Social Anxiety in Patients With Parkinson’s Disease
Two studies reported high frequencies of social anxiety in patients with Parkinson’s disease. Stein et al. 15 reported in a small sample (N=16) that 29% of the patients had social anxiety. In the second study, the frequency of social phobia was 11.5% in a large sample of patients with Parkinson’s disease (N=384). 16 Primary or secondary nature of social anxiety was not assessed in these studies. In other words, these studies did not differentiate whether social anxiety was secondary/related to Parkinson’s disease or not. On the other hand, none of the patients with Parkinson’s disease had social anxiety in a comparative study investigating psychiatric morbidity in patients with cervical dystonia and Parkinson’s disease. 17 Since secondary social anxiety in patients with Parkinson’s disease had not been investigated in previous studies, our present study aimed to examine the frequency and severity of social anxiety related to Parkinson’s disease in these patients. The effects of age, gender, education, the severity of Parkinson’s disease, and psychiatric morbidities on social anxiety were also evaluated.
METHODS
The study sample consisted of the first 50 patients with Parkinson’s disease who were consecutively admitted to the Movement Disorders Unit of a university hospital during the years 2005 and 2006. The comparison group (n=50) consisted of age-, sex-, and education-matched volunteers without Parkinson’s disease who were the relatives of clinical staff. The study design was approved by the ethics committee of the university. Written informed consent was obtained from all participants.
Neurological assessments of the patients were carried out by an experienced neurologist (MCA). Parkinson’s disease was diagnosed by United Kingdom Parkinson’s Disease Society Brain Bank Criteria. 18 The severity of parkinsonian symptoms was rated by the Unified Parkinson’s Disease Rating Scale (UPDRS). 19 , 20 In order to evaluate different manifestations of Parkinson’s disease, associated items were summed to obtain subscores. The subscore for bradykinesia is the sum of the scores of the items 23, 24, 25, 26, and 31; for tremor, the sum of items 21 and 22; for postural instability, the sum of items 28 and 30; and for rigidity, the score of item 22. The stage of Parkinson’s disease was determined by Hoehn-Yahr staging, and activities of daily living were assessed by the Schwab-England Activities of Daily Living Scale. 21 All patients were taking dopaminergic agents.
For the diagnosis of psychiatric disorders, the Turkish version of the Structured Clinical Interview for DSM-IV (SCID-I) 22 was administered. DSM-IV social anxiety A–G diagnostic criteria were used for the diagnosis of social anxiety, and the exclusion criterion (criterion H) of social anxiety related to a medical condition was disregarded. In other words, significant social anxiety, including social anxiety related to parkinsonian symptoms, which led to a decrement in social or occupational functioning and caused a certain amount of distress to the patient was considered as “social anxiety disorder.” In patients diagnosed with social anxiety disorder, the temporal relation between the social anxiety and Parkinson’s disease was also examined. The patients were asked about the onset of social anxiety symptoms and Parkinson’s disease as well as their attribution of social anxiety/avoidance to the symptoms of Parkinson’s disease. Therefore, social anxiety related to or secondary to Parkinson’s disease was differentiated.
Severity of social anxiety was measured by the Liebowitz Social Anxiety Scale (LSAS). The LSAS is a 24-item scale consisting of items about social/interactional situations (13 items) and performance situations (11 items). Each item is rated for fear and avoidance on a 4-point Likert scale. Higher scores indicate more severe social anxiety and avoidance. 23 The LSAS is a scale which sensitively and specifically measures the severity of social anxiety and avoidance. Different cutoff scores yielded different sensitivity and specificity values in previous studies, however consistently higher values (sensitivity of 90% and specificity of 88%–100%) were obtained. 23 – 25 The Turkish version of the LSAS was validated by Soykan et al., 26 and this scale was used in previous research investigating social anxiety secondary to physical diseases. 6 , 10 – 12
For the severity of anxiety and depressive symptoms, the Hamilton Depression Rating Scale (HAM-D) (21-item version) and Hamilton Anxiety Rating Scale (HAM-A) were used. Higher scores indicate more severe depression or anxiety. 27 , 28 Turkish versions of both scales have been validated. 29 , 30
Statistical Analysis
Between-group differences were tested by independent samples t test or Mann-Whitney U test, where appropriate. Linear relationships were analyzed by Spearman’s correlation test. The effects of age, severity of depression, and parkinsonian symptoms on the severity of social anxiety were tested by stepwise linear regression analysis. All statistics were carried out by SPSS 13.
RESULTS
Both groups consisted of 30 male and 20 female participants. There was no statistically significant difference between the two groups in terms of age and education. Total HAM-D, HAM-A, and LSAS scores were significantly higher in the patient group ( Table 1 ).
