Exaggerated Crying and Tremor With a Cerebellar Cyst
CASE REPORT
“Mr. K,” a 70-year-old right-handed farmer with the past medical history of atrial fibrillation, back pain, and childhood tremor, came for an evaluation of exaggerated and inappropriate crying spells that had been occurring for the past 5 years. He described crying episodes in situations that normally would not have caused him cry. For example, he would weep when he relayed telephone messages from other people to his wife, even when the message had no emotional significance. During these crying spells, he did not feel sad. His weeping spells, however, were much more intense when the context of the message was sad. In the last year, he also developed crying spells in reaction to good news and during happy events: he cried uncontrollably at humorous statements or jokes told by friends or neighbors. The severity of his crying spells worsened throughout the last years. He was aware that his emotional outbursts were socially inappropriate, and, as a result, began to restrict his social activities, such as going out to a restaurant or to church. He did not feel depressed and he did not admit to any of the neurovegetative signs of depression.
Five years ago, at about the same time that he developed pathological outbursts of crying, Mr. K noticed a worsening of his childhood tremor, most notably when he would reach or grab for utensils or similar objects. His childhood tremor was described as “shakiness” and was first noted when he was attending elementary school. However, both Mr. K and his wife reported that the worsening of his tremor 5 years ago coincided with the onset of his frequent spells of involuntary, uncontrollable and exaggerated crying. His problem of tremor had become so severe that he could not feed himself without dropping food or spilling liquids. A regimen of rimidone had been prescribed at a dose of 250 mg twice daily without effect. His other medications included warfarin and rosuvastatin.
On examination, Mr. K was a friendly man who responded to questions with complete and elaborate answers. He described himself as a happy person who enjoys life and appreciates his wife. His eye contact was appropriate and his emotional expression was normal except when he exhibited brief or prolonged episodes of tearing up and weeping during the interview. His cranial nerve exam was remarkable for bilateral nystagmus on lateral gaze. His coordination was abnormal with ataxia. He had abnormal finger-to-nose testing of both upper extremities as well as bilaterally abnormal intention tremor when he reached for objects. His tremor was not present at rest or in the first part of a voluntary movement, but was obvious when he reached for an object or when fine adjustments of the movement were required, such as in touching the object. He did not have a cerebellar gait or truncal ataxia. The remainder of his neurological examination, including gait and reflexes, was normal. Of note, his toes were downgoing.
Brain magnetic resonance imaging (MRI) revealed a cystic lesion within the inferior cerebellum ( Figure 1 ). Of note, this imaging study was performed about 5 years after the initiation of his pathological crying and almost the same number of years after worsening of his cerebellar tremor.
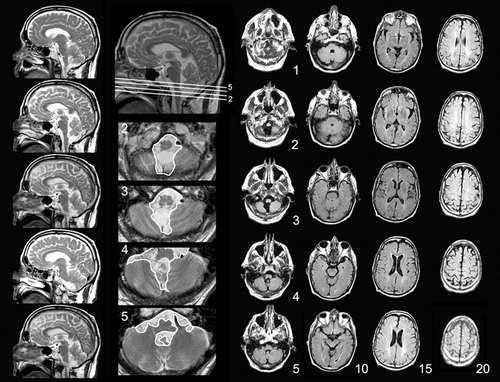
Brain MRIs were obtained 5 years after the onset of pathological crying and worsening of cerebellar signs. T2-weighted sagital, axial T2, and FLAIR images shown here reveal an arachnoid cyst in the midline cerebellum. The corpus callosum and the ventricles and sulci are normal in appearance and there is no lobar atrophy, white matter disease, or brainstem mass effect.
We discontinued the regimen of primidone because he did not benefit from it. Instead, we started him on a regimen of amitriptyline, 10 mg three times daily, because of evidence that it could alleviate pathological affect. 4 In the follow-up visits, he reported that his tremor had become better but he continued to be disabled by it. In addition, his crying spells diminished both in frequency and severity. We had observed multiple episodes of prolonged tearfulness during the initial visit, but we observed only one short episode of crying during the follow-up exam 4 weeks later. Although rating scales for pathological affect were not used at the time of the initial exam, we retrospectively reviewed our office notes and it seemed to us that he would have acquired scores of 19 on the Pathologic Crying and Laughing Scale (PLACS) 5 and 20 on the Center for Neurologic Study–Lability Scale (CNS-LS) 6 when he first was referred to us. Based on his reports, these scores would change to 14 and 15, respectively, after the initiation of amitriptyline.
Mr. K underwent a comprehensive neuropsychological assessment 6 months later. He was taking amitriptyline, 10 mg three times daily, in addition to his regimen of warfarin and rosuvastatin when this test was administered. The test revealed deficits in tasks requiring sustained attention and working memory, and on learning assignments that involved interference and distraction. He was weak in sequencing and switching from one cognitive set or strategy to another. He was inclined toward mild perseveration and “disinhibition” and showed lowered self-control and disregard for details of instruction. Learning and memory were intact and even sophisticated if the patient was not waylaid by distraction. Aberrations with visuospatial reproduction were also added to this picture, possibly due to deficits in executive functions.
