Facial Shape and Asymmetry by Three-Dimensional Laser Surface Scanning Covary With Cognition in a Sexually Dimorphic Manner
Abstract
The embryological unity over early fetal life of the anterior brain, neuroepithelium, neural crest, and facial ectoderm is responsible for facial dysmorphogenesis in disorders of early brain development, including schizophrenia. This study examined covariance of facial shape and asymmetry with cognition in a normal sample of 36 men and 51 women using geometric morphometrics. Facial shape and asymmetry covaried with verbal and visual spatial cognitive functions in a sexually dimorphic manner. Events over early fetal life may be an important determinant of sexually dimorphic covariance of anterior facial shape and asymmetry with aspects of cognition that involve the anterior brain.
Throughout early fetal life, the face and anterior brain evolve in exquisite embryological intimacy.1–3 This unity is responsible for facial dysmorphogenesis in disorders of early brain development with their associated cognitive and other deficits in adulthood.4,5 These disorders range from major chromosomal abnormalities such as Down’s syndrome to less readily recognizable conditions such as velocardiofacial syndrome. In a complementary manner, individuals with facial dysmorphogenesis such as cleft lip/palate have been reported recently to show abnormalities of brain structure6 in association with cognitive deficits.7
Recently, the application of anthropometric techniques has indicated that subtle facial dysmorphology is present also in schizophrenia.8–10 Such findings may be an important biological counterpart of epidemiological and other evidence that early developmental disturbances are involved in the origins of this disorder.9,11,12 It has also been reported that facial dysmorphology accompanies abnormalities of brain structure in a putative nonhuman primate model of schizophrenia involving early fetal insult.13 However, realization of the full potential of facial dysmorphology for informing incisively on the developmental biology of schizophrenia is predicated on clarification of a critical issue: in addition to its embryological relationship to brain structure, to what extent can it be shown that variation in facial morphology reflects variation in adult brain function? To address this challenge requires a technique for resolving and quantifying the detailed topography of facial [dys]morphology in a manner that allows it to be related to an index of brain function such as cognition.
As facial development is an intrinsically three-dimensional process, efforts to index facial shape with conventional approaches such as linear anthropometry fail to take into account fundamental geometric aspects of this process. As an interim approach, the authors have recently obtained useful additional information by comparing patients with schizophrenia and healthy subjects using three-dimensional facial configurations reconstructed from linear anthropometric measurements.14 However, three-dimensional digitization technologies now allow facial surfaces to be recorded and landmark coordinates obtained, while geometric morphometrics, which analyze three-dimensional landmark coordinates directly, provides the basis for relating shape to other biological variables both statistically and visually.14,15 Portable, hand-held three-dimensional laser surface scanning has become available, and the authors have recently applied this technique to specify statistically and anatomically the nature of sex differences in facial morphogenesis in human subjects.16 In the present study the authors have examined the relationship between cognitive ability and facial shape in a normal population, as captured using three-dimensional laser surface scanning, to establish a relationship between facial morphogenesis and adult brain function as a basis for conducting subsequent studies in schizophrenia.
METHOD
Subjects
The subjects were drawn from all grades of medical school staff, from ancillary to academic, who on interview gave (i) no personal or family history of serious mental illness or (ii) no personal history of early or later events/traumas that might alter facial configuration; they comprised 36 men (mean age = 32.4 years [SD = 9.3, range = 22–58]) and 51 women (mean age = 30.5 years [SD = 8.0, range = 20–59]). Following Research Ethics Committee approval and complete description of the study to the subjects, their written informed consent was obtained. To ensure ethnic homogeneity, all subjects were of Irish, Scottish, Welsh or English origin, as were their parents and grandparents.
Three-dimensional laser surface scanning.
