Acute and Chronic Lyme Disease: Controversies for Neuropsychiatry
Borrelia infection follows a similar clinical course and pattern as that of the more familiar spirochete, Treponema pallidum , responsible for syphilis. There are acute and chronic stages with vague and varied symptoms reports at each step. When neurological symptoms are present, the condition is called Lyme neuroborreliosis (LNB). There is also the possibility that much of the later presentations of Lyme disease may be attributable to secondary induction of the inflammatory or autoimmune cascades, similar again to that of syphilis. False positive test results have occurred in patients with syphilis, but a VDRL test will eliminate any confusion.
Early Localized and Early Disseminated Stages
The most common early manifestation (3–30 days after exposure) is a skin lesion at the site of the tick bite. This may be a diffusely reddened slowly expanding rash, or less commonly, the lesion may have a “bull’s eye” appearance (60–80% of cases). This rash is termed erythema migrans. This may be accompanied by fever, lymphadenopathy, arthralgia, and/or myalgia. 5 , 7 , 8 , 9 Later manifestations are due to spread of the spirochetes either under the skin or by way of the bloodstream to organs such as the brain, heart, and joints. 10 In the U.S., the causative agent is Borrelia burgdorferi sensu stricto. According to a recent review article, in these U.S. cases erythema migrans lesions are commonly present, less than 10% of Lyme disease cases develop Lyme neuroborreliosis, painful radiculitis is rare, Lyme arthritis is common, and meningitis is present in the majority. 4 In Europe, where most cases are due to Borrelia garinii or afzeli , erythema migrans lesions are uncommon, more than 35% of Lyme cases develop Lyme neuroborreliosis, painful radiculitis is common, Lyme arthritis is rare, and meningitis is present only in the minority of cases. 4
Lyme neuroborreliosis in the U.S. is characterized by a subacute meningitis (facial nerve palsy may be present) sometimes accompanied by headache, myalgia, malaise, numbness, tingling, and/or mild cognitive impairment. Lyme neuroborreliosis in Europe usually presents with a painful radiculitis (Bannwarth’s syndrome, Garin-Bujadoux syndrome). Peripheral neuropathy may be present with either form of Lyme neuroborreliosis. 4 Certainly, the cranial neuropathies are the most easily identified of the classic symptoms. Although seventh nerve palsy is the most frequent, other cranial nerve involvements have been reported, included cranial nerves 4, 5, 6, and 8. Understanding of this predilection to the cranial nerves has been slow in development, as the only known animal model to develop nervous system involvement (rhesus monkeys) developed peripheral nervous symptoms only. 6 In Europe, encephalomyelitis has been documented more frequently in both the acute and later stages of Lyme neuroborreliosis illness. MRI hyperintensities in cortical, subcortical, and/or cerebellar hemispheres are commonly reported. The CNS is more severely affected by the strains common in Europe. 6 , 11 , 12 , 13 For example, in one case report the patient had involvement of the spinal nerves and roots giving a polio-like presentation with flaccid paraparesis and MRI enhancement of the cord. 14
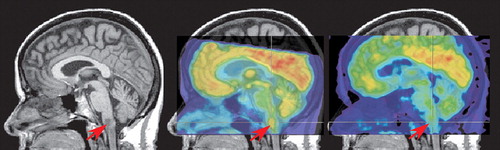
Multiple case reports have documented the diversity of clinical presentations that are possible in Lyme neuroborreliosis, as well as the challenges in differential diagnosis that result. CNS Lyme disease can result in a pleocytosis, high cell counts, and atypical lymphoid cell in the CSF. This can look remarkably similar to primary CNS lymphoma non-Hodgkin’s type on lumbar puncture. 9 , 15 Although less common, parenchymal involvement can and does occur, particularly if the initial infection is left untreated. 1 , 16 In both of these cases, a brainstem lesion was found that was hyperintense on T2 MRI, hypointense on T1 MRI, and hypermetabolic on [ 18 F] fluorodeoxyglucose positron emission tomography (FDG-PET), findings that are consistent with neoplasm ( Figure 2 ). Both cases were successfully treated with antibiotics and follow-up neuroimaging was performed. In one case, partial resolution of the lesion was found on MRI (PET was not performed). 16 In the other case, the lesion was still present on MRI, but was no longer hypermetabolic on PET ( Figure 2 ). 1 The authors of both studies noted that hypermetabolism can be associated with both neoplasm and inflammation. Other cases of brainstem Borrelia infection closely mimicking malignancy have included a child with a mass extending from the pons to C4. 17 Most interestingly, as the authors of this case reflect, this presentation was not only atypical in the mass evident on MRI, but also in the atypical clinical presentation (i.e., nausea, vomiting, weight loss, headache, and nuchal restriction). Ninety percent of childhood cases present with acute meningitis, facial paralysis, headache, and disturbed cognitive function.