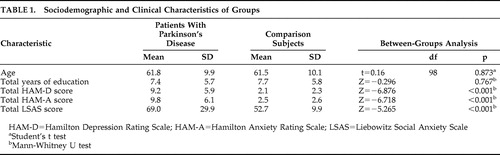 |
Social anxiety disorder was diagnosed in eight patients (16%) with Parkinson’s disease. These patients reported social anxiety related to Parkinson’s disease. None of these eight reported social anxiety disorder before the onset of Parkinson’s disease. Of the patients with Parkinson’s disease, 15 had a major depressive episode and two had comorbid generalized anxiety disorder. Two of the patients with secondary social anxiety also had a diagnosis of major depressive episode. In the comparison group, one patient had social anxiety disorder (2%) and one had a major depressive episode (2%).
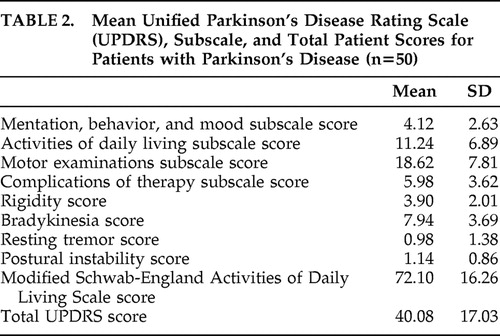
Mean UPDRS subscale and total scores of the patients are given in Table 2 . Forty-eight percent of patients (n=24) were at Hoehn-Yahr stage 2.5, 22% (n=11) were at stage 2, 22% (n=11) were at stage 3, 4% (n=2) were at stage 1, and 4% (n=2) were at stage 4.
 |
Total LSAS scores were negatively correlated with age and positively correlated with total HAM-D patient scores (R=−0.283, p=0.046; R=0.548, p<0.001). Total LSAS scores of the patients were positively correlated with the total UPDRS scores, as well as the subscores of mentation, behavior and mood, activities of daily living, and bradykinesia (R=0.409, p=0.003; R=0.623, p<0.001; R=0.345, p=0.014; R=0.322, p=0.023, respectively). Schwab-England Activities of Daily Living Scale scores were negatively correlated with total LSAS scores (R=−0.306, p=0.03). There was no significant correlation between total LSAS scores and other subscale scores of UPDRS (p>0.05). Education was not correlated with total LSAS scores either. There was no significant difference between male and female patients in terms of total LSAS scores (p>0.05). Also, there was a positive correlation between total UPDRS scores and total HAM-D scores of the patients (R=0.583, p<0.001).
Stepwise linear regression revealed that HAM-D total scores (standardized β=0.411, p=0.001) and age (standardized β=−0.425, p=0.001) predicted the severity of social anxiety, and this model explained 59.5% of the variance, while the total UPDRS score was not in the equation.
DISCUSSION
Consistent with previous research, we found a high frequency of social anxiety in patients with Parkinson’s disease. Current literature on social anxiety related to medical conditions clearly demonstrates the importance of this condition, since the rates reported in the present study, as well as in previous studies, are considerably higher than the rates of primary social anxiety disorder in the general population. 31 , 32
In a comparison study, we reported high frequencies of secondary social anxiety in patients with hyperkinetic movement disorders like essential tremor (30%), cervical dystonia (30%), and hemifacial spasm (20%). 11
In contrast with the classical notion that patients with Parkinson’s disease often avoid public situations so that their symptoms like tremor and dyskinesias will not be noticed, 33 the severity of social anxiety was not associated with the severity of Parkinson’s disease symptoms. Although there was positive correlation between UPDRS scores and LSAS patient scores, regression analysis revealed that only HAM-D scores and age were associated with LSAS scores. This discrepancy may be due to the fact that UPDRS patient scores were also positively correlated with HAM-D scores. In our previous study, the severity of social anxiety was not associated with the severity of the hyperkinesias either. In both of our studies severity was associated with younger age and depression. 11 Yolac Yarpuz et al. 12 also reported higher social anxiety and depression in patients with acne. Similarly, in acne patients social anxiety was negatively correlated with age, but it was not correlated with the severity of acne.