Facial surfaces were recorded using a portable, handheld Polhemus FastScan laser scanner;16 technical specifications are available at www.fastscan3d.com. A typical surface, consisting of ∼80,000 points, is shown in Figure 1. Twenty four three-dimensional landmarks, used routinely for linear measurements of interlandmark distances in fields such as reconstructive craniofacial surgery,17 were identified on these surfaces (see legend to Figure 1). The majority were located with the aid of conventional vertical and horizontal profiles; in addition, sublabiale and nasion were located using local surface profiles.16 They were located by the same investigator, blind to cognitive assessments; error in location was minimized by landmarking each facial scan four times: twice using the original image and twice with the facial image mirrored, to avoid systematic asymmetric landmark placement, with the results averaged. A previous study using such data demonstrated intraclass correlation coefficients for interlandmark distances of 0.88–0.99.16
Cognitive measures.
Cognitive testing was conducted blind to facial shape analysis. Subjects were assessed using (i) Trail Making Test Parts A and B, a test of spatial attention, visuomotor tracking and processing speed;18 mean time to complete test A was 27.9 sec (SD = 7.6) in men and 27.0 sec (SD = 6.0) in women, while mean time to complete test B was 54.5 sec (SD = 12.6) in men and 51.5 sec (SD = 12.4) in women; (ii) the Controlled Oral Word Association Test, a test of verbal fluency;19 mean production/3 minute was 45.7 words (SD = 18.8) in men and 44.1 words (SD = 14.3) in women.
Geometric morphometrics.
The form of an object is a combination of shape and size, such that a golf ball and a basketball have the same shape but different size, while a water-filled balloon retains a constant size when its shape is manipulated; the original landmark sets thus record facial form. Facial shape and size were analyzed separately by scaling the original landmark sets to unit size as measured by centroid size, which quantifies the dispersion of the configuration of the landmark set as the root mean square distance of the landmarks from their center.20
Covariance of facial size and shape with cognitive measures was analyzed using geometric morphometrics, which allows shape covariance to be expressed numerically and visually; the analytical strategy for this work is based on that developed for the study of sexual dimorphism, as previously described in detail,16 and summaries of this approach for biological practitioners are available.15 The sets of scaled landmark coordinates were aligned with a registration algorithm (Generalized Procrustes analysis) that has appropriate statistical properties. The transformed landmark coordinates were analyzed by principal component analysis to compute the major elements in shape variability within the sample, and principal components with eigenvalue greater than the mean value were selected.
To study the covariance of facial shape with continuous cognitive variables, regression models were produced by multivariate regression of principal components of shape onto cognitive measures; principal components that were insignificant (p > 0.05) were not included in the models. In order to visualize the models, β coefficients of multivariate regressions were used to compute the shape variation that correlated significantly with cognitive measures and this was added to the mean shape using dynamic three-dimensional graphics;15,16,21 R2, F, and p values for the models were calculated by multiple linear regression of cognitive measures onto significant principal components.21
To analyze the covariance of asymmetric face shape with cognitive measures, the 24 landmarks were divided into eight singular landmarks along the midline, and eight pairs of bilateral landmarks.14,22 “Mirrored” sets of landmark coordinates were produced by reversing the signs of the x coordinates of all landmarks and exchanging paired landmarks. The combined dataset, “mirrored” plus original (i.e., unmirrored), was aligned and a signed asymmetry vector for each subject was produced by subtracting the coordinates of the “mirrored” configuration from the original configuration. These signed asymmetry vectors were analyzed as described above for overall shape variance. The geometric meaning of the regression models was visualized in terms of the asymmetric shape variation that correlated significantly with cognitive measures; visualization was as dynamic three-dimensional graphics in terms of how asymmetric shape covaries along a shape axis with cognitive test score, about a mean of pure symmetry for each sample.22
RESULTS
Facial Size
As expected on the basis of smaller cerebral and cranial size in women,23,24 centroid size for these landmark coordinates was smaller (t=−14.7, df=129, p<0.001) in women (mean = 230.5 mm [SD=5.8]) than in men (mean=248.6 mm [SD=9.1]). Among men, increasing centroid size was associated with decreasing time to complete Trail-Making Test Part A [i.e., greater spatial attention, visuomotor tracking and processing speed] (r = −0.38, df = 33, p<0.05 with age as covariate) but not with time to complete Trail Making Test Part B (r=0.21, df=33, n.s.) or verbal fluency (r = 0.09, df = 31, n.s.); conversely, among women increasing centroid size was associated with increasing verbal fluency (r=0.47, df=48, p<0.001 with age as covariate) but not with time to complete Trail Making Test Part A (r=−0.10, df=54, n.s.) or B (r = 0.19, df=54, n.s.).