Structural neuroimaging in Lyme disease is not diagnostic, as many patients will have normal examinations. 18 , 19 , 20 , 21 , 22 Contrast enhancement of cranial nerves (particularly CN VII) has been reported. Small areas of abnormality in the white matter that are hyperintense on T2W MRI, similar to those seen in multiple sclerosis, may be present. A recent prospective study of patients with neuroborreliosis reported multiple sclerosis-like lesions in 80% (12/15) of neuroborreliosis patients with focal symptoms compared to 0% (0/5) of neuroborreliosis patients with nonfocal symptoms. 22 In stark contrast to what is found in multiple sclerosis, however, several measures of tissue integrity were normal in both areas of brain containing lesions and areas (both gray matter and white matter) that did not. Thus, the magnetization transfer ratio, mean diffusivity, and fractional anisotropy of these regions were not different between the patients with neuroborreliosis and the healthy comparison group. As the authors of the study noted, these findings suggest that neuroborreliosis is associated with little structural damage.
As is true in other conditions, functional imaging may show abnormalities not apparent on structural imaging. A recent retrospective study of patients with neurological symptoms attributed to Lyme disease found that 83% (19/23) had areas of mild-moderate hypometabolism on FDG-PET scans obtained either just prior to or just after commencement of antibiotic therapy. 20 The most common location was the temporal lobe (17/23) in one (5/17) or both (12/17) hemispheres. Hypometabolism in frontal and/or parietal cortex and/or subcortical areas was also present in some cases. The authors noted that they found no correlation between any specific metabolic pattern and age, duration of illness, prior treatment, or current medications. Follow-up PET scans obtained in three patients were congruent with clinical state: one patient had no significant changes in either symptoms or PET scan; two patients had a worsening of symptoms and greater areas of abnormality in their PET scans. Previous SPECT scans were available for nine patients. One was normal, as was the PET scan. Diffuse cortical hypoperfusion was present on the other eight SPECT scans. The PET findings were quite divergent in these cases: three were normal, three had bitemporal hypometabolism, one had hypometabolism only in the basal ganglia, and one had global hypometabolism. Thus, PET (cerebral metabolism) and SPECT (cerebral blood flow) did not provide identical information.
Late Disseminated Stage
The diverse clinical presentations that have been reported for Lyme disease create a diagnostic challenge. Multiple case reports document the later manifestations of untreated Lyme disease. Brainstem arteritis or cerebral vasculitis is not uncommon in Borrelia positive patients. Various presentations have included Avellis syndrome (paralysis of soft palate and vocal cords unilaterally and loss of pain/temperature sensation contralaterally) and transient ischemic attacks intermittently over a 2 year period due to middle cerebral artery vasculitis. 23 , 24 A presentation similar to supranuclear palsy (ataxic gait, inertia, memory disturbances) has also been reported. 25 In this case both structural and functional imaging were obtained prior to the correct diagnosis. Structural imaging (MRI and computed tomography [CT]) performed approximately 6 months after symptom onset showed only mild brain atrophy, and functional imaging (SPECT) was normal, leading to a diagnosis of spinocerebellar degeneration. A repeat SPECT scan 6 months later showed diffuse hypoperfusion in cerebral but not cerebellar cortex, bringing that diagnosis into question. Follow-up studies resulted in the diagnosis of Lyme neuroborreliosis.
