An Evidence-Based Review of the Psychopathology of Frontotemporal Dementia: A Report of the ANPA Committee on Research
Frontotemporal dementia results from frontotemporal lobar degeneration. On gross pathology, there is circumscribed and often asymmetric lobar atrophy of the frontal lobes, adjacent anterior temporal regions, or both. On microscopic examination, there are microvacuoles and gliosis with or without inclusion bodies. 8 – 10 The prior term of “Pick’s disease” referred to frontotemporal lobar degeneration with argentophilic, tau-positive intranuclear inclusions (Pick bodies). 11 , 12 Most patients with frontotemporal dementia and related syndromes, however, lack tau-positive inclusions and may have tau-negative, ubiquitin-positive inclusions with TDP-43 protein. 13 , 14 Some patients have additional involvement of other areas of the nervous system resulting in parkinsonism, motor neuron disease, or corticobasal degeneration. 4
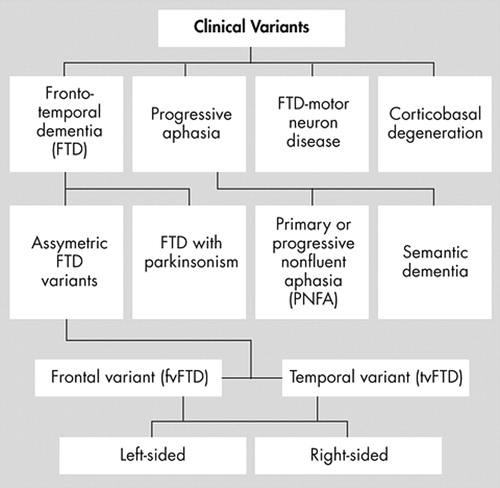
Clinically, frontotemporal lobar degenerations reflect the initial distribution of neuropathology and present as several clinical syndromes depending on their predominant presenting symptoms. 9 , 12 Neuropsychiatric behavioral changes define the clinical syndrome of frontotemporal dementia, which is the most common frontotemporal lobar degeneration. 1 Among 61 patients with pathologically proven frontotemporal lobar degeneration, 90% presented with behavioral disturbances consistent with frontotemporal dementia, including personality and social conduct disorder. 8 This review focuses on the clinical syndrome of frontotemporal dementia. Other frontotemporal lobar degeneration syndromes, such as primary or progressive nonfluent aphasia, semantic dementia, or several movement disorders, are discussed only where they overlap with frontotemporal dementia ( Figure 1 ). 15 , 16
This report specifically reviews the noncognitive neuropsychiatric features of frontotemporal dementia. In contrast to Alzheimer’s disease, during the first few years after onset, behavioral symptoms in frontotemporal dementia are more prominent and disruptive than memory or other cognitive deficits. 2 , 5 , 17 – 22 In the absence of diagnostic biomarkers, the clinical diagnosis depends on recognizing core, or necessary, behavioral features of frontotemporal dementia. 23 Yet, physicians frequently fail to recognize psychopathology as symptoms and misdiagnose these patients as having a primary psychiatric diagnosis. 23 – 26 For this and other reasons, this review of the psychopathology of frontotemporal dementia aims to comprehensively review the literature and characterize the psychopathology of this disorder.
METHOD
We searched MEDLINE using the strategy: “frontotemporal dementia” OR “Pick’s disease” OR “frontotemporal lobar degeneration” OR “frontal dementia” OR “frontal lobe dementia.” We also searched PsychInfo with the same strategy. The searches reviewed clinical papers on frontotemporal lobar degeneration if they included frontotemporal dementia or syndromes that overlapped with this disorder. The searches were complete to the end of April 2007 and were not limited to the English language. We also reviewed pertinent sections of selected books and references in our files.
We characterized our reports by type of behavior divided up into changes in personality, anxiety and compulsions, mood disorders, and psychosis. Using all pertinent studies, we summarized the evidence to date. This review focused on the clinical syndrome of frontotemporal dementia and not on the pathological diagnoses. Furthermore, we focused on personality, behavioral, and psychiatric manifestations rather than on memory, language, perceptual, executive, or other cognitive deficits. This review did not include most single case studies except where detailed studies were limited, as in the psychosis section.
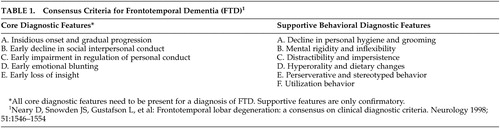
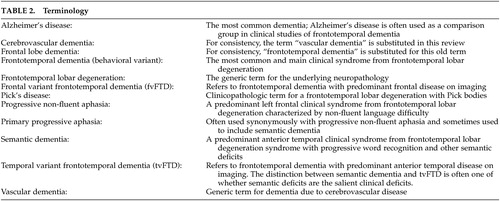
Although there were earlier behavioral descriptions, publications on the neuropsychiatric features of frontotemporal dementia did not appear in significant numbers until the early 1990s. The early case reports and papers culminated in the Clinical Consensus Criteria for FTD, 15 published in 1998, which, in effect, summarized the core and supportive behavioral features of frontotemporal dementia up to that date ( Table 1 ). There was still little systematic study of personality and behavior, however, until 2001 when Mychack et al. 27 outlined the need and potential methodologies for investigating the nature of personality and behavior in frontotemporal dementia. The subsequent years yielded a great many papers on the subject, employing a variety of measures and terminology ( Table 2 ). Much of this literature has struggled with the challenge of comparing frontotemporal dementia, a dementia whose criteria emphasize behavioral symptoms, with Alzheimer’s disease, a dementia whose criteria emphasizes cognitive deficits.
 |
 |
RESULTS
We obtained 504 citations from MEDLINE and PsychInfo. These citations were then reviewed for studies shedding light specifically on the noncognitive neuropsychiatric aspects of frontotemporal dementia and, with exceptions, not involving just a case report or a genetic kindred. We reviewed and included 192 articles as potentially relevant. After review, the studies were grouped into 10 behavioral categories, additional behaviors with limited literature, and treatment. The findings are summarized below.
Neuropsychiatric Symptoms
Apathy-Abulia
Apathy-abulia is the most common initial symptom of frontotemporal dementia and is present in the majority of patients as the disease progresses. 23 , 28 – 33 Among frontotemporal dementia patients, clinicians often mistake as depression the presence of apathy, or the lack of feeling or emotion, and abulia, or the loss of volition and initiative. 28 , 34 Miller et al. 21 initially reported two frontotemporal dementia patients with frontal hypoperfusion on single photon emission tomography (SPECT) who presented with “pseudodepression” attributable to apathy. The following year, Gustafson et al. 35 reviewed several hundred cases of dementias in a longitudinal prospective clinicopathological study. They identified 30 patients with frontotemporal dementia, all of whom developed apathy with amimia and mutism late in their course. In a careful retrospective review of nine patients with frontotemporal dementia, Galante et al. 36 confirmed that these patients became inactive with decreased behavioral initiation and spontaneity, loss of interest, and, ultimately, frank apathy. Eventually, several large series of outpatients, ranging from 50 to 74 patients, confirmed the presence of apathy-abulia in 62% to 89% of patients with frontotemporal dementia. 23 , 37 – 39
Comparative studies with the Neuropsychiatric Inventory show that apathy-abulia is more common, severe, and pervasive in frontotemporal dementia than in Alzheimer’s disease. Investigators developed the Neuropsychiatric Inventory to evaluate patients with Alzheimer’s disease on 12 behavioral features including delusions, hallucinations, agitation, dysphoria, anxiety, apathy, irritability, euphoria, disinhibition, aberrant motor behavior, night-time behavior disturbances, and appetite and eating abnormalities. 40 Levy et al. 41 first used the Neuropsychiatric Inventory to compare 22 patients with frontotemporal dementia with 30 patients with Alzheimer’s disease matched for dementia severity and found higher levels of apathy among those with frontotemporal dementia. Subsequently, Neuropsychiatric Inventory investigations reported apathy as one of the most common symptoms of frontotemporal dementia, present in approximately 95% of patients, 42 , 43 more severe and frequent in frontotemporal dementia compared to Alzheimer’s disease, 44 – 46 and present in both mild and moderate-severe frontotemporal dementia. 47 Furthermore, in a discriminant analysis, Perri et al. 48 combined the Neuropsychiatric Inventory with neuropsychological testing to correctly assign 73.7% of 19 frontotemporal dementia patients and 94.7% of 39 Alzheimer’s disease patients based on better Rey’s Figure A Copy, worse performance on the Initial Letter Verbal Fluency Test (FAS), and greater Neuropsychiatric Inventory apathy subscale scores among the frontotemporal dementia patients.
Additional scales and measures have further defined the frequency and nature of apathy-abulia in frontotemporal dementia. Bozeat et al. 49 reported apathy among 75% of 33 frontotemporal dementia patients using their own neuropsychiatric caregiver questionnaire. Kertesz et al. 50 confirmed that apathy and aspontaneity distinguish frontotemporal dementia from non-frontotemporal dementia groups using the Frontal Behavior Inventory, 51 – 52 an instrument that they felt would be more sensitive to frontotemporal dementia than the Neuropsychiatric Inventory. 50 Others found significantly more apathy and negative symptoms in frontotemporal dementia than in Alzheimer’s disease, dementia with Lewy bodies, or mixed dementias. 53 , 54 Rankin and colleagues 55 used the Interpersonal Adjectives Scales, a self-report and caregiver questionnaire, to measure personality change in 16 patients with frontal variant frontotemporal dementia (fvFTD), with greater frontal volume loss, and 13 with temporal variant frontotemporal dementia (tvFTD), with greater anterior temporal volume loss, and in a comparison group of 16 patients with Alzheimer’s disease. This, and a subsequent study with the Interpersonal Adjectives Scales, documented that fvFTD patients became aloof and introverted, unassured and socially submissive, and less dominant and assertive. 55 , 56
The presence of apathy-abulia in frontotemporal dementia correlates with orbitofrontal disease, extending from Brodmann’s area 10 to the anterior cingulate cortex, especially on the right. Liu et al. 57 studied 51 patients with frontotemporal dementia and 20 normal comparison subjects, as well as 22 patients with Alzheimer’s disease, using the Neuropsychiatric Inventory and MRI voxel-based morphometry. The fvFTD patients scored higher on apathy compared to the tvFTD patients or the Alzheimer’s disease patients. In voxel-based morphometry MRI studies, apathy scores on the Neuropsychiatric Inventory correlated not only with right lateral orbitofrontal atrophy but also with anterior cingulate cortex atrophy and, possibly, caudate head/ventral striatal atrophy. 58 , 59 In SPECT and positron emission tomography (PET) studies, apathy in frontotemporal dementia correlated with right frontal hypoperfusion or frontal hypometabolism ( Figure 2 ). 30 , 60 In addition, apathy in fvFTD may be associated with PET hypometabolism in the orbitofrontal cortex and anterior cingulate cortex. 31 , 61 Investigators further reported orbitofrontal hypometabolism specifically in the right medial Brodmann’s area 10 associated with apathy in a range of frontal-predominant disorders. 62
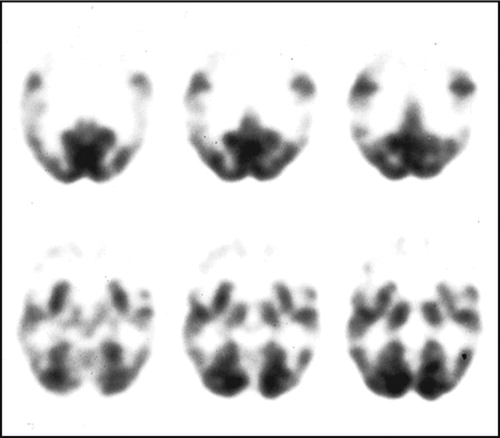
The PET shows prominent frontal lobe hypometabolism.
