Factor Analysis of the Rivermead Post-Concussion Symptoms Questionnaire in Mild-to-Moderate Traumatic Brain Injury Patients
Multiple scales have been used to assess depression in TBI patients, including the Hamilton Depression Rating Scale (HAM-D), 4 – 6 the Beck Depression Inventory (BDI), 7 and the Centre for Epidemiologic Studies-Dementia scale (CES-D). 8 While these scales are useful to quantify severity of depression, the impact of a TBI extends beyond mood symptoms. Many studies have observed a detrimental effect of TBI on cognitive function 9 , 10 and activities of daily living, 11 which can persist for many years following the injury. 12 The most common postconcussion symptoms are headaches, irritability, anxiety, dizziness, fatigue, and impaired concentration, 13 but a wider variety of symptoms that can include decreased libido, noise sensitivity, and social withdrawal have also been reported. 14 While it has recently been suggested that depression may increase the incidence of postconcussion symptoms, 15 little is known regarding the type of postconcussion symptoms experienced. A further exploration of the specific types and severities of postconcussion symptoms linked to the presence of depression would provide a better understanding of the impact of a mood disorder on TBI symptoms.
Factor analyses, using a variety of outcome scales, have been previously performed in TBI populations. A recent study looked at the factors of the Centre for Epidemiologic Studies-Dementia scale in mild-to-moderate TBI and found that the items of this scale compacted into four factors, where mood accounted for two factors (depressed and positive affect) followed by daily tasks functioning (somatic/reduced activity) and social functioning (interpersonal relationships). 8 Another study in a predominantly mild TBI group without a prior diagnosis of depression, performed a factor analysis of the Rivermead Post-Concussion Symptoms Questionnaire (RPCSQ) 16 and found that it formed three factors: cognitive, emotional, and somatic. 17
Our goal was to determine whether patients with post-TBI depression report greater postconcussion symptoms and to characterize the type of postconcussion symptoms reported. We hypothesized that, following factor analysis to define relevant, population-specific postconcussion symptoms clusters, post-TBI subjects with depression would report greater mood impairment and other postconcussion symptoms compared to nondepressed patients. Gaining a better understanding of the differences between these two groups may highlight the effects of depression in the TBI rehabilitation process and provide a rationale for the treatment of depression in conjunction with postconcussion symptoms.
METHODS
Participants
Two hundred seven mild-to-moderate TBI patients were recruited from the Traumatic Brain Injury Clinic at the Sunnybrook Health Sciences Centre from October 2003 to April 2007. Patients were either referred from the emergency department and trauma wards within the hospital (75%) or from external referrals from family physicians (25%). The patients recruited for this study were consecutive referrals who met the inclusion and exclusion criteria for study participation. Informed consent was obtained from all study subjects. The time of assessment was 113.6±92.7 days post-TBI. To be included, patients had to have experienced a mild-to-moderate TBI within 1 year. Patients were excluded from this study if they had a prior traumatic brain injury; a significant acute medical illness; or presence of a premorbid psychiatric diagnosis of schizophrenia, dementia, or bipolar disorder. The severity of the TBI (mild, moderate, or severe) was determined by the study physician, based on the guidelines of The American Congress of Rehabilitation Medicine. 18 Mild TBI is defined by a loss of consciousness that lasted less than 30 minutes, a Glasgow Coma Scale (GCS) at the emergency room score of 13–15, a duration of posttraumatic amnesia lasting less than 24 hours, and negative CT head scan results. Moderate TBI is defined as a GCS score of 9–12 and a posttraumatic amnesia lasting less than 1 week. Patients with indices of GCS and posttraumatic amnesia in the mild range who had focal injuries coded on their head CT were also categorized as moderate. Patients were diagnosed with a major depressive episode in the context of mood disorder secondary to TBI, based on the depression module of the Structured Clinical Interview for DSM-IV (SCID) 19 as administered by the study psychiatrist. The depression group consisted of individuals who were characterized as suffering from a major depression-like episode or depressive features. Individuals who met five of the nine categories of the depression module of the SCID were said to be suffering from a major depression-like episode. Individuals who did not meet the full criteria, but had a predominant mood of “depressed” or answered positive for the first question, “In the last month, has there been a period of time when you were feeling depressed or down most of the day nearly every day (what was that like?). If yes, how long did it last (as long as 2 weeks?)” were said to have depressive features. Information was recorded regarding the TBI, including details of accident, CT head scan results (classified as normal, atrophy, or focal injury), and any other injuries sustained during the accident. Three subjects who were initially classified as having a “moderate” TBI were later reclassified as “severe.” Baseline demographics, which included age, gender, number of years of education and marital status were also recorded.