More rarely, significant dementia or death has been documented. One such case included a previously healthy patient who presented with the classic rash, musculoskeletal pain, and joint swelling. 26 Despite multiple courses of antibiotics, he developed parkinsonian symptoms, eventual striatonigral degeneration, and death. The autopsy findings included mild atrophy of the basal ganglia, depigmentation of the substantia nigra, atrophy of the cerebellum, overall extensive neuronal loss, astrogliosis in the striatum, and substantia nigra. There were no Lewy bodies identified. Although multisystem atrophy commonly occurs in the absence of Lyme disease, it is worthy of note that the patient was very healthy with no signs of parkinsonism until the Lyme disease. He had repeated positive tests for the spirochetes. The authors of this article reviewed the few autopsy case reports in the literature and found no others with basal ganglia findings, but found commonly microgliosis, inflammation, vasculitis, and infarcts—primarily of the cortex, cerebellum, thalamus, and spinal cord.
A multitude of more specific neuropsychiatric symptoms have been reported in the literature resulting from Lyme disease. Some of these studies do not clearly distinguish between patients with untreated Lyme disease and patients with posttreatment residual symptoms. One summary of this literature notes that depression, decreased concentration, memory, and sleep disturbances occur early. With late stages, there are findings of significant worsening mood disorders, psychotic markers, panic attacks, severe dementias, personality changes, catatonia, and mania. 27
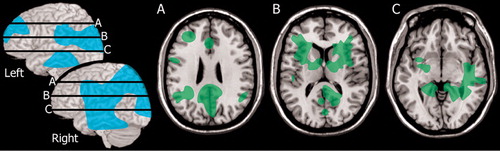
Only a few studies have utilized functional imaging in patients with late stage Lyme disease and neuropsychiatric symptoms. One prospective study specifically selected patients with possible Lyme encephalopathy based upon the presence of neuropsychiatric symptoms (present for 2 months to 12 years). 3 Neuropsychological testing, MRI, and SPECT were obtained from both the patients and a group of normal healthy individuals. More than half the patient group (13/22) were confirmed to have Lyme encephalopathy. Most of these (11/13) had normal MRI. Two had multiple areas of white matter abnormality. All (13/13) had multiple areas of hypoperfusion on SPECT, with both cortical and subcortical involvement. Group analysis indicated that the perfusion deficits clustered in lateral and medial frontal and medial temporal cortices, fronto-temporal white matter, and basal ganglia ( Figure 3 ). A 1-month course of antibiotic treatment was initiated. Most (11/13) of the Lyme encephalopathy group showed clear improvements in neuropsychiatric symptoms beginning 1–3 months after treatment. All had improved cerebral perfusion on repeat SPECT scans acquired at 6 months after treatment. One caveat on this study, however, is that only 2/13 had no previous antibiotic therapy and 5/13 had less than the recommended course. Thus, this group may include both late stage Lyme disease and post-treatment Lyme disease. A case study illustrates the diagnostic challenges. 28 The patient in this case study was initially diagnosed with anxiety and dysthymic disorder. Medication provided partial remission of anxiety symptoms, but did not alter the symptoms of depression, fatigue, or malaise. In addition, the patient’s arthritis symptoms continued to worsen. The patient was referred to internal medicine for evaluation of possible Lyme disease, which was confirmed. SPECT obtained at this time showed hypoperfusion of both temporal lobes. Antibiotic treatment resulted in gradual remission of symptoms. The authors of this case report noted the importance of considering the possibility of an infectious disease, particularly in the absence of any family history of psychiatric illness.
