM, Hershkowitz L: Postictal psychiatric symptoms in patients with poorly controlled seizures: a prevalence study. Epilepsia 1997; 38(suppl 8):155Google Scholar
35 Daly D: Ictal affect. Am J Psychiatry 1958; 115:97–108Crossref, Medline, Google Scholar
36 Weil AA: Depressive reactions associated with temporal lobe-uncinate seizure. J Nerv Ment Dis 1955; 121:505–510Crossref, Medline, Google Scholar
37 Anastassopoulos G, Kokkini D: Suicidal attempts in psychomotor epilepsy. Behav Neuropsychiatry 1969; 1:11–16Medline, Google Scholar
38 Hancock J, Bevilacqua A: Temporal lobe dysrhythmia and impulsive or suicidal behavior. South Med J 1971; 64:1189–1193Crossref, Medline, Google Scholar
39 Mendez MF, Lanska DJ, Manon-Espaillat R, et al: Causative factors for suicide attempts by overdose in epileptics. Arch Neurol 1989; 46:1065–1068Crossref, Medline, Google Scholar
40 Blumer D: Postictal depression: significance for the neurobehavioral disorder of epilepsy. J Epilepsy 1992; 5:214–219Crossref, Google Scholar
41 Kanner AM, Palac S: Depression in epilepsy: a common but often unrecognized comorbid malady. Epilepsy Behav 2000; 1:37–51Crossref, Medline, Google Scholar
42 Manchanda R: Psychiatric disorders in epilepsy: clinical aspects. Epilepsy Behav 2002; 3:39–45Crossref, Google Scholar
43 Blumer D, Montouris G, Hermann B: Psychiatric morbidity in seizure patients on a neurodiagnostic monitoring unit. J Neuropsychiatry 1995; 7:445–456Link, Google Scholar
44 Wiegartz P, Seidenberg M, Woodard A, et al: Co-morbid psychiatric disorder in chronic epilepsy: recognition and etiology of depression. Neurology 1999; 53(5 suppl 2):S3-S8Google Scholar
45 Mendez MF: Neuropsychiatric aspects of epilepsy, in Textbook of Geriatric Neuropsychiatry, Edited by Coffey CE, Cummings JL. Washington, DC, American Psychiatric Press, 1994, pp 510–521Google Scholar
46 Robertson MM: Depression in neurological disorders, in Depression and Physical Illness. Edited by Robertson MM, Katona CLE. Chichester, UK, John Wiley & Sons, 1997, pp 305–339Google Scholar
47 Strauss E, Wada J, Moll A: Depression in male and female subjects with complex partial seizures. Arch Neurol 1992; 49:391–392Crossref, Medline, Google Scholar
48 Hermann B, Whitman S: Psychosocial predictors of interictal depression. J Epilepsy 1989; 2:231–237Crossref, Google Scholar
49 Stevens JR: Interictal clinical manifestation of complex partial seizures. Adv Neurol 1975; 11:85–112Medline, Google Scholar
50 Victoroff JI, Benson F, Grafton ST, et al: Depression in complex partial seizures: electroencephalography and cerebral metabolic correlates. Arch Neurol 1994; 51:155–163Crossref, Medline, Google Scholar
51 Perini GI, Tosin C, Carraro C, et al: Interictal mood and personality disorder in temporal lobe epilepsy and juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry 1996; 61:601–605Crossref, Medline, Google Scholar
52 Altshuler L: Depression and epilepsy, in Epilepsy and Behavior. Edited by Devinsky O, Theodore W. New York, Wiley-Liss, 1991, pp 47–66Google Scholar
53 Indaco A, Carrieri PB, Nappi C, et al: Interictal depression in epilepsy. Epilepsy Res 1992; 12:45–50Crossref, Medline, Google Scholar
54 Septien L, Giroud M, Didi-Roy R, et al: Depression and partial epilepsy: relevance of laterality of the epileptic focus. Neurol Res 1993; 15:136–138Crossref, Medline, Google Scholar
55 Bromfield EB, Altshuler L, Leiderman DB, et al: Cerebral metabolism and depression in patients with complex partial seizures. Arch Neurol 1992; 49:617–623; correction, 49:976Crossref, Medline, Google Scholar
56 Schmitz EB, Moriarty J, Costa DC, et al: Psychiatric profiles and patterns of cerebral blood flow in focal epilepsy: interactions between depression, obsessionality, and perfusion related to the laterality of the epilepsy. J Neurol Neurosurg Psychiatry 1997; 62:458–463Crossref, Medline, Google Scholar
57 Stahl SM: Essential Psychopharmacology. New York, Cambridge University Press, 1996Google Scholar