Disinhibition-Impulsivity
Disinhibition-impulsivity is one of two major behavioral subtypes of frontotemporal dementia, along with apathy-abulia. 30 – 32 , 34 , 63 It is unclear from the literature how often disinhibition and impulsivity are dissociable or occur independently in frontotemporal dementia. Many clinical and clinicopathological reports describe disinhibition and a decreased ability to restrain impulses as prominent and early symptoms of frontotemporal dementia. 2 , 21 , 35 , 42 , 61 , 64 – 66 Among 63 frontotemporal lobar degeneration patients, Mourik et al. 43 described disinhibition in 52% of the patients on the Neuropsychiatric Inventory administered to their caregivers. Chow et al. 37 described disinhibition throughout the course of frontotemporal dementia among 62 patients evaluated retrospectively. Recently, Liscic et al. 67 reported their results on 48 autopsy-confirmed patients with frontotemporal lobar degeneration compared to 27 autopsy-confirmed patients with Alzheimer’s disease. The frontotemporal lobar degeneration patients, most of whom had frontotemporal dementia, had much more disinhibition-impulsivity (23–25 patients; about 50%) compared to the Alzheimer’s disease patients (1–3 patients; 4–11%).
Comparative studies using scales and measures show that disinhibition-impulsivity discriminates frontotemporal dementia from other dementias. Multiple investigations using the Neuropsychiatric Inventory to compare frontotemporal dementia with Alzheimer’s disease show higher disinhibition scores among the frontotemporal dementia patients, particularly with fvFTD, compared to patients with Alzheimer’s disease, dementia with Lewy bodies, and vascular dementia. 41 , 44 , 48 , 57 , 68 , 69 Lopez et al. 70 evaluated DSM-III-R diagnoses in 20 patients with frontotemporal dementia and 40 patients with Alzheimer’s disease and found disinhibition in six frontotemporal dementia patients (30%) compared to only two Alzheimer’s disease patients (5%). Bathgate et al. 71 found greater disinhibition among 30 frontotemporal dementia patients compared to 75 Alzheimer’s disease and 34 “cerebrovascular dementia” patients on a semistructured caregiver questionnaire. Bozeat et al. 49 found greater group levels of disinhibition among 33 frontotemporal dementia patients compared to 27 Alzheimer’s disease patients using their neuropsychiatric questionnaire.
The presence of disinhibition-impulsivity correlates with right-sided frontotemporal disease. Miller et al. 65 described behavioral disinhibition as one of the dominant, and often first, symptoms in five patients with predominant right frontotemporal involvement, and, in a retrospective comparison of 52 patients with frontotemporal dementia and 101 patients with Alzheimer’s disease, Lindau et al. 64 found that disinhibition was greatest in the frontotemporal dementia patients with asymmetric right-sided involvement. In addition, Liu et al. 57 found that disinhibition on the Neuropsychiatric Inventory is associated with decreased right frontal volumes in frontotemporal dementia. In contrast, other investigations have described prominent disinhibited and impulsive behavior or frivolousness and inappropriate humor among frontotemporal dementia patients with bilateral or predominantly right anterior temporal involvement. 60 , 72 – 75 Kertesz 28 suggested that the disinhibited subtype of frontotemporal dementia results specifically from both orbitofrontal and anterior temporal involvement. Consistent with this formulation, subsequent PET and voxel-based morphometry studies among disinhibited frontotemporal dementia patients show an interconnected right hemisphere region of involvement extending from the posterior orbitofrontal cortex, subgenu cingulate, to the anterior temporal pole. 1 , 30 , 31 , 59 , 61 This region may be somewhat more posterior than the region for apathy-abulia, but more research is needed to clarify this difference.
Loss of Insight and Self-Referential Behavior
Early in frontotemporal dementia, patients lose awareness of their disability or the consequences of their behavior and cannot see themselves from others’ point of view (perspective taking). Since being reported as part of the criteria for this disease, 5 , 15 , 38 , 71 , 76 , 77 there have been many clinical reports on loss of insight in frontotemporal dementia. Among 53 frontotemporal dementia patients, Mendez and Perryman 23 reported loss of insight in 58.5% at onset and in 100% 2 years later. Similarly, Passant et al. 25 found loss of insight in all 19 of their neuropathologically verified cases with frontotemporal dementia. Perrine et al. 78 showed loss of insight in frontotemporal dementia associated with loss of perspective taking (i.e., they cannot show how others see them). More recently, Evers et al. 79 evaluated the decreased insight in five of eight patients with frontotemporal dementia and suggested that there was primarily a loss of “emotional insight” from frontotemporal disease, rather than impairment in “cognitive insight.” This loss of emotional insight resembles more of an anosodiaphoria, or indifference towards their illness rather than a true anosognosia, or ignorance of their symptoms. 80
In comparative studies, the loss of insight or self-awareness is worse in frontotemporal dementia than in other dementia syndromes. Eslinger et al. 81 used the Brock Adaptive Function Inventory and Apathy Evaluation Scale to evaluate 27 frontotemporal lobar degeneration patients, 11 Alzheimer’s disease patients, and 11 comparison subjects. Their frontotemporal dementia sample as a whole showed significantly less behavioral self-awareness and self-knowledge than the Alzheimer’s disease and healthy comparison samples. Pijnenburg et al. 82 retrospectively reviewed the case notes of 46 patients diagnosed with frontotemporal lobar degeneration (21 with frontotemporal dementia). In contrast to the other patients, the majority of the frontotemporal dementia patients presented without complaints or awareness of symptoms. Moretti et al. 83 compared frontotemporal dementia versus vascular dementia (subcortical) and found that frontotemporal dementia had a total lack of insight, whereas it was more intact in vascular dementia. Finally, O’Keeffe et al. 84 investigated loss of insight in 14 frontotemporal dementia patients compared to 11 corticobasal degeneration and 10 progressive supranuclear palsy patients. Although all groups had decreased insight, the frontotemporal dementia patient group was most impaired in “emergent awareness” or detection of their errors.
The loss of insight in frontotemporal dementia is part of a spectrum of loss of self-referential behaviors such as self-consciousness, self-perception, stable self-concepts, self-criticism or reflection, awareness of personality changes, or self-conscious responses. 56 , 78 , 80 , 81 Snowden et al. 32 characterized frontotemporal dementia patients as lacking self-conscious emotions, and Bathgate et al. 71 characterized frontotemporal dementia patients as selfish. Using an autobiographical memory task, Piolino et al. 85 found a group deficit of autonoetic consciousness (self-perception) in 15 fvFTD patients. In a unique report, Miller et al. 86 showed that stable self-concepts, such as religion and political affiliation, can be altered with the development of frontotemporal dementia. Rankin et al. 56 used the Interpersonal Adjectives Scales to compare self-awareness of personality and personality changes in 12 fvFTD patients, 10 patients with Alzheimer’s disease, and 11 comparison subjects. In this and other studies, the frontotemporal dementia patients exaggerated positive qualities, minimized negative ones, and were generally unaware of their personality change (or current personality). 56 , 81 Finally, Sturm et al. 87 examined the response of 30 frontotemporal lobar degeneration patients and 23 cognitively normal comparison subjects to a loud, unexpected acoustic startle stimulus (115-dB burst of white noise). Results indicated that frontotemporal lobar degeneration patients and comparison subjects had similar responses to the startle except for significantly fewer facial signs of embarrassment or self-conscious responses among the frontotemporal lobar degeneration patients.
The presence of loss of insight and self-referential behavior correlates with right frontal involvement in frontotemporal dementia. 86 On SPECT scans of frontotemporal dementia patients, loss of insight is associated with hypoperfusion in the right hemisphere, particularly the frontal lobe. 60 , 80 Furthermore, loss of insight was significantly more common with right (54.6%) versus left (13.9%) temporal atrophy; 88 however, involvement of the left temporal lobe may result in anosognosia for their social disability. Sturm et al.’s 87 findings are consistent with neural loss in the medial prefrontal cortex, which may play an important role in the production of self-conscious emotions as self-awareness activates the medial prefrontal cortex during active recollection of one’s past and during passive self-reflection. 87 Finally, a recent multi-center study of lack of awareness in frontotemporal dementia compared to Alzheimer’s disease suggested that greater loss of awareness in frontotemporal dementia corresponds to the degree of frontally-mediated atrophy. 89
Decreased Emotion and Empathy
In general, frontotemporal dementia patients lack emotional warmth and appear emotionally shallow and indifferent. 32 , 65 , 90 , 91 , 92 In a large series, Le Ber et al. 61 document changes in affect and emotional development among patients with frontotemporal dementia, and Snowden et al. 32 found that emotional unresponsiveness, including reduced response to pain, was pervasive among frontotemporal dementia patients. Using the Interpersonal Reactivity Index, a measure of cognitive and emotional empathy, Lough et al. 93 reported decreased empathic concern and decreased perspective taking in frontotemporal dementia, and Eslinger et al. 94 reported decreased self-awareness of empathy. In experiments, the emotional deficit in frontotemporal dementia spares emotional reactivity and the recognition of happiness, but impairs the recognition of negative emotions such as sadness and fear and the experience of self-conscious emotions such as embarrassment and shame. 78 , 90
In comparative studies, emotional blunting and loss of empathy are worse in frontotemporal dementia than in Alzheimer’s disease and vascular dementia. Among 30 patients with frontotemporal dementia and 30 patients with Alzheimer’s disease, Miller et al. 46 reported more patients with emotional unconcern in the frontotemporal dementia group (N=24, 80.0%) compared to the Alzheimer’s disease group (N=6, 20.0%). 46 Barber et al. 95 found less emotional reaction to handicaps on a retrospective informant questionnaire in 18 subjects with pathologically proven frontotemporal dementia than in 20 subjects with Alzheimer’s disease. Likewise, among 30 frontotemporal dementia patients, 75 Alzheimer’s disease patients, and 34 vascular dementia patients, Bathgate et al. 71 reported greater loss of emotions among the frontotemporal dementia patients. Two studies that used the Scale for Emotional Blunting recorded higher emotional blunting scores in frontotemporal dementia than in Alzheimer’s disease. 53 , 75 Two other studies using the Interpersonal Reactivity Index reported significantly more impaired cognitive (perspective taking) and emotional (emotional contagion) empathy in frontotemporal dementia compared to other dementia groups. 96 , 97
In frontotemporal dementia, decreased empathy is associated with right anterior temporal disease. Case studies or series of tvFTD found loss of empathy, fixed facial expressions unresponsive to situations, and emotional distancing and blunting, particularly with right-sided disease, 72 , 73 , 98 , 99 and the speech of right hemispheric frontotemporal dementia patients was less relevant to emotionally loaded pictures than that of left hemisphere patients. 100 In studies using the Interpersonal Adjectives Scales or the Interpersonal Reactivity Index, Rankin et al. 55 showed that tvFTD patients were particularly prone to interpersonal coldness compared to fvFTD patients and had decreased emotional contagion or emotional response to another’s distress. 97 On voxel-based morphometry MRI, empathy correlated with a right medial frontotemporal network, particularly emotional empathy with the right ventromedial and anterior temporal areas. 96 , 97 Moreover, among artists with predominant right temporal frontotemporal dementia, Mendez and Perryman 101 report decreases in empathy reflected in alterations in their caricatures of others, and, using distorted and morphed face tasks, Mendez and Lim 102 showed that frontotemporal dementia patients with predominant right frontotemporal involvement tended to have decreased empathic awareness.