Assessments
All participants were asked to complete the Rivermead Post-Concussion Symptoms Questionnaire (RPCSQ). The RPCSQ is a 16-item self-reported questionnaire that assesses the severity of common symptoms that occur after a head injury or accident. 16 Questions examine the cognitive, somatic, and emotional symptoms concussion patients may experience following the injury in question. Responses range from 0 (not experienced at all) to 4 (a severe problem) and the maximum score that can be achieved is 64. The SCID was administered alongside the RPCSQ in order to determine if subjects met criteria for a major depressive episode.
Statistical Analysis
To determine the postconcussion symptoms experienced by those with depression, confirmatory factor analysis was conducted using SPSS for Windows, version 14.0. Varimax (orthogonal) rotation was used to generate the resulting three-factor solution. All three factors had eigenvalues greater than 1. Variables with factor loadings equal to or greater than a value of 0.4 were selected into a given model. The factor loadings were generated using the depressed patient group’s RPCSQ scores while nondepressed patients were excluded. Factors were named based on the underlying theme of the variables comprising the factor. Factor scores were generated using a weighted calculation. To test our hypothesis, these factor scores were compared between depressed and nondepressed TBI patients using an independent t-test. Given that comparison of depressed versus nondepressed patients indicated differences by race, the analyses were repeated in Caucasians and non-Caucasian groups separately. Given that three separate analyses were performed, a Bonferroni correction was utilized. A p value 0.017 (≤0.05/3) was considered significant and all tests were two-tailed.
RESULTS
Demographics
Of 207 participants initially consenting, seven depressed patients did not complete the RPCSQ, leaving 200 cases for analysis. Demographic information was recorded and compared between the two study groups (depressed, nondepressed) and there were no significant differences observed in age, gender, and marital status ( Table 1 ). However, race was significantly different between the two groups with Caucasians (Fisher’s exact 11.4, df=1, p<0.01) less likely to be depressed compared to non-Caucasians, while years of education demonstrated a trend (χ 2 , p=0.06).
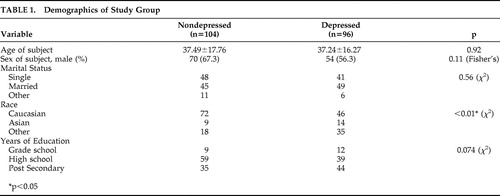 |
Factor Analysis
The 16 items of the RPCSQ formed a three factor model ( Table 2 ). The three factors consisted of mood/cognition, general somatic, and visual somatic. Factor 1: Mood/Cognition contained the variables fatigue, irritability, depression, frustration, forgetfulness, poor concentration, and taking longer to think. Factor 1 had an eigenvalue of 7.29 and it accounted for 45.7% of the variance. Factor 2: General Somatic contained the variables headache, feelings of dizziness, nausea, noise sensitivity, sleep disturbance, fatigue, and restlessness. Factor 2 had an eigenvalue of 1.81 and accounted for 11.29% of the variance. Factor 3: Visual Somatic contained the variables blurred vision, light sensitivity, and double vision. This factor had an eigenvalue of 1.20 and accounted for 7.49% of the variance in our study population.