Overall facial shape.
Independent of size and age, the shape component of facial form covaried with cognitive performance in a sexually dimorphic manner. Among men, the regression model for time to complete Trail Making Test Part A was associated with three principal components of facial shape, which explained 39% of variance in completion time (F=8.63, df=3, p<0.001); on visualization of this statistical model (Figure 2), increasing spatial attention, visuomotor tracking, and processing speed was associated primarily with reduced head width, reduced width of the eyes, and thicker lower lips; decreasing Trails B time was associated weakly with only a single principal component of facial shape. Conversely, verbal fluency was unrelated to any component of facial shape in men. In women, time to complete Trail Making Test Part A was associated weakly with only a single principal component, and time to complete Trail Making Test Part B with no principal component of facial shape. Conversely, the regression model for verbal fluency was associated with three principal components of facial shape in women, which explain 21% of variance in verbal fluency score (F=5.52, df=3, p<0.01); on visualization of this statistical model (Figure 2), increasing verbal fluency was associated primarily with higher and narrower upper face, wider and longer nose with reduced width and height of the mouth, and more prominent chin.
Asymmetric facial shape
Independent of size and age, time to complete Trail Making Test Part A among men was associated with two principal components of facial shape asymmetry, which explained 22% of variance in completion time (F = 5.58, df=2, p<0.01); on visualization of this statistical model (Figure 3), increasing spatial attention, visuomotor tracking, and processing speed was associated with nose set to the right (subject’s right, as per radiological convention) of the midline, and head width increased on the left and decreased on the right. Conversely, verbal fluency was unrelated to any principal component of facial shape asymmetry in men. In women, time to complete Trail Making Test Part A was associated with no principal component of facial shape asymmetry. Conversely, verbal fluency was associated with two principal components of facial shape asymmetry in women, which explain 19% of variance in completion time (F = 6.93, df=2, p<0.01); on visualization of this statistical model (Figure 3), increasing verbal fluency was associated with nose set to the right, and left eye and corner of mouth set high, back and to the left and the converse for the right eye and corner of mouth.
DISCUSSION
To establish that facial dysmorphology can inform incisively on the developmental pathobiology of schizophrenia, it is necessary to show, in addition to its embryological relationship to brain structure, that variation in facial morphology reflects variation in adult brain function. Here we describe the results of such a study using cognitive measures.
Though we note that larger centroid size of the anterior face in young adults is associated with greater cognitive performance, this finding is unlikely to indicate simply that a larger overlying face reflects a larger brain and that a larger brain is associated with greater cognitive performance; rather, it is likely to reflect integration of the multiplicity of processes that determine the size of the cerebral-craniofacial complex and its functional capacity over the lifetime trajectory from early fetal life through to adulthood.25,26 Only the early phase of this trajectory overlaps with the critical period during which cerebral-craniofacial shape and asymmetry are established.
More fundamentally, we found that variation in anterior facial shape and asymmetry, independent of size, is associated with variation in cognitive performance in a sexually dimorphic manner. The magnitude of facial shape variation associated with cognitive performance is unlikely to be observed qualitatively in any individual by another individual. However, using three-dimensional laser surface scanning and geometric morphometrics, double dissociations were consistently found: overall shape and asymmetry of the anterior face covaried with spatial attention, visuomotor tracking, and processing speed in men but not women, and with verbal fluency in women but not men. Like visceral anatomy, the craniofacies and CNS develop along a primarily female trajectory unless there is male gonadal hormone secretion after gestational week 10 to initiate sexual dimorphism;27 hence the sexes differ in cerebral morphology,28,29 facial shape16,25 and cognitive abilities.28,30 Thus, it is striking that these morphological dissociations involve for each sex the cognitive domain in which that sex shows, at least in large samples, preferential overall performance: in general, men perform better than women on visuospatial tasks, while women perform better than men on verbal tasks.28,30
Furthermore, this sexual dimorphism in relationships between facial indices and cognitive measures is strikingly similar to that reported previously for relationships between brain structural indices and IQ in normal individuals.39 In women, verbal IQ was found to correlate with the majority of brain structures examined (e.g., cranium, left and right cerebrum, cerebellum, left and right temporal lobes, left and right hippocampus), while correlations with performance IQ were limited; conversely, in men the majority of correlations were with performance rather than verbal IQ.