DISCUSSION
Our findings in Mr. K are notable for pathological crying, bilateral intention tremor, bilaterally abnormal finger-to-nose test, bilateral nystagmus, and deficits in prefrontal executive functions. The patient’s linguistic functions were normal except for a low average score on the Boston Naming Test, which could be attributable to his IQ of 95 (37%).
As shown in Figure 1 , the only structural abnormality seen in the patient’s MRIs is a cystic lesion impinging on the midline cerebellum and misplacing the inferior vermian structures without causing a mass effect on neighboring brainstem or supratentorial structures. The radiographic features of this cyst suggest that it is an arachnoid cyst. 7 These cysts, often found in the posterior fossa, 8 are known to be congenital lesions with subtle, if any, signs or symptoms during childhood, and may become symptomatically evident only in adult life. 9 , 10 According to Adams’ and Victor’s Textbook of Neurology, 10 arachnoid cysts in adults can simulate intracranial neoplasms in causing localized signs (i.e., localized pseudotumor). There are also numerous reports of elderly people who experienced focal or behavioral signs due to the presence of an arachnoid cyst. The age of these patients ranged from 50 to 70 years old, and in some of these cases the neurological deficits were reversed after draining the cyst. 11 – 19 This suggests that the cysts can indeed become symptomatic very late in life. Because Mr. K had had subtle intention tremor since childhood with clear worsening of his symptoms in the last 5 years, we believed that his cerebellar lesion was a congenital cyst that became more symptomatic in his adult life. Although a surgical approach to drain the cyst could prove our hypothesis, this procedure was not performed because of two reasons: first, the patient had atrial fibrillation and needed to be on warfarin and the possible benefits of the surgery may not have outweighed the risks associated with the procedure and the discontinuation of warfarin. Second, the patient was referred to our clinic because of his pathological emotionality, which he considered as more disabling than his tremor. This problem was treated medically and the patient was satisfied with his improvement on amitriptyline.
The physical cerebellar signs that we detected in our neurological exam included upper limb ataxia manifested with bilaterally abnormal intention tremor and a dysmetric performance in the finger-to-nose test in the absence of cerebellar gait or truncal ataxia. These findings are congruent with our current knowledge of a fractured somatotopic representation within the cerebellum. 20 Though disorders of gait are more prevalent with lesions in the territory of the superior cerebellar artery, 20 , 21 the involvement of the upper extremities point to the dysfunction of the inferior midline cerebellum, possibly in the lobule VIII of the posterior lobe. 20 As shown in Figure 1 , Mr. K had structural abnormalities involving the inferior, but not the superior, vermis.
Our clinical judgment is that the patient’s pathological crying late in life was also another late onset neuropsychiatric symptom referable to the same cerebellar lesion. Two lines of data suggest that this could indeed be the case. First, Mr. K suffered from no other neurological disorder that was known to be the cause of pathological crying, and there presently exist no clinical reports of the pathological crying syndrome in the absence of intercurrent neurological disease (for a review see Parvizi et al. 3 and Schiffer and Pope 22 ). Second, his problem of pathological crying started concurrently with worsening of the tremor, and the severity of his pathological crying also worsened in parallel with the worsening of his ataxia. Although it is possible that the patient’s recollection of the onset and severity of his symptoms might suffer from the limitations of recall and attribution bias, we believe the correlation between the worsening of cerebellar signs and the new development of pathological emotionality is compatible with previously reported cases. For instance, Doorenbos et al. 23 described a patient in whom pathological emotionality was specifically correlated with the activation of cerebellar signs. We have previously reported the case of a 51-year-old man who presented with sudden onset of pathological laughter and crying which correlated temporally with the onset of ataxia after punctuate lesions in the cortico-ponto-cerebellar pathways. 3 Similar to our neuropsychological findings in Mr. K, this patient had deficits in the prefrontal executive functions. An association between pathological emotional regulation and lesions in the cerebellopontine angle and in the midline cerebellum has also been described in the literature. For instance, Pollack et al. 24 described “emotional lability” in children with vermian neoplasms. Schmahmann and Sherman 25 also reported a patient with the resection of a vermian tumor who made sounds similar to crying and developed emotional lability and disinhibition. Children with posterior fossa lesions show an emotional fluctuation ranging from giggling almost uncontrollably to crying irritably and inconsolably. 26
In conclusion, we believe that the compromise of certain cerebellar networks in Mr. K may have caused his impaired modulation of emotional behavior, and consistent with the notion of diaschisis, may have contributed to some of his “prefrontal” deficits. This is consonant with the notion of cerebellar cognitive affective syndrome (CCAS), which includes changes in personality and inappropriate behavior, in addition to impairment of executive functions, spatial cognition, and language deficits. 25 However, we believe that involuntary, uncontrollable, and contextually inappropriate emotional expression with full awareness of the inappropriateness of the problem is different than the personality changes already reported in CCAS, and may be an additional problem attributable to the compromise of the cerebellum and its associated networks.