Chronic (Posttreatment) Lyme Disease
There is considerable controversy related to the possible existence of a chronic form of Lyme disease, and to the appropriate treatment approaches for symptoms that may remain following conventional antibiotic therapy. A recent “Point” and “Counterpoint” provides an overview for the interested reader. 29 , 30 Commonly attributable symptoms to chronic Lyme disease have included long-standing fatigue, chronic musculoskeletal pain, and subjective cognitive slowing. Some authors feel strongly that chronic Lyme disease symptoms are coincidental to the initial infection, brought on by stress of illness, or a result of an inflammatory state. 6 , 31 , 32 Others feel that the evidence is sufficient to cite Borrelia as a causative factor. A recent meta-analysis of reported late stage or post-treatment Lyme symptoms examined a total of 504 patients and 530 controls. 33 Identified symptoms for review included fatigue, musculoskeletal pain (swollen joints, muscle or joint pain, muscle aches), and cognitive deficits (memory, concentration, word finding, formulation of ideas/thoughts, judgment, and naming of objects). The authors concluded that fatigue, all three of the musculosketetal pain symptoms, and decreased memory, poor concentration, difficulties in formulating, ideas and difficulty in word finding were significantly more prominent in posttreatment Lyme disease patients than in controls. Challenges in interpreting these data include the inclusion of patients in various stages of infection/treatment, wide range of ages (adult and childhood intermixed data), and different levels of objective testing. The authors concluded, however, that after accounting for these challenges the data remain strong and significant that the above symptoms are more prominent in posttreatment Lyme disease patients. Of note, the above symptom patterns differ from those of chronic fatigue, fibromyalgia, and major depressive disorder. The interested reader is referred to the meta-analysis. 33
Most reports of structural imaging in the posttreatment stage are similar to what has been reported during the early disseminated stage (see section “Early Localized and Early Disseminated Stages”). 34 , 35 One study compared neuroimaging results between patients with focal (4/27) versus nonfocal (23/27) neurological deficits. 34 Patients with focal symptoms (e.g., cerebellar ataxia, sensory-motor deficits) had more widespread lesions in the white matter and a relapsing/remitting course similar to multiple sclerosis. Many (12/23) of the patients with nonfocal symptoms (e.g., fatigue, memory, depression) had normal MRI. A similar number (10/23) had punctate lesions in the white matter. Only one had multiple periventricular and subcortical lesions. Magnetization transfer imaging was obtained in the final eight patients with nonfocal symptoms. There were no differences between patients and normal healthy individuals in magnetization transfer ratio histograms. This is quite similar to the findings in the earlier stages of this disease (see section “Early Localized and Early Disseminated Stages”). The authors of this study note that this finding suggests that there is relatively little structural damage present.
Regional cerebral blood flow (rCBF) has been evaluated in a group of posttreatment Lyme disease (minimum 4 weeks of antibiotic therapy) patients utilizing xenon-133 and an external sensor array. 2 The major differences between the patient group and normal healthy individuals were in white matter perfusion. Patients had changes in the slower clearing compartment which is the white matter. Relatively lower perfusion was present in posterior temporal and parietal white matter ( Figure 3 ). The authors emphasized the potential for pathology in the white matter to disrupt processing within the cortical-subcortical networks that are critical for cognitive and emotional functioning.
Association of Lyme Disease With Other Conditions
Recent theoretical articles have proposed roles for Lyme borreliosis in the etiology of several CNS disorders. The parallel distribution of B. burgdorferi and multiple sclerosis prevalence has led to the suggestion that this pathogen, in either the spirochete or cystic form, may play a causative role in some cases of multiple sclerosis. 36 If this turns out to be correct, curative treatment may be possible. It has also been suggested that multiple sclerosis and Lyme borreliosis are associated with primary effusion lymphoma. 37 The possibility that Lyme disease may be involved in the etiology of autism spectrum disorders has been raised. 38 The similarity of symptoms between chronic Lyme disease and Gulf War syndrome has also been noted. 39 Most interestingly, one author has proposed a link to Alzheimer’s disease, suggesting the involvement of B. burgdorferi in genesis of plaques, neurofibrillary tangles, and trans-synaptic spread of infection. 40 , 41 As noted in all of the above articles, any firm links will certainly require more study.