Violation of Social and Moral Norms
Frontotemporal dementia patients have disturbed social and moral behavior. Social changes that differ from the patient’s premorbid behavior most commonly include social inadequacy or awkwardness, tactlessness, disagreeableness, decreased propriety and manners, unacceptable physical contact, or improper verbal or physical acts. 15 , 23 , 36 , 64 , 103 In a retrospective study of 19 neuropathologically verified patients with frontotemporal dementia, Passant et al. 25 found impaired social interactions in all patients, including offensive language in ten, physical aggression in eight, and many traffic violations. Mychack et al. 104 found that 11 of 12 right-sided and two of 19 left-sided frontotemporal dementia patients had socially undesirable behavior, such as criminality or socially deviant acts, as an early presenting symptom of frontotemporal dementia. Frontotemporal dementia patients may engage in minor theft or shoplifting, 76 , 105 driving violations, 106 inappropriate sexual behavior, 65 , 107 , 108 and acts of violence. 76 Miller et al. 109 found antisocial behavior in nearly 50% of patients with frontotemporal dementia, including stealing, hit and run accidents, physical assault, indecent exposure, sexual comments or advances, and public urination. Edwards-Lee et al. 73 described the stealing of small objects in six out of ten tvFTD patients.
Comparative studies indicate that social and moral behavioral changes can differentiate frontotemporal dementia from other dementias. Bozeat et al. 49 found that loss of social awareness helps differentiate frontotemporal dementia from Alzheimer’s disease, and Shinagawa et al. 33 found that changes in social behavior were more common initial symptoms in patients with frontotemporal dementia than in patients with Alzheimer’s disease or semantic dementia. A comparison of 21 frontotemporal dementia patients with 11 vascular dementia patients with a dominating frontal lobe syndrome found significantly less social awareness among the frontotemporal dementia patients. 110 Sociopathic acts are also more prominent in frontotemporal dementia compared to Alzheimer’s disease or other dementia illnesses. 46 , 105 , 108 Mendez et al. 111 reported that 16 frontotemporal dementia patients (57%) had sociopathic behavior, compared to only two Alzheimer’s disease patients (7%), and Diehl et al. 105 reported misdemeanor violations in 15 frontotemporal dementia patients (50%), compared to only one Alzheimer’s disease patient (3%). Finally, Mendez et al. 112 administered an inventory of moral knowledge and moral dilemmas to 26 patients with fvFTD, 26 patients with Alzheimer’s disease, and 26 normal control subjects. 113 Although all groups had knowledge of moral behavior, only the frontotemporal dementia patients were impaired in their ability to make immediate, emotionally based moral judgments.
In frontotemporal dementia, disturbed social and moral behavior correlates with right ventromedial-orbitofrontal-amygdalar disease. Miller et al. 114 suggested that the right hemisphere primarily controls social conduct in frontotemporal dementia, and various investigations have shown greater right-sided changes with social behavioral changes in frontotemporal lobar degeneration. 46 , 54 , 71 , 81 , 88 , 98 , 99 , 102 , 111 , 115 , 116 Sarazin et al. 62 studied 32 patients with frontal lobe pathologies with PET and found that hypometabolism, predominantly in the right orbitofrontal region, corresponded to an indifference to rules. Decreased agreeableness on the NEO Five-Factor Inventory in frontotemporal dementia also correlates with right orbitofrontal changes. 103 More recently, Nakano et al. 117 correlated antisocial behavior in frontotemporal dementia with decreased blood flow in the orbitofrontal cortex. In sum, frontotemporal dementia affects a socio-moral network that includes the right ventromedial region, which, as previously seen, emotionally tags social and moral situations (reexperiencing previously learned emotional responses in novel situations), the orbitofrontal cortex, which responds to social cues and mitigates impulsive reactions, and the amygdalae, which are necessary for threat detection and moral learning. 78 , 93 , 113
Changes in Dietary or Eating Behavior
Early changes in dietary and eating behavior are a common manifestation of frontotemporal dementia and include a spectrum from patients altering their dietary preferences to placing nonfood items in their mouths, consistent with the Klüver-Bucy syndrome. 35 , 118 Frontotemporal dementia patients develop gluttony, sweets and carbohydrate craving, increased weight, obsessions with particular foods, and occasional alcoholism.
Eating behavior changes occur in almost 80% of frontotemporal dementia patients. 47 Miller et al. 21 suggested that hyperorality was one of the most discriminating aspects of frontotemporal dementia, and, in an early clinicopathological study, Mendez et al. 2 described hyperoral behavior as one of the distinguishing features of frontotemporal dementia. Among 19 neuropathologically verified frontotemporal dementia cases, Passant et al. 25 found that 14 patients had alterations in dietary or oral behavior including two who started to drink alcohol. Other reports on frontotemporal dementia described hyperorality, 39 gluttony and indiscriminate eating, 32 , 71 , 119 sweet food preferences, 71 , 119 and changes in eating behaviors or eating disorders. 45 , 49 Snowden et al. 32 found that gluttony and early increased appetite, indiscriminate eating, and sweet cravings were the most characteristic dietary or oral symptoms of frontotemporal dementia, 120 whereas patients with semantic dementia were more likely to exhibit food fads or early changes in food preferences. 88 , 120 Finally, the Klüver-Bucy Syndrome, with generalized and potentially fatal hyperorality, is distinct from the other dietary and oral behaviors and only occurs in a minority of advanced frontotemporal dementia patients with bilateral temporal involvement. 32 , 118 , 121
Comparative studies show that frontotemporal dementia patients have more changes in dietary or eating behavior than patients with Alzheimer’s disease or vascular dementia. Miller et al. 122 queried the primary caregivers of 14 patients with frontotemporal dementia and 14 patients with Alzheimer’s disease on the occurrence of weight gain and sweet and carbohydrate craving. Weight gain in frontotemporal dementia patients amounted to 64% and carbohydrate craving was 79%, compared to 7% and 0%, respectively, for Alzheimer’s disease. Ikeda et al. 120 investigated the frequency of changes in eating behaviors in fvFTD (N=23), semantic dementia (N=25), and Alzheimer’s disease (N=43) using a caregiver questionnaire of swallowing problems, appetite change, food preference, eating habits, and other oral behaviors and found that the frequencies of symptoms in all domains except swallowing problems were higher in fvFTD than in Alzheimer’s disease. Srikanth et al. 69 compared 23 frontotemporal dementia patients, 44 Alzheimer’s disease patients, and 31 vascular dementia patients on the Neuropsychiatric Inventory and found that mean abnormal scores in the domain of appetite/eating behavior helped differentiate frontotemporal dementia from Alzheimer’s disease and vascular dementia. Recently, in Liscic et al.’s 67 clinicopathologic series, the 48 frontotemporal lobar degeneration patients had more hyperorality (N=6, 12.5%) than the 27 Alzheimer’s disease patients (0%).
In frontotemporal dementia, dietary or eating behavioral disturbances correlate with disease affecting lateral orbitofrontal cortex, especially on the right, and adjacent structures, especially the insula. In Neuropsychiatric Inventory studies of frontotemporal dementia, Liu et al. 57 showed that eating disorders correlated with frontal lobe involvement, and Short et al. 123 showed a right rather than a left frontal association with hyperphagia among 59 frontotemporal lobar degeneration patients. Rosen et al. 59 found that eating disorders were more specifically associated with changes in the lateral orbitofrontal cortex, ventral anterior cingulate cortex, and adjacent subfrontal gyrus, caudate head/ventral striatum, and insula. More recently, Whitwell et al. 124 differentiated carbohydrate cravings and hyperphagia in a voxel-based morphometry study of 16 frontotemporal lobar degeneration patients compared to nine normal control subjects. The development of a pathological sweet tooth was associated with gray matter loss in posterior lateral orbitofrontal cortices (Brodmann’s area 12/47) and right anterior insula, whereas the development of hyperphagia was associated with gray matter loss in anterolateral orbitofrontal cortices (Brodmann’s area 11). Seeley et al. 72 suggested that altered food preferences result from orbitofrontal derailment of satiety centers or from processing of insular disgust signals.
Repetitive Behaviors
Investigators have documented a range of repetitive behaviors in frontotemporal dementia. 15 , 35 , 41 , 65 , 122 , 125 – 127 Repetitive behaviors encompass simple repetitive acts and verbal or motor stereotypies such as lip smacking, hand rubbing or clapping, counting aloud, and humming. Repetitive behaviors also encompass complex repetitive motor routines such as counting, checking, cleaning, wandering a fixed route, repetitive trips to the bathroom, collecting and hoarding objects, pathological gambling, and rituals involving touching, grabbing, and superstitious acts. 21 , 114 , 126 – 128 Stereotypies or compulsive acts are often among the earliest and most salient presenting symptom of frontotemporal dementia and can be bizarre and severely disabling. 23 , 33 , 65 , 122
Similar to dietary and eating behaviors, about 80% of frontotemporal dementia patients eventually have repetitive behaviors, 125 which is more than in other dementias. Miller et al. 21 suggested that the best discriminants for frontotemporal dementia were stereotypical and perseverative behaviors along with hyperorality and loss of hygiene, and these investigators further reported repeated compulsive behaviors in 64% of frontotemporal dementia patients compared to 14% of Alzheimer’s disease patients. 122 Gustafson et al. 35 reviewed several hundred clinicopathological cases of dementia and concluded that frontotemporal dementia was a slowly progressive dementia characterized by stereotypy. Mendez et al. 126 evaluated compulsive behaviors as presenting symptoms in 29 patients with frontotemporal dementia compared to 48 patients with Alzheimer’s disease. Compulsive behaviors occurred in 11 frontotemporal dementia patients (38%) versus five Alzheimer’s disease patients (10%). Other repetitive behaviors reported in frontotemporal dementia include compulsive hoarding, pathological gambling, and self-injurious behaviors such as trichotillomania, picking at fingers to the point of excoriation, and self-biting. 25 , 129
The few studies that have used specific behavioral scales for repetitive behaviors have documented motor and verbal stereotypies as well as compulsions in frontotemporal dementia. 34 , 42 , 49 , 52 , 71 , 130 Shigenobu et al. 131 developed the Stereotypy Rating Inventory, which evaluates eating and cooking behaviors, roaming, speaking, movements, and daily rhythm; and assessed 26 frontotemporal lobar degeneration patients, 46 Alzheimer’s disease patients, 26 vascular dementia patients, and 40 normal controls subjects. The frontotemporal lobar degeneration group scored much higher on this instrument than all the other groups. Nyatsanza et al. 132 used the Stereotypic and Ritualistic Behavior subscale, an addendum to the Neuropsychiatric Inventory, to assess 18 fvFTD, 13 semantic dementia, and 28 Alzheimer’s disease patients. Both the fvFTD and the semantic dementia patients scored significantly higher on this subscale than the Alzheimer’s disease patients. Mendez et al. 133 used a stereotypy scale to evaluate 18 frontotemporal dementia and 18 Alzheimer’s disease patients. Of the frontotemporal dementia patients, eight had stereotypical behaviors (44.4%), such as frequent rubbing and self-injurious acts, compared to only one of the Alzheimer’s disease patients (5.6%). In addition, all of the frontotemporal dementia patients with stereotypical movements had compulsive-like behaviors, suggesting a similar pathophysiological cause.