The present findings, relating particularly to shape and asymmetry of the frontonasal prominences, eyes, and mouth, involve primarily anterior facial structures which emerge together with anterior brain structures about the midline in a coordinated sequence. This process of anterior facial-cerebral development begins between gestational weeks 6 through 12 and is then established through the next several weeks of fetal life to attain the fundamental arrangement of facial features characteristic of an adult. Thereafter, ultimate attainment of adult form derives more from increasing size than from alteration in shape. Facial shape and size stabilize after adolescence and changes in later life are largely due to soft tissue movements. Since the subjects were adults with a mean age in the 30s and because landmarks are mostly determined by bone and cartilage, the present analyses should be insensitive to postadolescent facial growth. Furthermore, the absence of subjects over age 60 precludes effects related to any involutional or other changes over senescence.
More specifically, over this period of fetal life the anterior brain, neuroepithelium, neural crest, and facial ectoderm constitute a unitary, sexually dimorphic three-dimensional developmental process.1–3,25,27 In contrast, development of the posterior face and brain are related less intimately and appear regulated by different mechanisms.2,4 For each sex, there was a distinct topography of facial shape that varied with increasing performance in its associated cognitive domain: reduced head width, reduced width of the eyes, and thicker lower lips in men; higher and narrower upper face, wider and longer nose with reduced width and height of the mouth, and more prominent chin in women. That these distinct topographies of variation in anterior facial shape between the sexes have correlates in anterior brain function would be in accordance with the above developmental process and complementary to sexual dimorphism in anterior cerebral structure.28,29
It is known that the human face as well as the brain is intrinsically asymmetric, with extent of facial asymmetry being an important determinant of perceived attractiveness by others.32 Though asymmetries are not identical mechanistically across all body regions, they share some regulatory processes,33–35 thus facial and cerebral asymmetries are also established over early fetal life.3,36,37 Critically, for each sex there was a distinct topography of anterior facial asymmetry that varied with increasing performance in its associated cognitive domain: nose set to the right of the midline, and head width increased on the left and decreased on the right in men; nose set to the right, and left eye and corner of mouth set high, back and to the left and the converse for the right eye and corner of mouth in women. These distinct topographies of variation in facial asymmetry between the sexes have correlates in brain function that are complementary to sexual dimorphism in cerebral asymmetry.29,35,38,39
CONCLUSION
The results of this study should be considered in relation to its strengths and limitations. Laser surface scanning and geometric morphometrics allow facial shape to be quantified and analyzed, both statistically and visually, in the three-dimensionality of the underlying developmental biology; in particular, these techniques allow for the independent resolution, analysis, and visualization of asymmetry, and its relationship to other psychological and biological variables. However, future studies should utilize a broader range of cognitive assessments to examine in greater detail the specificity of the functional correlates reported, and could apply these techniques to brain structure as well as function. Nevertheless, that the present findings took the form of double dissociations attests some robustness to the relationships reported.