Treatment
The Quality Standards Subcommittee of the American Academy of Neurology (AAN) recently published practice parameters for treatment of acute CNS Lyme disease. 42 They confirmed that doxycycline orally for 14 days was “comparably safe and effective” to intravenous cephalosporins if the parenchyma is not involved. If there is severe neurological disease, then parenteral antibiotics are recommended. Intravenous ceftriaxone is generally the drug of choice. It crosses the blood-brain barrier, has a long half-life, and has proven efficacy. Doses, treatment schedules, and alternatives for patients unable to take the treatment above are listed in the committee recommendations. 42 In addition to affirming the treatment for acute CNS Lyme disease, the committee further concluded that there is not sufficient evidence to warrant long term (months/years) of antibiotics for chronic Lyme syndrome symptoms. In review of the published studies, the risks of side effects, treatment complications, or adverse events are greater than the potential benefit. Antibiotics do not show sustained resolution of symptoms in previously fully-treated patients. Very recently, a double-blind, placebo-controlled trial of IV ceftriaxone versus placebo was undertaken in 37 patients with significant pain, fatigue, and mild-moderate cognitive deficits noted to be from chronic Lyme disease. 43 In agreement with the recommendations from the AAN, the risks of treatment were greater than the benefit, particularly for the cognitive symptoms. The patients with the most severe pain and fatigue noted improvement with the 10-week treatment. Patients with cognitive symptoms were improved at week 12 but lost all gains by week 24. The authors proposed that the temporary improvements in cognitive may be due to ceftriaxone’s generalized effect in sustained upregulation of the glutamate transporter.
CONCLUSIONS
In conclusion, tick-borne diseases have been identified since the early 20th century. The last 20 years have brought a more complete picture of the most commonly presenting acute tic-borne illnesses. Diagnostic triads and treatment recommendations have been published. More controversial are the answers to those patients who develop nonspecific and lingering neuropsychiatric symptoms postinfection. Questions remain as to whether or not these symptoms are a direct result of continued hidden infection or a result of a different secondary process (e.g., psychological reaction, inflammatory cascade induction, autoimmune). It is, however, prudent for the practicing clinician to be aware of Lyme disease in the differential for patients with new-onset neuropsychiatric symptoms in an endemic area. Awareness is particularly necessary if the patient also has an absence of a known psychiatric history, presence of fatigue and/or chronic musculoskeletal pain, nonspecific inflammatory white matter disease on standard imaging or hypometabolism/hypoperfusion on functional imaging.