In frontotemporal lobar degeneration, simple stereotypies correlate with right frontal involvement and complex compulsions with temporal involvement. The occurrence of stereotypies correlates with hypometabolism in the right orbitofrontal region, an area which regulates stimulus reinforcement associated with learning aberrant motor behavior. 62 Rosen et al. 59 further showed that the right lateral orbitofrontal cortex, dorsal anterior cingulate cortex, and insula, along with adjacent motor regions, participate in planning of motor acts in frontotemporal dementia. Consistent with this finding, McMurtray et al. 60 reported that the presence of right frontal hypoperfusion on SPECT predicted stereotyped behaviors in frontotemporal dementia. In contrast to simple verbal or motor stereotypies, frontotemporal lobar degeneration patients with complex compulsive behavior, or intentional and time-consuming repetitive acts, appear to have temporal lobe involvement. 32 Rosso et al. 134 found complex compulsions in 18 of 90 patients with frontotemporal dementia (21%) and an association between temporal lobe atrophy and complex compulsions. Others corroborate an association of prominent rigidity, complex compulsions, and a preoccupation with puzzles among patients with temporal predominant frontotemporal lobar degeneration. 72 , 88 Finally, some complex compulsions, such as pathological gambling, may require additional disinhibition from involvement in the adjacent orbitofrontal cortex. 135
Psychotic Symptoms
Clinicians can confuse frontotemporal dementia with schizophrenia or atypical psychosis, particularly when frontotemporal dementia occurs at a young age. 9 , 136 – 139 Delusional thoughts and hallucinations, however, are uncommon manifestations of frontotemporal dementia, particularly in comparison to Alzheimer’s disease. 4 A review of the world’s literature found 18 well-documented patients with possible frontotemporal dementia and delusions or hallucinations, 140 – 146 but only two cases were truly suggestive of this association. Miller et al. 65 and Edwards-Lee et al. 73 reported a 56-year-old woman who believed that she had contracted AIDS through her husband, became depressed, and showed severe hypoperfusion in the right frontal and temporal region and mild left frontotemporal hypoperfusion. Her subsequent course was consistent with frontotemporal dementia, but no pathology was reported. In a strong case for psychosis in frontotemporal dementia, Reischle et al. 147 reported a 53-year-old man with acute auditory and bizarre visual hallucinations with euphoria and self-overstimulation. Remission of the psychotic symptoms unmasked the clinical picture of a rapidly progressive frontotemporal dementia supported by the results of cerebral MRI and PET, but without pathological confirmation.
Although frontotemporal dementia can mimic schizophrenia, 38 the few psychiatric series suggest that frontotemporal dementia rarely manifests as psychosis. Among 68 fvFTD patients, only 5% had delusions and 2% had hallucinations. 61 Using the Comprehensive Psychiatric Rating Scale, Gregory 130 failed to find psychosis among 15 fvFTD patients. Although Gregory and Hodges 24 did not find psychosis in their prospective evaluation of 15 frontotemporal dementia patients, in their retrospective of 12 patients, one had been incorrectly diagnosed with a schizophreniform psychosis. Confirming frequent misdiagnoses during life, Passant et al. 25 found that most of their 19 autopsy-verified frontotemporal dementia patients had had an initial psychiatric diagnosis, including four with psychosis or schizophrenia, but on further review, none of these patients had actually manifested delusions or hallucinations.
In clinical reports, the prevalence of psychosis in frontotemporal dementia is much lower than in Alzheimer’s disease and appears overestimated with the Neuropsychiatric Inventory. Levy et al. 41 found delusions on the Neuropsychiatric Inventory in five of 22 frontotemporal dementia patients (23%) and 10 of 30 Alzheimer’s disease patients (33%), and Liu et al. 57 found delusions on the Neuropsychiatric Inventory in five of 23 fvFTD patients (22%) and five of 26 tvFTD patients (19%). Levy et al. 41 found hallucinations in none of the frontotemporal dementia patients and two of the Alzheimer’s disease patients (7%), and Liu et al. 57 found hallucinations in three of the fvFTD patients (13%) and none of the tvFTD patients. Using the Neuropsychiatric Inventory, Lopez et al. 70 also found much more delusional psychosis in Alzheimer’s disease than in frontotemporal dementia, and Hirono et al. 68 confirmed that there were fewer delusions in frontotemporal dementia compared to Alzheimer’s disease. Mourik et al. 43 initially reported delusions on the Neuropsychiatric Inventory in eight of 63 frontotemporal dementia patients (12.7%), along with four with hallucinations (6.3%), but it turned out that none of these patients had true “delusions” when specifically evaluated and queried. Finally, in Liscic et al.’s 67 clinicopathologic series, the 48 frontotemporal lobar degeneration patients had fewer hallucinations than the 27 Alzheimer’s disease patients (zero versus four, or 15%). Parenthetically, other investigators suggest that although delusions and hallucinations are rare in frontotemporal dementia, a low B 12 level may correlate with hallucinations in frontotemporal dementia. 54 , 148
Mood Disorders
Depressive symptoms occur in frontotemporal dementia. In early studies, Gustafson 107 reviewed the longitudinal course of 20 frontotemporal dementia patients and noted brief depressive reactions, and Miller et al. 21 reported that two of eight patients (25%) presented with depression. In autopsy-confirmed studies, Gustafson 76 found depressive episodes with occasional suicidal ideation and euphoria among 30 cases, and Mendez et al. 2 found depression among two of 21 frontotemporal dementia patients. Using the Comprehensive Psychiatric Rating Scale, Gregory 130 found three fvFTD patients out of 15 who reported sadness, but only one met criteria for DSM-IV major depressive episode. Among 63 frontotemporal dementia patients evaluated with the Neuropsychiatric Inventory, Mourik et al. 43 found depression in 10 cases (16%). Multidimensional scaling revealed a Cluster A was comprised of delusions, hallucinations, irritability, and agitation, and a Cluster B represented depression and anxiety. In some cases, depression may be a prodrome of frontotemporal dementia and a possible familial risk factor. 24 , 144 , 149 – 152
Depression in frontotemporal dementia has atypical features. Lopez et al. 70 prospectively evaluated DSM-III-R diagnoses in 20 patients with frontotemporal dementia (six autopsy-proven) ascertained by psychiatrists using a structured clinical interview and compared them to 40 patients with Alzheimer’s disease. The frontotemporal dementia patients had higher scores on the Hamilton Depression Rating Scale and exhibited significantly more DSM-III-R major depression (N=5, 25%) than the Alzheimer’s disease patients (N=2, 5%). However, among the frontotemporal dementia patients, only 40% expressed depressed mood, sleep disturbances were found in 30%, appetite changes in 25%, and low self-esteem in 10%, while irritable mood was found in 80% of patients, anergia in 75%, social withdrawal in 70%, psychomotor retardation in 35%, and suicidal ideation in 20%. Swartz et al., 153 using the Schedule for Clinical Assessment in Neuropsychiatry in a retrospective chart review, found that early episodic lability, sadness, anhedonia, increased appetite, loss of interest, and social withdrawal were common among 19 frontotemporal dementia patients, but hopelessness and suicidal ideation occurred in less than half, and themes of guilt were absent.
The relative prevalence of depression in frontotemporal dementia and Alzheimer’s disease may depend upon how depression is ascertained. Using their caregiver questionnaire, Bozeat et al. 49 found that mood changes in general were equally prevalent in frontotemporal dementia and Alzheimer’s disease and covaried with disease severity. Other investigations with other means or instruments have shown less depression, and possibly more euphoria, in frontotemporal dementia versus Alzheimer’s disease and most other dementias. 41 , 95 , 149 , 154 – 156 Using caregiver questionnaires and interviews, Barber et al. 95 found that mood changes were significantly more common in 20 Alzheimer’s disease than in 18 frontotemporal dementia patients. Snowden et al. 149 summarized their experiences in caring for approximately 200 frontotemporal lobar degeneration patients (40 autopsy-proven), and compared with approximately 400 patients with Alzheimer’s disease, depression was less common in frontotemporal dementia. Mendez et al. 154 found positive ratings on the Behavioral Pathology in Alzheimer's Disease (BEHAVE-AD) affective disturbances subscale in 25% of 29 frontotemporal dementia patients, compared to 38% of 29 Alzheimer’s disease patients, and Chiu and colleagues 157 did not find significant differences in the Behavioral Pathology in Alzheimer's Disease affective disturbances subscale between frontotemporal dementia and Alzheimer’s disease, vascular dementia, and dementia with Lewy bodies. In two studies using the Neuropsychiatric Inventory (154 patients), Levy et al. 41 , 156 found that patients with frontotemporal dementia had lower levels of depression or dysphoric mood (39%) than did those with Alzheimer’s disease (43%), Parkinson’s disease (55%), and Huntington’s disease (71%), but not progressive supranuclear palsy (18%). In addition, a neuropsychological study of 40 frontotemporal dementia patients and 40 subcortical vascular dementia patients found milder depressive symptoms in frontotemporal dementia than in vascular dementia. 83
In addition to depression, euphoria, hypomania, emotional lability, childish excitement, and acquired extroversion can occur in the context of frontotemporal dementia and can mimic bipolar disease. 2 , 21 , 46 , 65 , 75 , 158 In a longitudinal series of 20 patients with frontotemporal dementia, 107 euphoria occurred in 35% and ranged between 30% and 36% in two studies that used the Neuropsychiatric Inventory, 41 , 43 and Levy et al. 41 found that euphoria was more common in frontotemporal dementia (36% of 22 patients) compared to Alzheimer’s disease (7% of 30 patients). Although mania has not yet been reported in frontotemporal dementia, McMurtray et al. 60 reported hypomania-like behavior among tvFTD patients, and Thompson et al. 88 found hypomania retrospectively in 2.8% of 36 patients with left-lateralized tvFTD and 9.1% of 11 patients with right-lateralized tvFTD. The hypomanic patient in the latter study appeared to be elated with mild pressured speech and flight of ideas. Emotionalism or lability may occur in some patients with frontotemporal dementia. 15 , 76 , 159 Finally, peurile, childish, frivolous, or silly behavior occurs with right temporal, and probably adjacent orbitofrontal, involvement 75 and can be associated with moria, or foolish or silly euphoria, and Witzelsücht, or a tendency to tell inappropriate jokes. 74
Depression correlates with severe left frontotemporal disease, especially with temporal involvement, whereas other emotional disturbances correlate with early right temporal disease. In the study of Bozeat et al., 49 depression was present in 45% of their tvFTD patients but only 7% of their fvFTD patients. Similarly, Chow and Mendez 160 found depression in 19% of 16 patients with frontotemporal dementia, compared to 44% of nine patients with semantic dementia. Among tvFTD patients, Edwards-Lee et al. 73 found typical depression (anhedonia, feelings of worthlessness, crying) in two of five left tvFTD patients and none of five right tvFTD patients using the Neuropsychiatric Inventory. In contrast, among 74 frontotemporal dementia patients, McMurtray et al. 60 found hypomanic-like behavior associated with temporal hypoperfusion on SPECT, and Mendez et al. 75 found dysthymia and anxiety associated with right temporal hypoperfusion.