This study has some strengths and limitations. Social anxiety was investigated in a large sample of patients with Parkinson’s disease and compared with a matched comparison group. Moreover, this is the first study which specifically investigates social anxiety related to Parkinson’s disease. Since social anxiety was associated with depressive symptoms, the presence of depressive symptoms/major depressive disorder may have inflated the rate of secondary social anxiety disorder in this sample. On the other hand, our sample mainly consisted of patients with a mean age of 61 years old; therefore, the observed rate in the present study may be lower than the rate of social anxiety in patients with early-onset Parkinson’s disease (onset before the age of 40). In addition to that, most of the patients in this study had mild–moderate Parkinson’s disease, so the frequency of disease-related social anxiety might be lower than in severe forms of the disease. We did not examine the duration of Parkinson’s disease and disease-related disability which might affect the development of social anxiety. Although the duration of hyperkinesias or the disability level were not associated with the severity of social anxiety in our previous study, 11 both of these factors should be controlled in future studies. Furthermore, our comparison group consisted of healthy volunteers without any disabling medical illness; therefore, future studies may also compare social anxiety in Parkinson’s disease with social anxiety in other chronic medical illnesses.
Although clinical research on social anxiety in Parkinson’s disease patients is scarce, there is also neurobiological data which points out a common possible hypodopaminergic etiology in social anxiety and Parkinson’s disease. Striatal dopamine reuptake and D 2 receptor binding were lower in patients with social anxiety. 34 – 36 However, a recent study by Schneier et al. 37 found no difference between patients with generalized social anxiety (n=17) and healthy comparison subjects (n=13) in terms of striatal D 2 receptor or dopamine transporter availability. Generalizability of the findings in these studies is limited due to small sample sizes; therefore, future studies should investigate the possible biological link between the two diseases in larger samples.
Management of social anxiety and depressive coping style in these patients is necessary adjunct to the treatment of the primary disease since social anxiety is not associated with the objective severity of the disease. Therefore, neurologists dealing with these patients should be aware of social anxiety related to movement disorders and refer them to psychiatrists. Heinrichs et al. 38 reported a successful cognitive behavior therapy with a patient with Parkinson’s disease who also had social anxiety. However, there is no study investigating the treatment of social anxiety related to Parkinson’s disease or other medical conditions.
Since social anxiety in the context of a disability or disfigurement may present with symptoms similar in nature and severity to the social anxiety disorder defined in DSM-IV-TR, symptoms can be managed and treated the same way as in cases of “primary” social anxiety disorder. Revision of the criteria for social anxiety disorder in future diagnostic systems, such as DSM-V based on current evidence, is necessary. For example, exclusion of criterion H might be considered in the future DSM definition of the disorder. This would provide detection and management of these symptoms, as well as a basis for future research.
1. Reijnders JS, Ehrt U, Weber WE, et al: A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord 2008; 23:183–189Google Scholar
2. Lauterbach EC: The neuropsychiatry of Parkinson’s disease and related disorders. Psychiatr Clin North Am 2004; 27:801–825Google Scholar
3. Nuti A, Ceravolo R, Piccinni A, et al: Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur J Neurol 2004; 11:315–320Google Scholar
4. Oberlander EL, Schneier FR, Liebowitz MR: Physical disability and social phobia. J Clin Psychopharmacol 1994; 14:136–143Google Scholar
5. Stein MB, Baird A, Walker JR: Social phobia in adults with stuttering. Am J Psychiatry 1996; 153:278–280Google Scholar
6. Devrimci-Özgüven H, Kudakçi N, Boyvat A: Secondary social anxiety in patients with psoriasis. Turk Psikiyatri Derg 2000; 11:121–126Google Scholar
7. Gündel H, Wolf A, Xidara V, et al: Social phobia in spasmodic torticollis. J Neurol Neurosurg Psychiatry 2001; 71:499–504Google Scholar
8. Schneier FR, Barnes LF, Albert SM, et al: Characteristics of social phobia among persons with essential tremor. J Clin Psychiatry 2001; 62:367–372Google Scholar
9. Rosik CH: Psychiatric symptoms among prospective bariatric surgery patients: rates of prevalence and their relation to social desirability, pursuit of surgery, and follow-up attendance. Obes Surg 2005; 15:677–683Google Scholar