Biological variation in events over early fetal life may be an important determinant of sexually dimorphic covariance of facial shape and asymmetry with aspects of cognition. After this critical period of development, many additional biological and psychosocial factors are likely to operate across the sexes in sculpting cognitive performance to the level attained in adulthood. However, among these, covariance of cognitive performance with facial shape remains evident long after the early events which established that shape. In preliminary studies using indirect reconstruction of facial configurations from linear anthropometric measurements, we have reported recently that subtle facial dysmorphology noted in schizophrenia8–10 includes sexually dimorphic disruption to facial asymmetries,14 in elaboration of disruption to cerebral asymmetries.40 However, the extent to which such aspects of facial dysmorphogenesis reflect brain dysfunction has been unknown. The present study indicates that variation in facial shape and asymmetry reflects variation in adult brain function. This attests the approach of direct three-dimensional laser surface scanning of facial dysmorphogenesis for informing on the developmental pathobiology of psychotic and other illnesses and their clinical correlates. On this basis we are currently conducting such studies in schizophrenia. Studies in first-episode psychosis may be particularly informative.
ACKNOWLEDGMENTS
This study was presented at the XIIth Biennial Winter Workshop on Schizophrenia, Davos, Switzerland, February 7–13, 2004.
This study was supported by the Stanley Medical Research Institute.
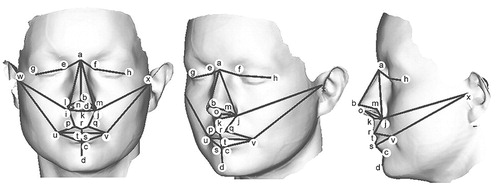
FIGURE 1. Typical Laser Surface Scan Showing the 24 3D Landmarksa
aLines drawn between superimposed landmarks are indicated in coronal, oblique and sagittal planes to aid interpretation of Figures 2 and 3. Landmarks: a=soft tissue nasion; b=pronasale; c=sublabiale; d=pogonion; e/f=inner canthus; g/h= outer canthus; i/j=alar crest; k=subnasale; l/m=alare; n/o=columella breakpoint; p/q=christa philtrum; r=labiale superius; s=labiale inferius; t=stomion; u/v= cheilion; w/x=tragion.
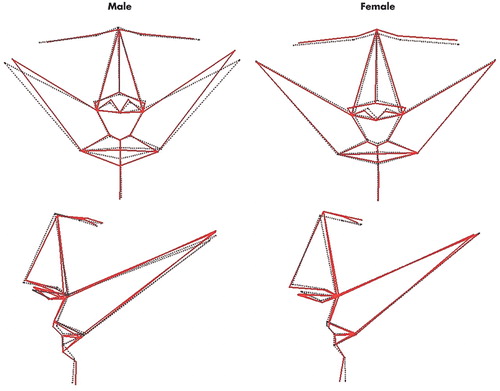
FIGURE 2. Visualization of Multivariate Regressions of Total Facial Shape Onto Cognitive Measures for 36 Men and 51 Womena
aUpper row: coronal images of the regression models; lower row: sagittal images of the regression models. Dotted black lines join the mean coordinates for each sample. For men (left) solid red lines indicate aspects of total shape associated with a reduction in time to complete Trail Making Test Part A (i.e. greater cognitive performance) of 5 seconds, exaggerated by a factor of 10 for clarity. For women (right) solid red lines indicate aspects of total shape associated with an increase in score on the verbal fluency test (i.e. greater cognitive performance) of 10 words, exaggerated by a factor of 10 for clarity.
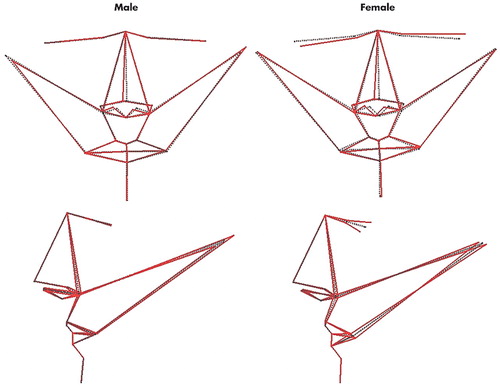
FIGURE 3. Visualization of Multivariate Regressions of Asymmetric Facial Shape Onto Cognitive Measures for 36 Men and 51 Womena
aUpper row: coronal images of the regression models; lower row: sagittal images of the regression models. Dotted black lines indicate pure symmetry for each sample. For men (left) solid red lines indicate aspects of asymmetric shape associated with a reduction in time to complete Trail Making Test Part A (i.e. greater cognitive performance) of 5 seconds, exaggerated by a factor of 10 for clarity. For women (right) solid red lines indicate aspects of asymmetric shape associated with an increase in score on the verbal fluency test (i.e. greater cognitive performance) of 10 words, exaggerated by a factor of 10 for clarity.