Anxiety, Irritability, and Aggression
Anxiety symptoms, usually assessed with the Neuropsychiatric Inventory, may be more frequent among frontotemporal dementia patients than has been previously appreciated. In Lopez et al.’s 70 study, 45% of their 20 frontotemporal dementia patients had anxiety, compared to only 10% of their 40 Alzheimer’s disease patients. Neuropsychiatric Inventory studies show more anxiety in patients with fvFTD than those with tvFTD or Alzheimer’s disease. 43 , 57 Porter et al. 161 specifically assessed the prevalence of anxiety among 115 patients with Alzheimer’s disease, as compared with 33 patients with frontotemporal dementia, 43 patients with vascular dementia, and 40 normal control subjects. Anxiety was significantly more common in patients with frontotemporal dementia and vascular dementia than in patients with Alzheimer’s disease. More recently, Le Ber et al. 61 evaluated 68 frontotemporal dementia patients and reported anxiety symptoms in 15% of them. Among frontotemporal dementia patients, anxiety correlated with right temporal hypoperfusion, 60 and increased neuroticism and personal distress correlated with right temporal atrophy. 99 Finally, several investigators have commented on the presence of hypochondriacal complaints among frontotemporal dementia patients. 15 , 35 , 41 , 65 , 76 , 130
Irritability and aggression are systematically described in only a few publications on frontotemporal dementia. Mendez et al. 154 applied the Behavioral Pathology in Alzheimer's Disease rating scale and found more anger and aggressive outbursts in frontotemporal dementia compared to Alzheimer’s disease, and Lopez et al. 70 evaluated DSM-III-R diagnoses and found significantly more irritability and agitation in frontotemporal dementia compared to Alzheimer’s disease. Passant et al. 25 reported physical aggression and signs of hostility in eight of their 19 neuropathologically confirmed frontotemporal dementia patients. Edwards-Lee et al. 73 described irritability and aggressive behavior as particularly associated with right temporal involvement, but Thompson et al. 88 indicated that irritability was more common in left than in right temporal frontotemporal lobar degeneration.
Additional Behaviors with Limited Literature
Common additional noncognitive behaviors in frontotemporal dementia and related syndromes include declines in hygiene and self-care, behavioral eccentricities, fixed stare and/or smile, aberrant motor behavior, sleep disturbances, and increased artistic creativity. Most of these behaviors are difficult to entirely distinguish from previously discussed neuropsychiatric features of frontotemporal dementia; however, criteria and investigators have specifically referred to these behaviors as characteristic of this disorder. Loss of hygiene and self-care are common in frontotemporal dementia but are difficult to dissociate from apathy-abulia, disinhibition-impulsivity, and loss of insight and self-referential behavior. Several investigators have emphasized the eccentric behaviors that can emerge, particularly in right tvFTD, including bizarre alterations in mode of dress and marked changes in religious or political orientation. 73 , 86 An “alien stare” or peculiar physical bearing (sustained stare or fixed fatuous smile) is associated with right frontotemporal disease. 75 Affect in frontotemporal dementia is sometimes jocular and facial expressions are often fatuous with grinning and inappropriate giggling. 149 Many of the Neuropsychiatric Inventory studies describe aberrant motor behavior in patients with frontotemporal dementia as compared to other conditions, 41 , 43 , 44 , 48 , 57 , 68 , 69 but these can be repetitive stereotypies or compulsions, disinhibition-impulsivity, irritability, or other behaviors. Finally, Liu et al. 57 described sleep disturbances in tvFTD and among 47 semantic dementia patients, and Thompson et al. 88 described those with predominant right-side involvement as more prone to sleep problems.
One of the most interesting and intriguing behavioral changes among frontotemporal dementia patients is an increase in artistic creativity. Several case reports describe an increase in primarily visual artistic performance among well-diagnosed patients with frontotemporal dementia. 162 – 166 These authors suggest the possibility of a release of perceptual abilities with progressive impairments of language and other left hemisphere functions.
Treatment
There are few studies dedicated to the evaluation of drug treatments in frontotemporal dementia ( Table 3 ). 167 Although there is no specific cure for frontotemporal dementia and related syndromes, symptomatic therapies can be very helpful. Serotonin binding is decreased in frontotemporal dementia, 168 and selective serotonin reuptake inhibitors (SSRIs) can decrease the neuropsychiatric symptoms of frontotemporal dementia. 160 Disinhibition-impulsivity, depressive symptoms, carbohydrate craving, and repetitive behaviors may respond to SSRIs such as sertraline, paroxetine, or fluoxetine 169 and trazodone may help for some of these behaviors. 170 In one study, most frontotemporal dementia patients had a decrease in their stereotypical movements with the administration of sertraline. 133 In another study, four of five frontotemporal dementia patients treated with fluoxetine, sertraline, or paroxetine for a minimum of 3 months had improvement in depressive symptoms. 169 SSRIs, however, may produce side-effects in these patients. 171 For example, treatment of pathological laughter in a 61-year-old man with frontotemporal dementia was complicated by intermittent rhythmic myoclonus, reliably evoked independently by fluoxetine or trazodone. 172 In another example, treatment with paroxetine was associated with impairment on paired associates learning, reversal learning, and delayed pattern recognition. 173 In two patients who failed to respond to SSRIs for depression, preliminary evidence suggested that coadministration of lithium might be helpful, 174 and fluvoxamine helped some patients with compulsions. 175 Frontotemporal dementia is associated with some reduction in dopaminergic function, suggesting an as yet unstudied role for drugs such as selegiline and amantadine. 168 Marked disinhibition, aggressive behavior, or verbal outbursts may respond to small doses of atypical antipsychotics such as risperidone, olanzapine, quetiapine, or aripiprazole. Caution is advised, however, as occasionally, similar to patients with dementia with Lewy bodies, patients with frontotemporal dementia may manifest neuroleptic hypersensitivity and elements of malignant neuroleptic syndrome. 176 Theoretically, carbamazepine, valproate, or lamotrigine may diminish symptoms as well, but no dedicated studies are available. One study has shown some efficacy of a single 40 mg methylphenidate dose in improved decision-making behavior on a gambling task, but not on other behavioral or cognitive measures. 177
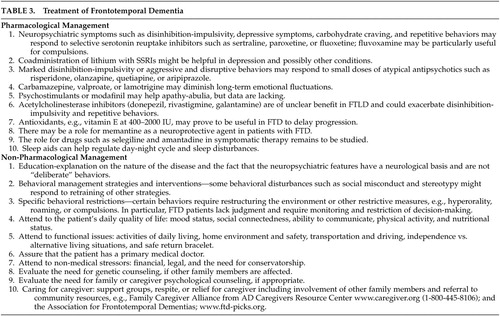 |
The role of medications used to treat Alzheimer’s disease for the treatment of frontotemporal dementia is unclear. Unlike Alzheimer’s disease patients, frontotemporal dementia patients have normal cholinergic function. 168 There is questionable benefit from acetylcholinesterase inhibitors such as donepezil, rivastigmine, or galantamine and recent evidence that they may exacerbate disinhibition-impulsivity and repetitive behaviors. 178 Others have suggested improvement among patients with frontotemporal dementia; 179 – 181 however, those studies probably included significant numbers of Alzheimer’s disease patients among those with so-called “FTD.” For example, in the largest study to date on acetylcholinesterase inhibitors in frontotemporal dementia, all of the patients uncharacteristically had significant memory, cognitive impairments, and behavioral disorders about 7 months into their disease and were older by about 10 years than the average age of onset for this disease. Antioxidant supplements, such as vitamin E, may be helpful in delaying progression, and the potential role of memantine in frontotemporal lobar degeneration is currently under active investigation. There is some rationale for the use of this putative neuroprotective agent in patients with frontotemporal dementia.
The nonpharmacological management of patients with frontotemporal dementia focuses on education and behavioral interventions. Clinicians help caregivers by explaining that the neuropsychiatric features have a neurological basis and by designing behavioral management strategies. 160 , 182 Some behavioral disturbances such as social misconduct and stereotypy might respond to rehabilitation techniques or to retraining via preserved procedural memory. 183 , 184 When hyperorality is present, dietary and other restrictions may prevent excessive weight gain or the dangerous placement of nonfood items in the mouth. 118 Similarly, other specific interventions should be employed to target other neuropsychiatric behaviors. In general, frontotemporal dementia is very stressful to the caregiver and support for the family, as well as continued advice on the management of disturbed behaviors, is critically important. Depression, anxiety, disinhibition, apathy, agitation, and psychosis should be detected and treated since these are particularly associated with high levels of caregiver distress. 43 , 44
DISCUSSION
The current review indicates that frontotemporal dementia patients can develop several noncognitive neuropsychiatric behaviors that are strikingly different from the patient’s premorbid behavior. The most common and early indication of frontotemporal dementia is subtle apathy, abulia, or a decreased pursuit of usual activities to the intensity present before. A second major subgroup is first evident when a patient suddenly performs uncharacteristically disinhibited or impulsive acts. Several investigators have suggested that these two presenting subgroups constitute major behavioral subtypes of frontotemporal dementia. 28 , 32 , 185 Another characteristic of frontotemporal dementia is an early decrease in self-referential behavior which extends to not only decreases in insight and disease awareness but an inability to see themselves through others’ eyes. They cannot recognize others’ feelings, attitudes, or beliefs. Their emotions are blunted, and they become unempathic to the point of not responding to the emotional needs of their family and friends. Perhaps most strikingly there is a decline in social behavior ranging from a loss of a sense of social propriety and manners to frank sociopathic acts. There are major changes in dietary or eating behavior as well, including indiscriminate eating and a craving for sweets or the development of food fads. Frontotemporal dementia patients often manifest a range of repetitive behaviors from simple motor stereotypies to complex compulsions. Other neuropsychiatric manifestations may occur to varying degrees including euphoria and mood lability in about a third of patients; dysphoria or depression, including as a prodrome to frontotemporal dementia; and anxiety, irritability and aggressiveness. However, delusions and hallucinations are rare.
Patients with frontotemporal dementia present with neuropsychiatric features which vary depending on the regions that are involved earliest in the disease. 60 , 75 In the earliest stages, frontotemporal dementia is associated with significant hemispheric asymmetry as well as a different extent of pathology in frontal and anterior temporal regions. 9 Consequently, the presence of specific regional involvement on functional neuroimaging may suggest which neuropsychiatric features are most likely manifestations of frontotemporal dementia. For example, patients with predominantly frontal frontotemporal dementia may manifest apathy-abulia, decreased social dominance, and aspontaneity, 55 , 57 , 104 and those with predominantly temporal frontotemporal dementia may have impairments in emotional processing, complex compulsions, and anxiety or hypomania-like behavior. 55 , 60 , 186 Patients with right frontal frontotemporal dementia can violate social and moral norms, manifest disinhibition-impulsivity, lose insight, and have stereotypies and indiscriminate eating. 57 , 65 , 73 , 104 , 187 Patients with right temporal frontotemporal dementia can have blunted emotions and nonempathic affect and interpersonal coldness. These and other neuropsychiatric-neuroimaging correlations are useful in the early diagnosis of frontotemporal dementia.
In sum, frontotemporal dementia is a devastating neurodegenerative disease, and the early diagnosis of this disorder is critical for developing management strategies and interventions for these patients. Within the first few years after onset, the neuropsychiatric behaviors reviewed here usually precede or overshadow any cognitive disabilities. 2 , 5 , 21 , 22 , 73 In the absence of a biomarker, however, frontotemporal dementia remains difficult to diagnose and may be confused with other neurological or psychiatric disorders. 2 , 23 , 25 , 188 – 190 During the first 2 years of their disease, these patients may see many different doctors and undergo extensive evaluations with significant delays in diagnosis of 3–4 years or more. 8 , 25 , 190 Moreover, clinicopathologic studies report a wide range (0%–71%) of clinical accuracy for diagnosing frontotemporal dementia. 2 , 3 , 6 , 8 , 12 , 13 , 188 , 189 Even at tertiary centers, the diagnostic rate for frontotemporal dementia reaches only about 80%–85% by the time of death. 13 , 191 , 192 Recognizing these noncognitive neuropsychiatric symptoms of frontotemporal dementia is critical to recognizing the clinical disease.