58 Burley ES, Ferrendelli JA: Regulatory effects of neurotransmitters on electroshock and pentylenetetrazol seizures. Fed Proc 1984; 43:2521–2524Medline, Google Scholar
59 Craig CR: Evidence for a role of neurotransmitters in the mechanism of topical convulsant models. Fed Proc 1984; 43:2525–2528Medline, Google Scholar
60 Killam EK, Killam KF Jr: Evidence for neurotransmitter abnormalities related to seizure activity in the epileptic baboon. Fed Proc 1984; 43:2510–2515Medline, Google Scholar
61 McNamara JO: Role of neurotransmitters in seizure mechanisms in the kindling model of epilepsy. Fed Proc 1984; 43:2516–2520Medline, Google Scholar
62 Jobe PC, Dailey JW, Wernicke JF: A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol 1999; 13:317–356Crossref, Medline, Google Scholar
63 Schmitz B, Robertson MM, Trimble MR: Depression and schizophrenia in epilepsy: social and biological risk factors. Epilepsy Res 1999; 35:59–68Crossref, Medline, Google Scholar
64 Devinsky O: Cognitive and behavioral effects of antiepileptic drugs. Epilepsia 1995; 36(suppl 2):S46-S65Google Scholar
65 Besag FM: Behavioural effects of the new anticonvulsants. Drug Saf 2001; 24:513–536Crossref, Medline, Google Scholar
66 Barabas G, Matthews WS: Barbiturate anticonvulsants as a cause of severe depression. Pediatrics 1988; 82:284–285Medline, Google Scholar
67 McConnell HW, Duncan D: Behavioral effects of antiepileptic drugs, in Psychiatric Comorbidity in Epilepsy, Edited by McConnell HW, Snyder PJ. Washington, DC, American Psychiatric Press, 1998, pp 205–244Google Scholar
68 Ring HA, Reynolds EH: Vigabatrin and behaviour disturbance (letter). Lancet 1990; 335:970Crossref, Medline, Google Scholar
69 Chengappa KNR, Gershon S, Levine J: The evolving role of topiramate among other mood stabilizers in the management of bipolar disorder. Bipolar Disord 2001; 3:215–232Crossref, Medline, Google Scholar
70 McConnell HW, Duffy J, Cress K: Behavioral effects of felbamate. J Neuropsychiatry Clin Neurosci 1994; 6:323Google Scholar
71 Modighy K, Robak OH Vestergaard P: Anticonvulsants in Psychiatry. Petersfield, UK, Wrightson Biomedical, 1995Google Scholar
72 Ryback RS, Brodsky L, Munasifi F: Gabapentin in bipolar disorder (letter). J Neuropsychiatry Clin Neurosci 1997; 9:301Link, Google Scholar
73 Semenchuk MR, Labiner DM: Gabapentin and lamotrigine: prescribing guidelines for psychiatry. J Practical Psychiatry and Behavioral Health 1997; 12:334–342Google Scholar
74 Charles LL, Stoesz L, Tollefson G: Zonisamide-induced mania. Psychosomatics 1990; 31:214–217Crossref, Medline, Google Scholar
75 Schlatter FJ, Soutullo CA, Cervera-Enguix S: First break of mania associated with topiramate treatment. J Clin Psychopharmacol 2001; 21:464–466Crossref, Medline, Google Scholar
76 Robertson MM: Carbamazepine and depression. Int Clin Psychopharmacol 1987; 1:23–35Google Scholar
77 Glosser G, Zwil AS, Glosser DS, et al: Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry 2000; 68:53–58Crossref, Medline, Google Scholar
78 Kellet MW, Smith DF, Baker GA, et al: Quality of life after epilepsy surgery. J Neurol Neurosurg Psychiatry 1997; 63:52–58Crossref, Medline, Google Scholar
79 Betts T: Epilepsy, Psychiatry and Learning Difficulty. London, Martin Dunitz, 1998Google Scholar
80 Robertson MM: Depression in patients with epilepsy: an overview and clinical study, in The Psychopharmacology of Epilepsy. Edited by Trimble MR. New York, John Wiley & Sons, 1985, pp 65–82Google Scholar
81 Edwards JG: Antidepressants and seizures: epidemiological and clinical aspects. Ibid, pp 119–139Google Scholar
82 Lejoyeux M, Rouillon F, Ades J, et al: Neural symptoms induced by tricyclic antidepressants: phenomenology and pathophysiology. Acta Psychiatr Scand 1992; 85:249–256Crossref, Medline, Google Scholar
83 Jabbari B, Bryan GE, Marsh EE, et al: Incidence of seizures with tricyclic and tetracyclic antidepressants. Arch Neurol 1985; 43:480–481Crossref, Google Scholar
84 Henry JA, Antao CA: Suicide and fatal antidepressant poisoning. Eur J Med 1992; 1:343–348Medline, Google Scholar