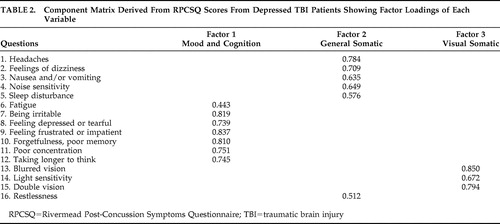 |
Weighted factor scores were generated for each patient and compared between the two study groups ( Table 3 ). The depressed TBI group had higher scores on all three factors of postconcussion symptoms (Bonferonni corrected p<0.017); Factor 1: Mood and Cognition (F=−11.19, df=198, p<0.01), Factor 2: General Somatic (F=−8.476, df=198, p<0.01), and Factor 3: Visual Somatic (F=−6.68, df=198, p<0.01). A breakdown of how the patients answered all 16 questions of the RPCSQ is also summarized in Table 4 .
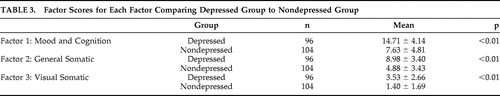 |
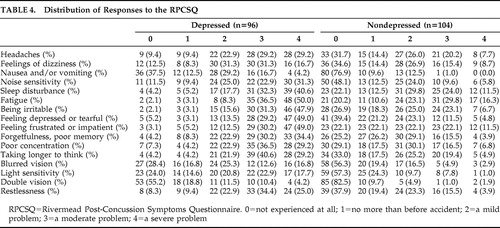 |
Subanalyses by Race
In order to further explore the relationship of race with depression, we looked at depressed compared to nondepressed in the Caucasian and non-Caucasian subpopulations separately. Our subanalysis found that factor scores of all three factor groups remained significantly different between depressed and nondepressed in both Caucasians [Factor 1: Mood and Cognition (F=2.126, df=116, p<0.01), Factor 2: General Somatic (F=0.274, df=116, p<0.01), and Factor 3: Visual Somatic (F=29.8, df=65.9, p<0.01)] and non-Caucasians [Factor 1: Mood and Cognition (F=2.569, df=74, p<0.01), Factor 2: General Somatic (F=1.398, df=74, p<0.01), and Factor 3: Visual Somatic (F=4.265, df=71.86, p<0.01)].
DISCUSSION
The main objective of this study was to determine if depression would influence how subjects reported an array of symptoms experienced post-TBI. The RPCSQ and the three distinct factors found in this study outline the impact of depression on the perception of postconcussion symptoms. We have found that depressed patients scored higher in all factor scores versus nondepressed patients. More interestingly, despite the high prevalence of somatic symptoms linked to mild-to-moderate TBI, somatic symptoms were still reported as more severe by those suffering from depression.
Factor analyses have been performed previously in TBI patients using a variety of validated scales. One such example is the Center for Epidemiologic Studies-Depression scale (CES-D), where four separate factors were found: depressed affect, positive affect, somatic/reduced activity, and interpersonal relationships. A factor analysis of the RPCSQ in a TBI population without a diagnosis of depression found that the 16 questions compacted best into three separate factors: somatic, emotional, and cognitive. 17 The results from our own study were similar to this study, in that we found three distinct factors. However, it must be noted that the individual variables were grouped into mood/cognitive, general somatic and visual somatic symptoms.