10. Topcuoglu V, Bez Y, Sahin Bicer D, et al: Social phobia in essential tremor. Turk Psikiyatri Derg 2006; 17:93–100Google Scholar
11. Ozel-Kizil ET, Akbostanci MC, Ozguven HD, et al: Secondary social anxiety in hyperkinesias. Mov Disord 2008; 23:641–645Google Scholar
12. Yolaç Yarpuz A, Demirci Saadet E, Erdi Sanli H, et al: Social anxiety in acne vulgaris patients and relationship with clinical variables. Turk Psikiyatri Derg 2008; 19:29–37Google Scholar
13. Ozel-Kizil ET: Social anxiety secondary to a general medical condition, in Social Phobia: Etiology, Diagnosis, and Treatment. Edited by Axelby CP. New York, Nova Publishers, 2009, pp 261–268Google Scholar
14. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
15. Stein MB, Heuser IJ, Juncos JL, et al: Anxiety disorders in patients with Parkinson’s disease. Am J Psychiatry 1990; 147:217–220Google Scholar
16. de Rijk C, Bijl RV: Prevalence of mental disorders in persons with Parkinson’s disease. Ned Tijdschr Geneeskd 1998; 142:27–31Google Scholar
17. Lauterbach EC, Freeman A, Vogel RL: Differential DSM-III psychiatric disorder prevalence profiles in dystonia and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2004; 16:29–36Google Scholar
18. Hughes AJ, Daniel SE, Kilford L, et al: Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184Google Scholar
19. Stern MB: The clinical characteristics of Parkinson’s disease and Parkinsonian syndromes: a diagnosis and assessment, in The Comprehensive Management of Parkinson’s Disease. Edited by Stern MB, Hurting HI. New York, PMA Publishing, 1988, pp 3–50Google Scholar
20. Akbostancı MC, Balaban H, Atbaşoğlu C: Interrater reliability study of motor examination subscale of UPDRS and AIMS. Parkinson Hast Hareket Boz Der 2000; 3:7–13Google Scholar
21. Akbostanci MC, Kocatürk PA, Tan FU, et al: Erythrocyte superoxide dismutase activity differs in clinical subgroups of Parkinson’s disease patients. Acta Neurol Belg 2001; 101:180–183Google Scholar
22. Corapcioglu A, Aydemir O, Yildiz M, et al: DSM-IV eksen I bozuklukları için yapılandırılmış klinik görüşme, SCID-I, Klinik Versiyon. Hekimler Yayın Birliği, Ankara, 1999Google Scholar
23. Heimberg RG, Horner KJ, Juster HR, et al: Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med 1999; 29:199–212Google Scholar
24. Rytwinski NK, Fresco DM, Heimberg RG, et al: Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depress Anxiety 2009; 26:34–38Google Scholar
25. Mennin DS, Fresco DM, Heimberg RG, et al: Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. J Anxiety Disord 2002; 16:661–673Google Scholar
26. Soykan C, Ozguven HD, Gencoz T: Liebowitz Social Anxiety Scale: the Turkish version. Psychol Rep 2003; 93(part 2):1059–1069Google Scholar
27. Williams BW: A structured interview guide for Hamilton Depression Rating Scale. Arch Gen Psychiatr 1978; 45:742–747Google Scholar
28. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Google Scholar
29. Akdemir A, Orsel S, Dag I, et al: The reliability and validity of Turkish version of Hamilton Depression Rating Scale. Compr Psychiatry 2001; 42:161–165Google Scholar
30. Yazıcı MK, Demir B, Tanrıverdi N, et al: Hamilton Anxiety Rating Scale: interrater reliability and validity study. Türk Psikiyatri Derg 1998; 9:114–117Google Scholar
31. Wacker HR, Müllejans R, Klein KH, et al: Identification of cases of anxiety disorders and affective disorders in the community according to ICD-10 and DSM-III-R by using the Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 1992; 2:91–100Google Scholar
32. Magee WJ, Eaton WW, Wittchen HU, et al: Agoraphobia, simple phobia and social phobia in the national comorbidity survey. Arch Gen Psychiatry 1996; 53:159–168Google Scholar
33. Weintraub D, Stern B: Disorders of mood and affect in Parkinson’s disease, in Handbook of Clinical Neurology: Parkinson’s Disease and Related Disorders Part I. Edited by Koller W, Melamed E. Edinburgh, Elsevier, 2007, pp 421–433Google Scholar
34. Tiihonen J, Kuikka J, Bergström K, et al: Dopamine reuptake site densities in patients with social phobia. Am J Psychiatry 1997; 154:239–242Google Scholar
35. Schneier FR, Liebowitz MR, Abi-Dargham A, et al: Low dopamine D 2 receptor binding potential in social phobia. Am J Psychiatry 2000; 157:457–459 Google Scholar
36. Schneier FR, Martinez D, Abi-Dargham A, et al: Striatal dopamine D 2 receptor availability in OCD with and without comorbid social anxiety disorder: preliminary findings. Depress Anxiety 2008; 25:1–7 Google Scholar
37. Schneier FR, Abi-Dargham A, Martinez D, et al: Dopamine transporters, D 2 receptors, and dopamine release in generalized social anxiety disorder. Depress Anxiety 2009; 26:411–418 Google Scholar
38. Heinrichs N, Hoffman EC, Hofmann SG: Cognitive-behavioral treatment for social phobia in Parkinson’s disease: a single-case study. Cogn Behav Pract 2001; 8:328–335Google Scholar