85 Milne RJ, Goa KL: Citalopram: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depressive illness. Drugs 1991; 41:450–477Crossref, Medline, Google Scholar
86 McConnell HW, Duncan D: Treatment of psychiatric comorbidity in epilepsy, in Psychiatric Comorbidity in Epilepsy. Edited by McConnell HW, Snyder PJ. Washington, DC, American Psychiatric Press, 1998, pp 245–361Google Scholar
87 Karnaze DS: Depression in epilepsy may be safely treated with antidepressant drugs. Epilepsia Suppl 1997; 8:182Google Scholar
88 Richou H, Ruimy P, Charbaut H, et al: A multicentre, double-blind, clomipramine-controlled efficacy and safety study of Org 3770. Hum Psychopharmacol 1995; 10:263–271Crossref, Google Scholar
89 Shukla S, Mukherjee S, Decina P: Lithium in the treatment of bipolar disorders associated with epilepsy: an open study. J Clin Psychopharmacol 1988; 8:201–204Crossref, Medline, Google Scholar
90 Blackwood DH, Cull RE, Freeman CP, et al: A study of the incidence of epilepsy following ECT. J Neurol Neurosurg Psychiatry 1980; 43:1098–1102Crossref, Medline, Google Scholar
91 Sackeim HA: The anticonvulsant hypothesis of the mechanisms of action of ECT: current status. J ECT 1999; 15:5–26Medline, Google Scholar
92 Coffey CE, Lucke J, Weiner RD, et al: Seizure threshold in electroconvulsive therapy (ECT), II: the anticonvulsant effect of ECT. Biol Psychiatry 1995; 37:777–788Crossref, Medline, Google Scholar
93 George MS, Wassermann EM, Williams WA, et al: Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6:1853–1856Crossref, Medline, Google Scholar
94 Pascual-Leone A, Rubio B, Pallardo F, et al: Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996; 348:233–237Crossref, Medline, Google Scholar
95 George MS, Wasserman EM, Kimbrell TA, et al: Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997; 154:1752–1756Crossref, Medline, Google Scholar
96 George MS, Belmaker RH (eds): Transcranial Magnetic Stimulation in Neuropsychiatry. Washington, DC, American Psychiatric Press, 1999Google Scholar
97 Wassermann EM: Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 1998; 108:1–16Crossref, Medline, Google Scholar
98 Wassermann EM, Grafman J, Berry C, et al: Use and safety of a new repetitive transcranial magnetic stimulator. Electroencephalogr Clin Neurophysiol 1996; 101:412–417Crossref, Medline, Google Scholar
99 Dhuna AK, Gates JR, Pascual-Leone A: Transcranial magnetic stimulation in patients with epilepsy. Neurology 1991; 41:1067–1071Crossref, Medline, Google Scholar
100 Pascual-Leone A, Houser CM, Reese K, et al: Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalogr Clin Neurophysiol 1993; 89:120–130Crossref, Medline, Google Scholar
101 Wassermann EM, Cohen LG, Flitman SS, et al: Seizures in healthy people with repeated “safe” trains of transcranial magnetic stimuli (letter). Lancet 1996; 347:825–826Crossref, Medline, Google Scholar
102 Rush AJ, George MS, Sackheim HA, et al: Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry 2000; 47:276–286Crossref, Medline, Google Scholar
103 George MS, Sackheim HA, Rush AJ, et al: Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry 2000; 47:287–295Crossref, Medline, Google Scholar
104 Handforth A, DeGiorgio CM, Schacter SC, et al: Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 1998; 51:48–55Crossref, Medline, Google Scholar
105 Vagus Nerve Stimulation Study Group: A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995; 45:224–230Crossref, Medline, Google Scholar
106 Harden CL, Pulver MC, Nikolov B, et al: Effect of vagus nerve stimulation on mood in adult epilepsy patients (abstract). Neurology 1999; 52 (suppl 2):A238-P03122Google Scholar
107 Naritoku DK, Terry WJ, Helfert RH: Regional induction of Fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res 1995; 22:53–62Crossref, Medline, Google Scholar
108 Ben-Menachem E, Hamberger A, Hedner T, et al: Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res 1995; 20:221–227Crossref, Medline, Google Scholar