Directions for Future Research
Clinical investigators still have far to go in defining the neuropsychiatric features of frontotemporal dementia and arriving at better diagnostic criteria. First, as new and better diagnostic criteria are developed, investigators need to specifically include the neuropsychiatric behaviors reviewed here. In the absence of biomarkers for frontotemporal dementia and related disorders, clinicians rely on behavioral criteria for diagnosis during life. Second, the neuropsychiatric features need to undergo more rigorous examination. The current literature is often confounded by a lack of systematic assessment of specific behavioral disorders using valid and reliable criteria and by a lack of uniform assessment methodology. Many studies are retrospective investigations that do not use a gold standard technique for diagnosing frontotemporal dementia and rely on chart notes for documentation of behavioral disturbances. Some studies appear to use strictly clinical impressions without defining behavioral criteria. Others use behavioral scales, such as the Neuropsychiatric Inventory, rather than formal diagnostic criteria for psychiatric symptoms. There is a clear need for the systematic assessment of neuropsychiatric behaviors using valid and reliable diagnostic criteria with a view toward defining their prevalences, correlates (neurological, functional, imaging, prognostic, pathological), and treatment outcomes. Finally, there is a striking lack of clinical drug trials for the frontotemporal lobar degenerations. Both therapeutic and symptomatic drug trials are desperately needed in frontotemporal dementia. These trials should employ well-diagnosed patients and conditions and utilize adequate outcome measures. Nevertheless, the exponential increase in research studies in frontotemporal dementia in recent years is an indication that the future promises to reveal much more about the neuropsychiatry of frontotemporal dementia and its treatment.
1 . Johnson JK, Diehl J, Mendez MF, et al: Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol 2005; 62:925–930Google Scholar
2 . Mendez MF, Selwood A, Mastri AR, et al: Pick’s disease versus Alzheimer’s disease: a comparison of clinical characteristics. Neurol 1993; 43:289–292Google Scholar
3 . Pasquier F, Delacourte A: Non-Alzheimer degenerative dementias. Curr Opin Neurol 1998; 11:417–427Google Scholar
4 . Mendez MF, Cummings JL: Dementia: A Clinical Approach, 3rd ed. Philadelphia, Butterworth-Heinemann (Elsevier), 2003Google Scholar
5 . Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 1994; 57:416–418Google Scholar
6 . Ratnavalli E, Brayne C, Dawson K, et al: The prevalence of frontotemporal dementia. Neurology 2002; 58:1615–1621Google Scholar
7 . Robert PH, Lafont V, Snowden JS, et al: [Diagnostic criteria for fronto-temporal lobe degeneration.] Encephale 1999; 25:612–621 (French)Google Scholar
8 . Hodges JR, Davies RR, Xuereb JH, et al: Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004; 56:399–406Google Scholar
9 . McKhann GM, Albert MS, Grossman M, et al: Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on frontotemporal dementia and Pick’s disease. Arch Neurol 2001; 58:1803–1809Google Scholar
10 . Mott RT, Dickson DW, Trojanowski JQ, et al: Neuropathologic, biochemical, and molecular characterization of the frontotemporal dementias. J Neuropathol Exp Neurol 2005; 64:420–428Google Scholar
11 . Pick A: Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prag Med Wochenschr 1892; 17:165–167Google Scholar
12 . Kertesz A, Munoz DG: Frontotemporal dementia. Med Clin North Am 2002; 86:501–518, ivGoogle Scholar
13 . Forman MS, Farmer J, Johnson JK, et al: Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006; 59:952–962Google Scholar
14 . Josephs KA, Petersen RC, Knopman DS, et al: Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006; 66:41–48Google Scholar
15 . Neary D, Snowden JS, Gustafson L, et al: Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554Google Scholar
16 . Kertesz A, Davidson W, Munoz DG: Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia and corticobasal degeneration: the Pick complex. Dement Geriatr Cogn Disord 1999; 10(suppl 1):46–49Google Scholar
17 . Duara R, Barker W, Luis CA: Frontotemporal dementia and Alzheimer’s disease: differential diagnosis. Dement Geriatr Cogn Disord 1999; 10(suppl 1):37–42Google Scholar
18 . Diehl J, Mayer T, Kurz A, et al: [Features of frontotemporal dementia from the perspective of a special family support group]. Nervenarzt 2003; 74:445–449 (German)Google Scholar
19 . Filley CM, Kleinschmidt-De Masters BK, Gross KF: Non-Alzheimer fronto-temporal degenerative dementia:a neurobehavioral and pathologic study. Clin Neuropathol 1994; 13:109–116Google Scholar
20 . Hooten WM, Lyketsos CG: Frontotemporal dementia: a clinicopathological review of four postmortem studies. J Neuropsychiatry Clin Neurosci 1996; 8:10–19Google Scholar
21 . Miller BL, Cummings JL, Villanueva-Meyer J, et al: Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology 1991; 41:1374–1382Google Scholar
22 . Pasquier F, Petit H: Frontotemporal dementia: its rediscovery. Eur Neurol 1997; 38:1–6Google Scholar
23 . Mendez MF, Perryman KM: Neuropsychiatric features of frontotemporal dementia: evaluation of consensus criteria and review. J Neuropsychiatry Clin Neurosci 2002; 14:424–429Google Scholar
24 . Gregory CA, Hodges JR: Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm Suppl 1996; 47:103–123Google Scholar
25 . Passant U, Elfgren C, Englund E, et al: Psychiatric symptoms and their psychosocial consequences in frontotemporal dementia. Alzheimer Dis Assoc Disord 2005; 19(suppl 1):S15–18Google Scholar
26 . Mendez MF, Shapira JS, McMurtray A, et al: Accuracy of the clinical evaluation for frontotemporal dementia. Arch Neurol 2007; 64:830–835Google Scholar
27 . Mychack P, Rosen H, Miller BL: Novel applications of social-personality measures to the study of dementia. Neurocase 2001; 7:131–143Google Scholar
28 . Kertesz A: Pick complex: an integrative approach to frontotemporal dementia: primary progressive aphasia, corticobasal degeneration, and progressive supranuclear palsy. Neurologist 2003; 9:311–317Google Scholar
29 . Snowden JS, Neary D, Mann DM: Frontotemporal dementia. Br J Psychiatry 2002; 180:140–143Google Scholar
30 . Franceschi M, Anchisi D, Pelati O, et al: Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol 2005; 57:216–225Google Scholar
31 . Peters F, Perani D, Herholz K, et al: Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord 2006; 21:373–379Google Scholar
32 . Snowden JS, Bathgate D, Varma A, et al: Distinct behavioral profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 2001; 70:323–332Google Scholar
33 . Shinagawa S, Ikeda M, Fukuhara R, et al: Initial symptoms in frontotemporal dementia and semantic dementia compared with Alzheimer’s disease. Dement Geriatr Cogn Disord 2006; 21:74–80Google Scholar
34 . Kertesz A, Davidson W, McCabe P, et al: Behavioral quantitation is more sensitive than cognitive testing in frontotemporal dementia. Alzheimer Dis Assoc Disord 2003; 17:223–229Google Scholar
35 . Gustafson L, Brun A, Passant U: Frontal lobe degeneration of non-Alzheimer type. Baillieres Clin Neurol 1992; 1:559–582Google Scholar
36 . Galante E, Muggia S, Spinnler H, et al: Degenerative dementia of the frontal type: clinical evidence from nine cases. Dement Geriatr Cogn Disord 1999; 10:28–39Google Scholar
37 . Chow TW, Miller BL, Boone K, et al: Frontotemporal dementia classification and neuropsychiatry. Neurologist 2002; 8:263–269Google Scholar
38 . Diehl J, Kurz A: Frontotemporal dementia: patient characteristics, cognition, and behaviour. Int J Geriatr Psychiatry 2002; 17:914–918Google Scholar
39 . Pasquier F, Lebert F, Lavenu I, et al: The clinical picture of frontotemporal dementia: diagnosis and follow-up. Dement Geriatr Cogn Disord 1999; 10(suppl 1):10–14Google Scholar
40 . Cummings JL: The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 1997; 48:S10–16Google Scholar
41 . Levy ML, Miller BL, Cummings JL, et al: Alzheimer disease and frontotemporal dementias: behavioral distinctions. Arch Neurol 1996; 53:687–690Google Scholar
42 . Ikeda M, Tanabe H: [Neuropsychology of frontal type dementia]. Seishin Shinkeigaku Zasshi 2000; 102:113–124 (Japanese)Google Scholar
43 . Mourik JC, Rosso SM, Niermeijer MF, et al: Frontotemporal dementia: behavioral symptoms and caregiver distress. Dement Geriatr Cogn Disord 2004; 18:299–306Google Scholar
44 . de Vugt ME, Riedijk SR, Aalten P, et al: Impact of behavioral problems on spousal caregivers: a comparison between Alzheimer’s disease and frontotemporal dementia. Dement Geriatr Cogn Disord 2006; 22:35–41Google Scholar
45 . Jenner C, Reali G, Puopolo M, et al: Can cognitive and behavioral disorders differentiate frontal variant-frontotemporal dementia from Alzheimer’s disease at early stages? Behav Neurol 2006; 17:89–95Google Scholar
46 . Miller BL, Ikonte C, Ponton M, et al: A study of the Lund-Manchester research criteria for frontotemporal dementia: clinical and single-photon emission CT correlations. Neurology 1997; 48:937–942Google Scholar
47 . Diehl-Schmid J, Pohl C, Perneczky R, et al: Behavioral disturbances in the course of frontotemporal dementia. Dement Geriatr Cogn Disord 2006; 22:352–357Google Scholar
48 . Perri R, Koch G, Carlesimo GA, et al: Alzheimer’s disease and frontal variant of frontotemporal dementia: a very brief battery for cognitive and behavioral distinction. J Neurol 2005; 252:1238–1244Google Scholar
49 . Bozeat S, Gregory CA, Ralph MA, et al: Which neuropsychiatric and behavioral features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry 2000; 69:178–186Google Scholar
50 . Kertesz A, Davidson W, Fox H: Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 1997; 24:29–36Google Scholar
51 . Marczinski CA, Davidson W, Kertesz A: A longitudinal study of behavior in frontotemporal dementia and primary progressive aphasia. Cogn Behav Neurol 2004; 17:185–190Google Scholar
52 . Kertesz A, Nadkarni N, Davidson W, et al: The frontal behavioral inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6:460–468Google Scholar
53 . Boone KB, Miller BL, Swartz R, et al: Relationship between positive and negative symptoms and neuropsychological scores in frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc 2003; 9:698–709Google Scholar
54 . Engelborghs S, Maertens K, Nagels G, et al: Neuropsychiatric symptoms of dementia: cross-sectional analysis from a prospective, longitudinal Belgian study. Int J Geriatr Psychiatry 2005; 20:1028–1037Google Scholar
55 . Rankin KP, Kramer JH, Mychack P, et al: Double dissociation of social functioning in frontotemporal dementia. Neurology 2003; 60:266–271Google Scholar
56 . Rankin KP, Baldwin E, Pace-Savitsky C, et al: Self awareness and personality change in dementia. J Neurol Neurosurg Psychiatry 2005; 76:632–639Google Scholar