Regarding the mood/cognition factor, it is expected that individuals with depression, based on the SCID, would report more severe mood symptoms, which included irritability, feelings of depression or tearfulness, and feelings of frustration or impatience. The RPCSQ depressive symptoms subgroup thus confirms the results of the SCID. The cognitive factor group consisted of poor memory, poor concentration, and longer time to think. The depressed group reported higher impairment with these questions, which is consistent with previous studies: TBI patients with a diagnosis of depression have been observed to perform poorly on cognitive testing when compared to nondepressed patients. 2 , 10 Somatic symptoms have generally been grouped together in previous factor analyses. 8 , 20 However, we found that visual somatic, which dealt with blurred vision, light sensitivity, and double vision, formed its own factor that was separate from the general somatic symptoms. Visual impairment has been previously observed in patients with a moderate-to-severe TBI. 21 , 22 A recent study found that individuals with a mild TBI were more likely to develop, among other illnesses, depression and visual imperceptions relative to healthy comparison subjects. 23 The visual somatic factor scores were significantly different between depressed and nondepressed patients in the present study, indicating that depressed patients reported more severe visual symptoms. Although the visual somatic symptoms experienced by our study group may be considered nominal, a study looking at postoperative cataract patients found an association between visual impairment and depressive symptoms. 24 , 25
General somatic symptoms, which included headaches, dizziness, nausea, noise sensitivity, sleep disturbance, fatigue, and restlessness formed the final factor. Common somatic symptoms observed in post-TBI include headaches, dizziness and nausea, which can occur immediately after the accident and persist for up to 6 months. 26 – 28 Comparing factor scores between the two groups, the depressed group reported greater somatic impairment. This could indicate that the presence of depression may be contributing to the severity of somatic symptoms, which is consistent with previous observations of depressed TBI patients when compared to nondepressed TBI patients. 29 Although RPCSQ scores have been previously compared between depressed and nondepressed TBI patients, this study goes one step further by examining the differences in specific symptom clusters in a larger subject group. The individual items of the RPCSQ were grouped into mood/behavior, general somatic, and visual somatic symptoms and scores were then compared on this basis. These findings suggest that depressed TBI patients report more severe postconcussion symptoms compared to their nondepressed counterparts. Since many postconcussion symptoms overlap with those of depression, depressed TBI patients may be experiencing a cumulative effect of head injury and depressive symptoms, therefore increasing the severity of symptoms reported by the depressed group. It has yet to be determined if this could influence the overall rehabilitation of this patient cohort. Another study found that the presence of secondary depression was associated with medical comorbidities, although this was observed in postmyocardial infarction patients. 30 With regard to the general depressed population, it was found that depressed individuals tend to report greater somatic symptoms. 31 , 32
Focusing on the demographic information of our study sample, race was significantly different between our study groups. It was found that a lower proportion of individuals who identified themselves as Caucasian were susceptible to developing depression compared to the Asian and other subgroups. However, self-reporting of postconcussion symptoms between depressed and nondepressed subjects was similarly greater in those with depression, irrespective of race. The number of years of education displayed a trend between the depressed and nondepressed groups, where individuals with a high school education or less were shown to be less likely to develop depression when compared to individuals with postsecondary education. A previous study has reported the opposite, showing that fewer years of education was a risk factor for the development of depression. 33 These factors may also be important in determining both depressive and other postconcussion symptoms.
Although our findings for this study highlight the differences between depressed and nondepressed TBI patients, some caution must be taken in interpreting our results. A potential limitation of the RPCSQ, a self-report scale, is that patients with depression typically report more severe postconcussion symptoms despite having similar severity of injury as nondepressed patients. 34 Utilizing a scale that would be administered by a trained interviewer or a physician may yield different results. Additionally, the validity of the RPCSQ as a scale has been recently questioned. A review found that the RPCSQ scale had good test-retest reliability and had the best construct reliability as two separate scales, where headache, dizziness, and nausea formed its own subscale. 35 Performing our factor analysis, we have essentially looked at the RPCSQ not as one score, but instead as a group of subscores pertaining to a specific symptom group. A second limitation of this study is the sample size. Although our sample group is 200 patients and is similar in size to other factor analyses, 17 , 36 a larger sample size would be helpful to confirm these results. It is also important to consider that this study was performed in a regional trauma center, and the patient population may differ from a community population. A final limitation to this study is the diagnosis of a mild and moderate TBI. Within our own study, 55% of the study population met criteria for a moderate TBI while 45% were considered mild. To diagnose our study participants, we have used the guidelines put forth by The American Congress of Rehabilitation Medicine. There is some controversy regarding the classification of TBI severity, particularly with the length of posttraumatic amnesia. While we characterized a moderate TBI as a posttraumatic amnesia of 1–7 days, other groups would consider this a severe TBI 37 or have alternatively opted to use only GCS scores. 38 Recently, a new guideline, the Mayo TBI Severity Classification System, considers a posttraumatic amnesia greater than 24 hours a characteristic of a moderate-severe TBI. 39 The variation in guideline use may lead to different conclusions when comparing TBI studies to one another. 40
Despite these limitations, a strength of this study is that there was an objective diagnosis of depression using the well-validated SCID and this has allowed us to determine how depressed patients view their own somatic symptoms compared with nondepressed patients.