1 Diewert VM, Lozanoff S, Choy V: Computer reconstructions of human embryonic craniofacial morphology showing changes in relations between the face and brain during primary palate formation. J Craniofac Genet Dev Biol 1993; 13:193–201Medline, Google Scholar
2 Schneider RA, Hu D, Rubenstein JLR, et al: Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development 2001; 128:2755–2767Medline, Google Scholar
3 Carstens MH: Development of the facial midline. J Craniofac Surg 2002; 13:129–187Crossref, Medline, Google Scholar
4 Kjaer I: Human prenatal craniofacial development related to brain development under normal and pathological conditions. Acta Odontol Scand 1995; 53:135–143Crossref, Medline, Google Scholar
5 Cohen MM: Malformations of the craniofacial region. Am J Med Genet 2002; 115:245–268Crossref, Medline, Google Scholar
6 Nopoulos P, Berg S, Canady J, et al: Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genet Med 2002; 4:1–9Crossref, Medline, Google Scholar
7 Nopoulos P, Berg S, Van Demark D, et al: Cognitive dysfunction in adult males with non-syndromic clefts of the lip and/or palate. Neuropsychologia 2002; 40:2178–2184Crossref, Medline, Google Scholar
8 Lane A, Kinsella A, Murphy P, et al: The anthropometric assessment of dysmorphic features in schizophrenia as an index of its developmental origins. Psychol Med 1997; 27:1155–1164Crossref, Medline, Google Scholar
9 Waddington JL, Lane A, Larkin C, et al: The neurodevelopmental basis of schizophrenia: clinical clues from cerebro-craniofacial dysmorphogenesis, and the roots of a lifetime trajectory of disease. Biol Psychiatry 1999; 46:31–39Crossref, Medline, Google Scholar
10 McGrath JC, El-Saadi O, Grim V, et al: Minor physical anomalies and quantitative measures of the head and face in psychosis. Arch Gen Psychiatry 2002; 59:458–464Crossref, Medline, Google Scholar
11 Weinberger DR: Schizophrenia: from neuropathology to neurodevelopment. Lancet 1995; 346:552–557Crossref, Medline, Google Scholar
12 Arnold SE, Rioux L: Challenges, status, and opportunities for studying developmental neuropathology in adult schizophrenia. Schizophr Bull 2001; 27:395–416Crossref, Medline, Google Scholar
13 Gelowitz DL, Rakic P, Goldman-Rakic PS, et al: Craniofacial dysmorphogenesis in fetally irradiated nonhuman primates: implications for the neurodevelopmental hypothesis of schizophrenia. Biol Psychiatry 2002; 52:716–720Crossref, Medline, Google Scholar
14 Hennessy RJ, Lane A, Kinsella A, et al: 3D morphometrics of craniofacial dysmorphology reveals sex-specific asymmetries in schizophrenia. Schizophr Res 2004; 67:261–268Crossref, Medline, Google Scholar
15 O’Higgins P: The study of morphological variation in the hominid fossil record: biology, landmarks and geometry. J Anat 2000; 197:103–120Crossref, Medline, Google Scholar
16 Hennessy RJ, Kinsella A, Waddington JL: 3D laser surface scanning and geometric morphometric analysis of craniofacial shape as an index of cerebro-craniofacial morphogenesis: initial application to sexual dimorphism. Biol Psychiatry 2002; 51:507–514Crossref, Medline, Google Scholar
17 Farkas LG: Anthropometry of the Head and Face. New York, Raven Press, 1994Google Scholar
18 Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, Neuropsychology Press, 1985Google Scholar
19 Benton A, Hamsher K: Multilingual Aphasia Examination. Iowa City, University of Iowa, 1976Google Scholar
20 Hennessy RJ, Moss JP: Facial growth: separating shape from size. Eur J Orthodont 2001; 23:275–285Crossref, Medline, Google Scholar