57 . Liu W, Miller BL, Kramer JH, et al: Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 2004; 62:742–748Google Scholar
58 . Rosen HJ, Pace-Savitsky K, Perry RJ, et al: Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dement Geriatr Cogn Disord 2004; 17:277–281Google Scholar
59 . Rosen HJ, Allison SC, Schauer GF, et al: Neuroanatomical correlates of behavioral disorders in dementia. Brain 2005; 128:2612–2625Google Scholar
60 . McMurtray AM, Chen AK, Shapira JS, et al: Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology 2006; 66:517–522Google Scholar
61 . Le Ber I, Guedj E, Gabelle A, et al: Demographic, neurological and behavioral characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain 2006; 129:3051–3065Google Scholar
62 . Sarazin M, Michon A, Pillon B, et al: Metabolic correlates of behavioral and affective disturbances in frontal lobe pathologies. J Neurol 2003; 250:827–833Google Scholar
63 . Neary D, Snowden J, Mann D: Frontotemporal dementia. Lancet Neurol 2005; 4:771–780Google Scholar
64 . Lindau M, Almkvist O, Kushi J, et al: First symptoms: frontotemporal dementia versus Alzheimer’s disease. Dement Geriatr Cogn Disord 2000; 11:286–293Google Scholar
65 . Miller BL, Chang L, Mena I, et al: Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia 1993; 4:204–213Google Scholar
66 . Snowden JS, Neary D, Mann DM: Autopsy proven sporadic frontotemporal dementia due to microvacuolar-type histology, with onset at 21 years of age. J Neurol Neurosurg Psychiatry 2004; 75:1337–1339Google Scholar
67 . Liscic RM, Storandt M, Cairns NJ, et al: Clinical and psychometric distinction of frontotemporal and Alzheimer dementias. Arch Neurol 2007; 64:535–540Google Scholar
68 . Hirono N, Mori E, Tanimukai S, et al: Distinctive neurobehavioral features among neurodegenerative dementias. J Neuropsychiatry Clin Neurosci 1999; 11:498–503Google Scholar
69 . Srikanth S, Nagaraja AV, Ratnavalli E: Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci 2005; 236:43–48Google Scholar
70 . Lopez OL GM, Becker JT, Reynolds CF, et al: Symptoms of depression and psychosis in Alzheimer’s disease and frontotemporal dementia. Neuropsychiatry Neuropsychol Behav Neurol 1996; 9:154–161Google Scholar
71 . Bathgate D, Snowden JS, Varma A, et al: Behavior in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand 2001; 103:367–378Google Scholar
72 . Seeley WW, Bauer AM, Miller BL, et al: The natural history of temporal variant frontotemporal dementia. Neurology 2005; 64:1384–1390Google Scholar
73 . Edwards-Lee T, Miller BL, Benson DF, et al: The temporal variant of frontotemporal dementia. Brain 1997; 120:1027–1040Google Scholar
74 . Mendez MF: Moria and Witzelsucht from frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2005; 17:429–430Google Scholar
75 . Mendez MF, McMurtray A, Chen AK, et al: Functional neuroimaging and presenting psychiatric features in frontotemporal dementia. J Neurol Neurosurg Psychiatry 2006; 77:4–7Google Scholar
76 . Gustafson L: Clinical picture of frontal lobe degeneration of non-Alzheimer type. Dementia 1993; 4:143–148Google Scholar
77 . Kertesz A: Behavioral and psychological symptoms and frontotemporal dementia (Pick’s disease). Int Psychogeriatrics 2000; 12:183–187Google Scholar
78 . Perrine R, Schmidt C, Hogge M, et al: Social mind representation: where does it fail in frontotemporal dementia? J Cog Neurosci 2007; 19:671–683Google Scholar
79 . Evers K, Kilander L, Lindau M: Insight in frontotemporal dementia: conceptual analysis and empirical evaluation of the consensus criterion “loss of insight” in frontotemporal dementia. Brain Cogn 2007; 63:13–23Google Scholar
80 . Mendez MF, Shapira JS: Loss of insight and functional neuroimaging in frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2005; 17:413–416Google Scholar
81 . Eslinger PJ, Dennis K, Moore P, et al: Metacognitive deficits in frontotemporal dementia. J Neurol Neurosurg Psychiatry 2005; 76:1630–1635Google Scholar
82 . Pijnenburg YA, Gillissen F, Jonker C, et al: Initial complaints in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 2004; 17:302–306Google Scholar
83 . Moretti R, Torre P, Antonello RM, et al: Frontal lobe dementia and subcortical vascular dementia: a neuropsychological comparison. Psychol Rep 2005; 96:141–151Google Scholar
84 . O’Keeffe FM, Murray B, Coen RF, et al: Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain 2007; 130:753–764Google Scholar
85 . Piolino P, Desgranges B, Belliard S, et al: Autobiographical memory and autonoetic consciousness: triple dissociation in neurodegenerative diseases. Brain 2003; 126:2203–2219Google Scholar
86 . Miller BL, Seeley WW, Mychack P, et al: Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology 2001; 57:817–821Google Scholar
87 . Sturm VE, Rosen HJ, Allison S, et al: Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain 2006; 129:2508–2516Google Scholar
88 . Thompson SA, Patterson K, Hodges JR: Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 2003; 61:1196–1203Google Scholar
89 . Salmon E, Perani D, Collett F, et al: A comparison of unawareness in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2008; 79:176–179Google Scholar
90 . Werner KH, Roberts NA, Rosen HJ, et al. Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology 2007; 69:148–155Google Scholar
91 . Basun H, Almkvist O, Axelman K, et al: Clinical characteristics of a chromosome 17-linked rapidly progressive familial frontotemporal dementia. Arch Neurol 1997; 54:539–544Google Scholar
92 . Brun A: Frontal lobe degeneration of non-Alzheimer type revisited. Dementia 1993; 4:126–131Google Scholar
93 . Lough S, Kipps CM, Treise C, et al: Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia 2006; 44:950–958Google Scholar
94 . Eslinger PJ, Moore P, Troiani V, et al: Oops! Resolving social dilemmas in frontotemporal dementia. J Neurol Neurosurg Psychiatry 2007; 78:457–460Google Scholar
95 . Barber R, Snowden JS, Craufurd D: Frontotemporal dementia and Alzheimer’s disease: retrospective differentiation using information from informants. J Neurol Neurosurg Psychiatry 1995; 59:61–70Google Scholar
96 . Rankin KP, Gorno-Tempini ML, Allison SC, et al: Structural anatomy of empathy in neurodegenerative disease. Brain 2006; 129:2945–2956Google Scholar
97 . Rankin KP, Kramer JH, Miller BL: Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol 2005; 18:28–36Google Scholar
98 . Perry RJ, Rosen HR, Kramer JH, et al: Hemispheric dominance for emotions, empathy and social behavior: evidence from right and left handers with frontotemporal dementia. Neurocase 2001; 7:145–160Google Scholar
99 . Gorno-Tempini ML, Rankin KP, Woolley JD, et al: Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex 2004; 40:631–644Google Scholar
100 . Tallberg IM, Persson H, Wangmar L, et al: Semantic range and relevance of emotive utterances in patients with frontotemporal degeneration. Brain Lang 2002; 82:146–158Google Scholar
101 . Mendez MF, Perryman KM: Disrupted facial empathy in drawings from artists with frontotemporal dementia. Neurocase 2003; 9:44–50Google Scholar
102 . Mendez MF, Lim GT: Alterations of the sense of “humanness” in right hemisphere predominant frontotemporal dementia patients. Cogn Behav Neurol 2004; 17:133–138Google Scholar
103 . Rankin KP, Rosen HJ, Kramer JH, et al: Right and left medial orbitofrontal volumes show an opposite relationship to agreeableness in FTD. Dement Geriatr Cogn Disord 2004; 17:328–332Google Scholar
104 . Mychack P, Kramer JH, Boone KB, et al: The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology 2001; 56:S11–15Google Scholar
105 . Diehl J, Ernst J, Krapp S, et al: [Misdemeanor in frontotemporal dementia.] Fortschr Neurol Psychiatr 2006; 74:203–210 (German)Google Scholar
106 . Frisoni GB, Pizzolato G, Geroldi C, et al: Dementia of the frontal type: neuropsychological and [99Tc]-HM-PAO SPECT features. J Geriatr Psychiatry Neurol 1995; 8:42–48Google Scholar
107 . Gustafson L: Frontal lobe degeneration of non-Alzheimer type II: clinical picture and differential diagnosis. Arch Gerontol Geriatr 1987; 6:209–223Google Scholar
108 . Mendez MF, Chow T, Ringman J, et al: Pedophilia and temporal lobe disturbances. J Neuropsychiatry Clin Neurosci 2000; 12:71–76Google Scholar
109 . Miller BL, Darby A, Benson DF, et al: Aggressive, socially disruptive and antisocial behaviour associated with fronto-temporal dementia. Br J Psychiatry 1997; 170:150–154Google Scholar
110 . Sjogren M, Wallin A, Edman A: Symptomatological characteristics distinguish between frontotemporal dementia and vascular dementia with a dominant frontal lobe syndrome. Int J Geriatr Psychiatry 1997; 12:656–661Google Scholar
111 . Mendez MF, Chen AK, Shapira JS, et al: Acquired sociopathy and frontotemporal dementia. Dement Geriatr Cogn Disord 2005; 20:99–104Google Scholar
112 . Mendez MF, Anderson E, Shapira JS: An investigation of moral judgement in frontotemporal dementia. Cogn Behav Neurol 2005; 18:193–197Google Scholar
113 . Mendez MF: What frontotemporal dementia reveals about the neurobiological basis of morality. Med Hypotheses 2006; 67:411–418Google Scholar
114 . Miller BL, Diehl J, Freedman M, et al: International approaches to frontotemporal dementia diagnosis: from social cognition to neuropsychology. Ann Neurol 2003; 54(suppl 5):S7–10Google Scholar
115 . Keane J, Calder AJ, Hodges JR, et al: Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia 2002; 40:655–665Google Scholar
116 . Lough S, Gregory C, Hodges JR: Dissociation of social cognition and executive function in frontal variant frontotemporal dementia. Neurocase 2001; 7:123–130Google Scholar
117 . Nakano S, Asada T, Yamashita F, et al: Relationship between antisocial behavior and regional cerebral blood flow in frontotemporal dementia. Neuroimage 2006; 32:301–306Google Scholar
118 . Mendez MF, Foti DJ: Lethal hyperoral behavior from the Klüver-Bucy syndrome. J Neurol Neurosurg Psychiatry 1997; 62:293–294Google Scholar
119 . Lough S, Hodges JR: Measuring and modifying abnormal social cognition in frontal variant frontotemporal dementia. J Psychosom Res 2002; 53:639–646Google Scholar
120 . Ikeda M, Brown J, Holland AJ, et al: Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2002; 73:371–376Google Scholar
121 . Cummings JL, Duchen LW: Klüver-Bucy syndrome in Pick disease: clinical and pathologic correlations. Neurology 1981; 31:1415–1422Google Scholar
122 . Miller BL, Darby AL, Swartz JR, et al: Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia 1995; 6:195–199Google Scholar
123 . Short RA, Broderick DF, Patton A, et al: Different patterns of magnetic resonance imaging atrophy for frontotemporal lobar degeneration syndromes. Arch Neurol 2005; 62:1106–1110Google Scholar
124 . Whitwell JL, Sampson EL, Loy CT, et al: VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage 2007; 35:207–213Google Scholar