1 . Plotkin M, Hautzel H, Krause BJ, et al: Fluorine-18-labeled fluorodeoxyglucose-positron emission tomography studies of acute brainstem Lyme neuroborreliosis [corrected] case report. J Neurosurg 2005; 102:927–929Google Scholar
2 . Fallon BA, Keilp J, Prohovnik I, et al: Regional cerebral blood flow and cognitive deficits in chronic Lyme disease. J Neuropsychiatry Clin Neurosci 2003; 15:326–332Google Scholar
3 . Logigian EL, Johnson KA, Kijewski MF, et al: Reversible cerebral hypoperfusion in Lyme encephalopathy. Neurology 1997; 49:1661–1670Google Scholar
4 . Pachner AR, Steiner I: Lyme neuroborreliosis: infection, immunity, and inflammation. Lancet Neurol 2007; 6:544–552Google Scholar
5 . Stonehouse A, Studdiford JS, Henry CA: An update on the diagnosis and treatment of early Lyme disease: “Focusing on the bull’s eye, you may miss the mark.” J Emerg Med 2007 (epub ahead of print)Google Scholar
6 . Halperin JJ: Central nervous system Lyme disease. Curr Neurol Neurosci Rep 2005; 5:446–452Google Scholar
7 . Oschmann P, Dorndorf W, Hornig C, et al: Stages and syndromes of neuroborreliosis. J Neurol 1998; 245:262–272Google Scholar
8 . Anslow P: Cranial bacterial infection. Eur Radiol 2004; 14:E145–E154Google Scholar
9 . Greer DM, Schaefer PW, Plotkin SR, et al: Case records of the Massachusetts General Hospital: case 11–2007: a 59-year-old man with neck pain, weakness in the arms, and cranial-nerve palsies. N Engl J Med 2007; 356:1561–1570Google Scholar
10 . Gunther G, Haglund M: Tick-borne encephalopathies: epidemiology, diagnosis, treatment and prevention. CNS Drugs 2005; 19:1009–1032Google Scholar
11 . Steinbach JP, Melms A, Skalej M, et al: Delayed resolution of white matter changes folling therapy of B. burgdorferi encephalitis. Neurology 2005; 64:758–759Google Scholar
12 . Van der Stappen A, De Cauwer H, Van den Hauwe L: MR findings in acute cerebellitis. Eur Radiol 2005; 15:1071–1072Google Scholar
13 . Rovers JM, Louwerse ES, de Jager CP: Complete recovery from an unusual cause of coma. Intensive Care Med 2007; 33:542–544Google Scholar
14 . Charles V, Duprez TP, Kabamba B, et al: Poliomyelitis-like syndrome with matching magnetic resonance features in a case of Lyme neuroborreliosis. J Neurol Neurosurg Psychiatry 2007; 78:1160–1161Google Scholar
15 . Bahrain H, Laureno R, Krishnan J, et al: Lyme disease mimicking central nervous system lymphoma. Cancer Invest 2007; 25:336–339Google Scholar
16 . Kalina P, Decker A, Kornel E, et al: Lyme disease of the brainstem. Neuroradiology 2005; 47:903–907Google Scholar
17 . Latsch K, Tappe D, Warmuth-Metz M, et al: Central nervous system borreliosis mimicking a pontine tumour. J Med Microbiol 2006; 55:1597–1599Google Scholar
18 . Halperin JJ: Abnormalities of the nervous system in Lyme disease: response to antimicrobial therapy. Rev Infect Dis 1989; 11:S1499–S1504Google Scholar
19 . Kruger H, Heim E, Schuknecht B, et al: Acute and chronic neuroborreliosis with and without CNS involvement: a clinical, MRI, and HLA study of 27 cases. J Neurol 1991; 238:271–280Google Scholar
20 . Newberg A, Hassan A, Alavi A: Cerebral metabolic changes associated with Lyme disease. Nucl Med Commun 2002; 23:773–777Google Scholar
21 . Latchaw RE, Silva P, Saraf-Lavi E, et al: Intracranial infections in the immunocompetent host, in Imaging of the Nervous System: Diagnostic and Therapeutic Applications. Edited by Latchaw RE, Kucharczyk J, Moseley ME. Philadelphia, Elsevier Mosby, 2005, pp 905–934Google Scholar
22 . Agosta F, Rocca MA, Benedetti B, et al: MR imaging assessment of brain and cervical cord damage in patients with neuroborreliosis. Am J Neuroradiol 2006; 27:892–894Google Scholar