In summary, we found that depressed patients scored higher than nondepressed on self-reported mood, cognitive, somatic, and visual postconcussion symptoms. This study emphasizes the need to treat depression concurrently with rehabilitation, and suggests treatment response may improve postconcussion symptoms. This is consistent with other illnesses, where researchers have proposed treating patients for their depression in order to improve symptoms related to the primary illness. 41 By alleviating these depressive symptoms, TBI patients may more quickly regain their ability to lead an independent life style.
1. Thurman DJ, Alverson C, Dunn KA, et al: Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 1999; 14:602–615Google Scholar
2. Rapoport MJ, McCullagh S, Shammi P, et al: Cognitive impairment associated with major depression following mild and moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci 2005; 17:61–65Google Scholar
3. Waraich P, Goldner EM, Somers JM, et al: Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry 2004; 49:124–138Google Scholar
4. Lee H, Kim SW, Kim JM, et al: Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol 2005; 20:97–104Google Scholar
5. Rapoport MJ, Chan F, Lanctot K, et al: An open-label study of citalopram for major depression following traumatic brain injury. J Psychopharmacol 2008; 22:860–864Google Scholar
6. Fann JR, Uomoto JM, Katon WJ: Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 2000; 12:226–232Google Scholar
7. Rowland SM, Lam CS, Leahy B: Use of the Beck Depression Inventory-II (BDI-II) with persons with traumatic brain injury: analysis of factorial structure. Brain Inj 2005; 19:77–83Google Scholar
8. McCauley SR, Pedroza C, Brown SA, et al: Confirmatory factor structure of the center for epidemiologic studies-depression scale (CES-D) in mild-to-moderate traumatic brain injury. Brain Inj 2006; 20:519–527Google Scholar
9. Rapoport MJ, Herrmann N, Shammi P, et al: Outcome after traumatic brain injury sustained in older adulthood: a one-year longitudinal study. Am J Geriatr Psychiatry 2006; 14:456–465Google Scholar
10. Chamelian L, Feinstein A: The effect of major depression on subjective and objective cognitive deficits in mild to moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci 2006; 18:33–38Google Scholar
11. Mazaux JM, Masson F, Levin HS, et al: Long-term neuropsychological outcome and loss of social autonomy after traumatic brain injury. Arch Phys Med Rehabil 1997; 78:1316–1320Google Scholar
12. Ponsford J, Draper K, Schonberger M: Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J Int Neuropsychol Soc 2008; 14:233–242Google Scholar
13. Ingebrigtsen T, Waterloo K, Marup-Jensen S, et al: Quantification of postconcussion symptoms 3 months after minor head injury in 100 consecutive patients. J Neurol 1998; 245:609–612Google Scholar
14. Rees RJ, Bellon ML: Postconcussion syndrome ebb and flow: longitudinal effects and management. NeuroRehabilitation 2007; 22:229–242Google Scholar
15. Suhr JA, Gunstad J: Postconcussive symptom report: the relative influence of head injury and depression. J Clin Exp Neuropsychol 2002; 24:981–993Google Scholar
16. King NS, Crawford S, Wenden FJ, et al: The Rivermead Post-Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995; 242:587–592Google Scholar
17. Potter S, Leigh E, Wade D, et al: The Rivermead Post-Concussion Symptoms Questionnaire: a confirmatory factor analysis. J Neurol 2006; 253:1603–1614Google Scholar
18. Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of Mild Traumatic Brain Injury. J Head Trauma Rehabil 1993; 8:86–87Google Scholar
19. First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0). New York, Biometrics Research Department, NY State Psychiatric Institute, 1996Google Scholar