125 . Ames D, Cummings JL, Wirshing WC, et al: Repetitive and compulsive behavior in frontal lobe degenerations. J Neuropsychiatry Clin Neurosci 1994; 6:100–113Google Scholar
126 . Mendez MF, Perryman KM, Miller BL, et al: Compulsive behaviors as presenting symptoms of frontotemporal dementia. J Geriatr Psychiatry Neurol 1997; 10:154–157Google Scholar
127 . Tonkonogy JM, Smith TW, Barreira PJ: Obsessive-compulsive disorders in Pick’s disease. J Neuropsychiatry Clin Neurosci 1994; 6:176–180Google Scholar
128 . Snowden JS, Neary D, Mann DM, et al: Progressive language disorder due to lobar atrophy. Ann Neurol 1992; 31:174–183Google Scholar
129 . Mendez MF, Bagert BA, Edwards-Lee T: Self-injurious behavior in frontotemporal dementia. Neurocase 1997; 3:231–236Google Scholar
130 . Gregory CA: Frontal variant of frontotemporal dementia: a cross-sectional and longitudinal study of neuropsychiatric features. Psychol Med 1999; 29:1205–1217Google Scholar
131 . Shigenobu K, Ikeda M, Fukuhara R, et al: The stereotypy rating inventory for frontotemporal lobar degeneration. Psychiatry Res 2002; 110:175–187Google Scholar
132 . Nyatsanza S, Shetty T, Gregory C, et al: A study of stereotypic behaviours in Alzheimer’s disease and frontal and temporal variant frontotemporal dementia. J Neurol Neurosurg Psychiatry 2003; 74:1398–1402Google Scholar
133 . Mendez MF, Shapira JS, Miller BL: Stereotypical movements and frontotemporal dementia. Mov Disord 2005; 20:742–745Google Scholar
134 . Rosso SM, Roks G, Stevens M, et al: Complex compulsive behavior in the temporal variant of frontotemporal dementia. J Neurol 2001; 248:965–970Google Scholar
135 . Lo Coco D, Nacci P: Frontotemporal dementia presenting with pathological gambling. J Neuropsychiatry Clin Neurosci 2004; 16:117–118Google Scholar
136 . Stone J, Griffiths TD, Rastogi S, et al: Non-Pick’s frontotemporal dementia imitating schizophrenia in a 22-year-old man. J Neurol 2003; 250:369–370Google Scholar
137 . Lamote H, Tan KL, Verhoeven WM: [Frontotemporal dementia in a young woman with apparent schizophrenia.] Ned Tijdschr Geneeskd 1998; 142:1962–1965 (Dutch)Google Scholar
138 . Salmon E, Degueldre C, Franco G, et al: Frontal lobe dementia presenting as personality disorder. Acta Neurol Belg 1996; 96:130–134Google Scholar
139 . Goldstein K, Katz SE: The psychopathology of Pick’s disease. Arch Neurol Psychiatry 1937; 38:473–490Google Scholar
140 . Neary D, Snowden JS, Northen B, et al: Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry 1988; 51:353–361Google Scholar
141 . Waddington JL, Youssef HA, Farrell MA, et al: Initial “schizophrenia-like” psychosis in Pick’s disease: case study with neuroimaging and neuropathology, and implications for frontotemporal dysfunction in schizophrenia. Schizophr Res 1995; 18:79–82Google Scholar
142 . Kitabayashi Y, Otakara C, Hirosawa R, et al: Frontotemporal dementia complicated with schizophrenia. Psychiatry Clin Neurosci 2005; 59:749–750Google Scholar
143 . Lamote H, Tan KL, Verhoeven WM: [Frontotemporal dementia in a young woman with apparent schizophrenia]. Ned Tijdschr Geneeskd 1998; 142:1962–1965 (Dutch)Google Scholar
144 . Dell DL, Halford JJ: Dementia presenting as postpartum depression. Obstet Gynecol 2002; 99:925–928Google Scholar
145 . Vanderzeypen F, Bier JC, Genevrois C, et al: [Frontal dementia or dementia praecox? A case report of a psychotic disorder with a severe decline.] Encephale 2003; 29:172–180 (French)Google Scholar
146 . Kerssens CJ, Pijnenburg YA, Schouws S, et al: [The development of psychotic symptoms in later life: late-onset schizophrenia or frontotemporal dementia? A case study.] Tijdschr Psychiatr 2006; 48:739–744 (Dutch)Google Scholar
147 . Reischle E, Sturm K, Schuierer G, et al: [A case of schizophreniform disorder in frontotemporal dementia (FTD)]. Psychiatr Prax 2003; 30(suppl 2):S78–82 (German)Google Scholar
148 . Engelborghs S, Maertens K, Marien P, et al: Behavioural and neuropsychological correlates of frontal lobe features in dementia. Psychol Med 2006; 36:1173–1182Google Scholar
149 . Snowden JS, Neary D, Mann DM: Fronto-Temporal Lobar Degeneration: Fronto-Temporal Dementia, Progressive Aphasia, Semantic Dementia. New York, Churchill Livingstone, 1996Google Scholar
150 . Luaute JP, Favel P, Remy C, et al: [Affective disorders and dementia of the frontal lobe type: hypothesis of a pathogenic relationship.] Encephale 1994; 20:27–36 (French)Google Scholar
151 . Suzuki H, Kuroda S, Ishizu H, et al: Depression in the early stages of Pick’s disease. Acta Med Okayama 1999; 53:253–257Google Scholar
152 . Chow TW, Miller BL, Hayashi VN, et al: Inheritance of frontotemporal dementia. Arch Neurol 1999; 56:817–822Google Scholar
153 . Swartz JR, Miller BL, Lesser IM, et al: Behavioral phenomenology in Alzheimer’s disease, frontotemporal dementia, and late-life depression: a retrospective analysis. J Geriatr Psychiatry Neurol 1997; 10:67–74Google Scholar
154 . Mendez MF, Perryman KM, Miller BL, et al: Behavioral differences between frontotemporal dementia and Alzheimer’s disease: a comparison on the BEHAV-AD rating scale. Int Psychogeriatr 1998; 10:155–162Google Scholar
155 . Pfeffer A, Luczywek E, Golebiowski M, et al: Frontotemporal dementia: an attempt at clinical characteristics. Dement Geriatr Cogn Disord 1999; 10:217–220Google Scholar
156 . Levy ML, Cummings JL, Fairbanks LA, et al: Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Google Scholar
157 . Chiu MJ, Chen TF, Yip PK, et al: Behavioral and psychologic symptoms in different types of dementia. J Formos Med Assoc 2006; 105:556–562Google Scholar
158 . Mendez MF, Chen AK, Shapira JS, et al: Acquired extroversion associated with bitemporal variant of frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2006; 18:100–107Google Scholar
159 . Lebert F, Pasquier F, Petit H: Personality traits and frontal lobe dementia. Int J Geriatr Psychiatry 1995; 10:1047–1049Google Scholar
160 . Chow TW, Mendez MF: Goals in symptomatic pharmacologic management of frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen 2002; 17:267–272Google Scholar
161 . Porter VR, Buxton WG, Fairbanks LA, et al: Frequency and characteristics of anxiety among patients with Alzheimer’s disease and related dementias. J Neuropsychiatry Clin Neurosci 2003; 15:180–186Google Scholar
162 . Mendez MF: Dementia as a window to the neurology of art. Med Hypotheses 2004; 63:1–7Google Scholar
163 . Mell JC, Howard SM, Miller BL: Art and the brain: the influence of frontotemporal dementia on an accomplished artist. Neurology 2003; 60:1707–1710Google Scholar
164 . Miller BL, Cummings J, Mishkin F, et al: Emergence of artistic talent in frontotemporal dementia. Neurology 1998; 51:978–982Google Scholar
165 . Miller BL, Boone K, Cummings JL, et al: Functional correlates of musical and visual ability in frontotemporal dementia. Br J Psychiatry 2000; 176:458–463Google Scholar
166 . Miller BL, Hou CE: Portraits of artists: emergence of visual creativity in dementia. Arch Neurol 2004; 61:842–844Google Scholar
167 . Litvan I: Therapy and management of frontal lobe dementia patients. Neurol 2001; 56:S41–45Google Scholar
168 . Huey ED, Putnam KT, Grafman J: A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 2006; 66:17–22Google Scholar
169 . Swartz JR, Miller BL, Lesser IM, et al: Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997; 58:212–216Google Scholar
170 . Lebert F, Stekke W, Hasenbroekx C, et al: Frontotemporal dementia: a randomized, controlled trial with trazodone. Dement Geriatr Cogn Disord 2004; 17:355–359Google Scholar
171 . Chow TW: Frontotemporal dementias: clinical features and management. Semin Clin Neuropsychiatry 2003; 8:58–70Google Scholar
172 . Lauterbach EC: Reversible intermittent rhythmic myoclonus with fluoxetine in presumed Pick’s disease. Mov Disord 1994; 9:343–346Google Scholar
173 . Deakin JB, Rahman S, Nestor PJ, et al: Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl) 2004; 172:400–408Google Scholar
174 . Anderson IM, Scott K, Harborne G: Serotonin and depression in frontal lobe dementia. Am J Psychiatry 1995; 152:645Google Scholar
175 . Ikeda M, Shigenobu K, Fukuhara R, et al: Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord 2004; 17:117–121Google Scholar
176 . Mendez MF, Lipton A: Emergent neuroleptic hypersensitivity as a herald of presenile dementia. J Neuropsychiatry Clin Neurosci 2001; 13:347–356Google Scholar
177 . Rahman S, Robbins TW, Hodges JR, et al: Methylphenidate (“Ritalin”) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology 2006; 31:651–658Google Scholar
178 . Mendez MF, Shapira JS, McMurtray A, et al: Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry 2007; 15:84–87Google Scholar
179 . Procter AW, Qurne M, Francis PT: Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord 1999; 10(suppl 1):80–84Google Scholar
180 . Moretti R, Torre P, Antonello RM, et al: Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging 2004; 21:931–937Google Scholar
181 . Proctor R, Burns A, Powell HS, et al: Behavioral management in nursing and residential homes: a randomized controlled trial. Lancet 1999; 354:26–29Google Scholar
182 . Perry RJ, Miller BL: Behavior and treatment in frontotemporal dementia. Neurology 2001; 56(suppl 4):S46–51Google Scholar
183 . Ikeda M, Tanabe H, Horino T, et al: [Care for patients with Pick’s disease—by using their preserved procedural memory]. Seishin Shinkeigaku Zasshi 1995; 97:179–192 (Japanese)Google Scholar
184 . Robinson KM: Rehabilitation applications in caring for patients with Pick’s disease and frontotemporal dementias. Neurology 2001; 56:S56–58Google Scholar
185 . Perry RJ, Graham A, Williams G, et al: Patterns of frontal lobe atrophy in frontotemporal dementia: a volumetric MRI study. Dement Geriatr Cogn Disord 2006; 22:278–287Google Scholar
186 . Rosen HJ, Hartikainen KM, Jagust W, et al: Utility of clinical criteria in differentiating frontotemporal lobar degeneration (FTLD) from AD. Neurology 2002; 58:1608–1615Google Scholar
187 . Razani J, Boone KB, Miller BL, et al: Neuropsychological performance of right- and left-frontotemporal dementia compared to Alzheimer’s disease. J Int Neuropsychol Soc 2001; 7:468–480Google Scholar
188 . Fujihara S, Brucki SM, Rocha MS, et al: Prevalence of presenile dementia in a tertiary outpatient clinic. Arq Neuropsiquiatr 2004; 62:592–595Google Scholar
189 . Litvan I, Agid Y, Sastry N, et al: What are the obstacles for an accurate clinical diagnosis of Pick’s disease? A clinicopathologic study. Neurology 1997; 49:62–69Google Scholar
190 . Roberson ED, Hesse JH, Rose KD, et al: Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology 2005; 65:719–725Google Scholar
191 . Knopman DS, Petersen RC, Edland SD, et al: The incidence of frontotemporal lobar degeneration in Rochester, MN, 1990 through 1994. Neurology 2004; 62:506–508Google Scholar
192 . Lopez OL, Litvan I, Catt KE, et al: Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology 1999; 53:1292–1299Google Scholar