23 . Heinrich A, Khaw AV, Ahrens N, et al: Cerebral vasculitis as the only manifestation of Borrelia burgdorferi infection in a 17-year-old patient with basal ganglia infarction. Eur Neurol 2003; 50:109–112Google Scholar
24 . Habek M, Mubrin Z, Brinar VV: Avellis syndrome due to borreliosis. Eur J Neurol 2007; 14:112–114Google Scholar
25 . Sumiya H, Kobayashi K, Mizukoshi C, et al: Brain perfusion SPECT in Lyme neuroborreliosis. J Nucl Med 1997; 38:1120–1122Google Scholar
26 . Cassarino DS, Quezado MM, Ghatak NR, et al: Lyme-associated parkinsonism: a neuropathologic case study and review of the literature. Arch Pathol Lab Med 2003; 127:1204–1206Google Scholar
27 . Paparone PW: Neuropsychiatric manifestations of Lyme disease. J Am Osteopath Assoc 1998; 98:373–378Google Scholar
28 . Rachman M, Garfield DA: Lyme disease and secondary depression: universal lessons from an uncommon case. Psychosomatics 1998; 39:301–302Google Scholar
29 . Auwaerter PG: Point: antibiotic therapy is not the answer for patients with persisting symptoms attributable to Lyme disease. Clin Infect Dis 2007; 45:143–148Google Scholar
30 . Stricker RB: Counterpoint: long-term antibiotic therapy improves persistent symptoms associated with Lyme disease. Clin Infect Dis 2007; 45:149–157Google Scholar
31 . Schneider RK, Robinson MJ, Levenson JL: Psychiatric presentations of non-HIV infectious disease: neurocysticercosis, Lyme disease, and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection. Psychiatr Clin North Am 2002; 25:1–16Google Scholar
32 . Feder HM, Johnson BJ, O’Connell S, Ad Hoc International Lyme Disease Group: a critical appraisal of “chronic Lyme disease.” N Engl J Med 2007; 357:1422–1430Google Scholar
33 . Cairns V, Godwin J: Post-Lyme borreliosis syndrome: a meta-analysis of reported symptoms. Int J Epidemiol 2005; 34:1340–1345Google Scholar
34 . Morgen K, Martin R, Stone RD, et al: FLAIR and magnetization transfer imaging of patients with post-treatment Lyme disease syndrome. Neurology 2001; 11:1980–1985Google Scholar
35 . Aalto A, Sjowall J, Davidsson PL, et al: Brain magnetic resonance imaging does not contribute to the diagnosis of chronic neuroborreliosis. Acta Radiol 2007; 48:755–762Google Scholar
36 . Fritzsche M: Chronic Lyme borreliosis at the root of multiple sclerosis—is a cure with antibiotics attainable? Med Hypotheses 2005; 64:438–448Google Scholar
37 . Batinac T, Petranovic D, Zamolo G, et al: Lyme borreliosis and multiple sclerosis are associated with primary effusion lymphoma. Med Hypotheses 2007; 69:117–119Google Scholar
38 . Bransfield RC, Wulfman JS, Harvey WT, et al: The association between tick-borne infections, Lyme borreliosis and autism spectrum disorders. Med Hypotheses 2007 (epub ahead of print)Google Scholar
39 . Owen DC: Is Gulf War syndrome actually chronic Lyme disease? Med Hypotheses 2005; 64:717–720Google Scholar
40 . MacDonald AB: Plaques of Alzheimer’s disease orginate from cysts of borrelia burgdorferi, the Lyme disease spirochete. Med Hypotheses 2006; 67:592–600Google Scholar
41 . MacDonald AB: Alzheimer’s neuroborreliosis with trans-synaptic spread of infection and neurofibrillary tangles derived from intraneuronal spirochetes. Med Hypotheses 2007; 68:822–825Google Scholar
42 . Halperin JJ, Shapiro E, Logigian EL, et al: Practice Parameter: treatment of nervous system Lyme disease (an evidence-based review): report of the Quality Standards Subcommitee of the American Academy of Neurology. Neurology 2007; 69:91–102Google Scholar
43 . Fallon BA, Keilp JG, Corbera KM, et al: A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2007 (epub ahead of print)Google Scholar