21 Penin X, Berge C, Baylac M: Ontogenetic study of the skull in modern humans and the common chimpanzees: neotenic hypothesis reconsidered with a tridimensional procrustes analysis. Am J Phys Anthropology 2002; 118:50–62Crossref, Medline, Google Scholar
22 Klingenberg CP, Barluenga M, Meyer A: Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 2002; 56:1909–1920Crossref, Medline, Google Scholar
23 Swaab DF, Hofman MA: Sexual differentiation of the human brain: a historical perspective. Prog Brain Res 1984; 61:361–374Crossref, Medline, Google Scholar
24 Edland SD, Xu Y, Plevak M, et al: Total intracranial volume: normative values and lack of association in Alzheimer’s disease. Neurol 2002; 59:272–274Crossref, Medline, Google Scholar
25 Enlow DH, Hans MG: Essentials of Facial Growth. Philadelphia, WB Saunders, 1996Google Scholar
26 Drachman DA: Hat size, brain size, intelligence, and dementia: what morphometry can tell us about brain function and disease. Neurol 2002; 59:156–157Crossref, Medline, Google Scholar
27 Breedlove SM: Sexual dimorphism in the vertebrate nervous system. J Neurosci 1992; 12:4133–4142Crossref, Medline, Google Scholar
28 Gur RC, Turetsky BI, Matsui M, et al: Sex differences in brain grey and white matter in healthy young adults: correlations with cognitive performance. J Neurosci 1999; 19:4065–4072Crossref, Medline, Google Scholar
29 Goldstein JM, Seidman LJ, Horton NJ, et al: Normal sexual dimorphism of the adult human brain assessed by in-vivo magnetic resonance imaging. Cerebral Cortex 2001; 11:490–497Crossref, Medline, Google Scholar
30 Caplan PJ, Crawford M, Hyde JS, et al: Gender Differences in Human Cognition. New York, Oxford University Press, 1997Google Scholar
31 Andreasen NC, Flaum M, Swayze V, et al: Intelligence and brain structure in normal individuals. Am J Psychiatry 1993; 150:130–134Crossref, Medline, Google Scholar
32 Little AC, Burt DM, Penton-Voak IS, et al: Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proc R Soc Lond B 2001; 268:39–44Crossref, Medline, Google Scholar
33 Kennedy DN, O’Craven KM, Ticho BS, et al: Structural and functional brain asymmetries in human situs inversus totalis. Neurol 1999; 53:1260–1265Crossref, Medline, Google Scholar
34 Bisgrove B, Essner JE, Yost HJ: Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development 2000; 127:3567–3579Medline, Google Scholar
35 Toga AW, Thompson PM: Mapping brain asymmetry. Nat Rev Neurosci 2003; 4:37–48Crossref, Medline, Google Scholar
36 De Lacoste M-C, Horvath DS, Woodward DJ: Possible sex differences in the developing human fetal brain. J Clin Exp Neuropsychol 1991; 13:831–846Crossref, Medline, Google Scholar
37 McCartney G, Hepper P: Development of lateralized behaviour in the human fetus from 12 to 27 weeks’ gestation. Dev Med Child Neurol 1999; 41:83–86Crossref, Medline, Google Scholar
38 Bear D, Schiff D, Saver J, et al: Quantitative analysis of cerebral asymmetries : fronto-occipital correlation, sexual dimorphism and association with handedness. Arch Neurol 1986; 43:598–603Crossref, Medline, Google Scholar
39 Good CD, Johnsrude I, Ashburner J, et al: Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 564 normal adult human brains. NeuroImage 2001; 14:685–700Crossref, Medline, Google Scholar
40 Sommer I, Aleman A, Ramsey N, et al: Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta analysis. Br J Psychiatry 2001; 178:344–351Crossref, Medline, Google Scholar