1. Oppenheim H, Siemerling E: Mitteilungen uber Pseudobulbarparalyse und akute Bulbarparalyse. Berl kli Woch 1886; 46Google Scholar
2. Wilson SAK: Some problems in neurology, II: pathological laughing and crying. J Neurol Psychopathol 1924; IV:299–333Google Scholar
3. Parvizi J, Anderson SW, Martin C, et al: Pathological laughter and crying: a link to the cerebellum. Brain 2001; 124:1708–1719Google Scholar
4. Schiffer RB, Herndon RM, Rudick RA: Treatment of pathologic laughing and weeping with amitriptyline. N Engl J Med 1985; 312:1480–1482Google Scholar
5. Robinson RG, Parikh RM, Lipsey JR, et al: Pathological laughing and crying following stroke: validation of a measurement scale and a double-blind treatment study. Am J Psychiatry 1993; 150:286–293Google Scholar
6. Smith RA, Berg JE, Pope LE, et al: Validation of the CNS emotional lability scale for pseudobulbar affect (pathological laughing and crying) in multiple sclerosis patients. Mult Scler 2004; 10:679–685Google Scholar
7. Nelson MD Jr, Maher K, Gilles FH: A different approach to cysts of the posterior fossa. Pediatr Radiol 2004; 34:720–732Google Scholar
8. Jallo GI, Woo HH, Meshki C, et al: Arachnoid cysts of the cerebellopontine angle: diagnosis and surgery. Neurosurgery 1997; 40:31–37Google Scholar
9. Boes CJ, Capobianco DJ, Cutrer FM, et al: Headache and other craniofacial pain, in Neurology in Clinical Practice, 4th ed. Edited by Bradley WG, Daroff RB, Fenichel GM, et al. Philadelphia, Butterworth Heinemann, 2003, pp 2057–2058Google Scholar
10. Adams RD, Victor M: Intracranial neoplasms: principles of neurology, in Adams’ and Victors’ Manual of Neurology, 5th ed. New York, McGraw-Hill, 1993, p 573Google Scholar
11. Miyamoto T, Ebisudani D, Kitamura K, et al: Surgical management of symptomatic intrasellar arachnoid cysts–two case reports. Neurol Med Chir (Tokyo) 1999; 39:941–945Google Scholar
12. Hayashi T, Nishijima M, Umezawa K, et al: Interhemispheric cyst causing leg monoparesis in the elderly: case report. Neurol Med Chir (Tokyo) 2001; 41:463–465Google Scholar
13. Topsakal C, Kaplan M, Erol F, et al: Unusual arachnoid cyst of the quadrigeminal cistern in an adult presenting with apneic spells and normal pressure hydrocephalus: case report. Neurol Med Chir (Tokyo) 2002; 42:44–50Google Scholar
14. Hishikawa T, Chikama M, Tsuboi M, et al: [Two cases of symptomatic arachnoid cysts in elderly patients—a comparison and analysis with child cases]. No Shinkei Geka 2002; 30:959–965Google Scholar
15. Yamasaki F, Kodama Y, Hotta T, et al: Interhemispheric arachnoid cyst in the elderly: case report and review of the literature. Surg Neurol 2003; 59:68–74Google Scholar
16. Kandenwein JA, Richter HP, Borm W: Surgical therapy of symptomatic arachnoid cysts: an outcome analysis. Acta Neurochir (Wien) 2004; 146:1317–1322Google Scholar
17. Raeder MB, Helland CA, Hugdahl K, et al: Arachnoid cysts cause cognitive deficits that improve after surgery. Neurology 2005; 64:160–162Google Scholar
18. Watanabe M, Kameyama S, Takeda N, et al: Two cases of symptomatic interhemispheric arachnoid cyst in the elderly. Surg Neurol 1994; 42:346–351Google Scholar
19. Hirohata M, Matsuo H, Miyagi J, et al: [Interhemispheric arachnoid cyst; report of three cases]. No Shinkei Geka 1992; 20:701–705Google Scholar
20. Manni E, Petrosini L: A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci 2004; 5:241–249Google Scholar
21. Muley SA, Bushara KO: Isolated gait ataxia due to cerebellar vermis infarct. Arch Neurol 2004; 61:1461Google Scholar
22. Schiffer R, Pope LE: Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci 2005; 17:447–454Google Scholar
23. Doorenbos DI, Haerer AF, Payment M, et al: Stimulus-specific pathologic laughter: a case report with discrete unilateral localization. Neurology 1993; 43:229–230Google Scholar
24. Pollack IF, Polinko P, Albright AL, et al: Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 1995; 37:885–893Google Scholar
25. Schmahmann JD, Sherman JC: The cerebellar cognitive affective syndrome. Brain 1998; 121:561–579Google Scholar
26. Levisohn L, Cronin-Golomb A, Schmahmann JD: Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 2000; 123:1041–1050Google Scholar