20. Rhee SH, Petroski GF, Parker JC, et al: A confirmatory factor analysis of the center for epidemiologic studies depression scale in rheumatoid arthritis patients: additional evidence for a four-factor model. Arthritis Care Res 1999; 12:392–400Google Scholar
21. Rutner D, Kapoor N, Ciuffreda KJ, et al: Occurrence of ocular disease in traumatic brain injury in a selected sample: a retrospective analysis. Brain Inj 2006; 20:1079–1086Google Scholar
22. Poggi G, Calori G, Mancarella G, et al: Visual disorders after traumatic brain injury in developmental age. Brain Inj 2000; 14:833–845Google Scholar
23. Vanderploeg RD, Curtiss G, Luis CA, et al: Long-term morbidities following self-reported mild traumatic brain injury. J Clin Exp Neuropsychol 2007; 29:585–598Google Scholar
24. Walker JG, Anstey KJ, Hennessy MP, et al: The impact of cataract surgery on visual functioning, vision-related disability and psychological distress: a randomized controlled trial. Clin Experiment Ophthalmol 2006; 34:734–742Google Scholar
25. Walker JG, Anstey KJ, Lord SR: Psychological distress and visual functioning in relation to vision-related disability in older individuals with cataracts. Br J Health Psychol 2006; 11:303–317Google Scholar
26. Walker WC, Seel RT, Curtiss G, et al: Headache after moderate and severe traumatic brain injury: a longitudinal analysis. Arch Phys Med Rehabil 2005; 86:1793–1800Google Scholar
27. Maskell F, Chiarelli P, Isles R: Dizziness after traumatic brain injury: results from an interview study. Brain Inj 2007; 21:741–752Google Scholar
28. De Kruijk JR, Leffers P, Menheere PP, et al. Prediction of posttraumatic complaints after mild traumatic brain injury: early symptoms and biochemical markers. J Neurol Neurosurg Psychiatry 2002; 73:727–732Google Scholar
29. Rapoport MJ, McCullagh S, Streiner D, et al: The clinical significance of major depression following mild traumatic brain injury. Psychosomatics 2003; 44:31–37Google Scholar
30. Watkins LL, Schneiderman N, Blumenthal JA, et al: Cognitive and somatic symptoms of depression are associated with medical comorbidity in patients after acute myocardial infarction. Am Heart J 2003; 146:48–54Google Scholar
31. Katon W, Sullivan M, Walker E: Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med 2001; 134:917–925Google Scholar
32. Wells KB, Stewart A, Hays RD, et al: The functioning and well-being of depressed patients: results from the medical outcomes study. JAMA 1989; 262:914–919Google Scholar
33. Deb S, Lyons I, Koutzoukis C, et al: Rate of psychiatric illness 1 year after traumatic brain injury. Am J Psychiatry 1999; 156:374–378Google Scholar
34. Fann JR, Katon WJ, Uomoto JM, et al: Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. Am J Psychiatry 1995; 152:1493–1499Google Scholar
35. Eyres S, Carey A, Gilworth G, et al: Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clin Rehabil 2005; 19:878–887Google Scholar
36. de Coster L, Leentjens AF, Lodder J, et al: The sensitivity of somatic symptoms in poststroke depression: a discriminant analytic approach. Int J Geriatr Psychiatry 2005; 20:358–362Google Scholar
37. Rao V, Lyketsos C: Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics 2000; 41:95–103Google Scholar
38. Jacobsson LJ, Westerberg M, Lexell J: Demographics, injury characteristics and outcome of traumatic brain injuries in northern Sweden. Acta Neurol Scand 2007; 116:300–306Google Scholar
39. Malec JF, Brown AW, Leibson CL, et al: The Mayo classification system for traumatic brain injury severity. J Neurotrauma 2007; 24:1417–1424Google Scholar
40. Sherer M, Struchen MA, Yablon SA, et al: Comparison of indices of traumatic brain injury severity: Glasgow Coma Scale, length of coma, posttraumatic amnesia. J Neurol Neurosurg Psychiatry 2007; 79:678–685Google Scholar
41. de Jonge P, Ormel J, van den Brink RH, et al: Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry 2006; 163:138–144Google Scholar



