Utility of the Clock Drawing Test in the Assessment of Catatonia
Catatonia—a neuropsychiatric syndrome first described by Kahlbaum in 18741—involves an underlying cognitive impairment that can be difficult to appreciate in the presence of unusual behavior and abnormal movements.2,3 When catatonia is suspected, the peculiar behaviors and motoric abnormalities are systematically examined, for which standardized rating scales exist. The most widely used assessment measure is the Bush-Francis Catatonia Rating Scale (BFCRS),4 a 23-item scale that includes a 14-item screening instrument typically used to assess and rate the severity of catatonia. Thereafter, a response to the lorazepam challenge test is observed. Brief clinical improvement of symptoms 5–10 minutes postintravenous administration of lorazepam is considered to be a positive response and consistent with a diagnosis of catatonia.3,5,6 However, routine evaluation of catatonia does not always include screening for cognitive dysfunction.
The Clock Drawing Test (CDT) can illustratively provide a window into the cognitive dysfunction in catatonia.7 The CDT is an ideal test when lengthy neuropsychological evaluation is not possible, given its effectiveness, acceptability, and fast and easy administration.8 In fact, the CDT has been used for more than 100 years, dating back to 1915, and has become one of the most widely utilized cognitive screening instruments.8 Despite its simplicity, the CDT can be used to assess many cognitive domains, including language comprehension, visuospatial abilities, reconstruction skills, concentration, numerical knowledge, visual memory, and executive function.9 To date, it is an important test for detecting cognitive dysfunction in Alzheimer’s disease and other dementias10 as well as a broad range of brain disorders, including, but not limited to, acute stroke,11 traumatic brain injury,12 delirium,13 Huntington’s disease,14 Parkinson’s disease,10 multiple sclerosis,15 bipolar disorder,16 and schizophrenia.17
We present a case that pictorially documents the dramatic response that can be observed with a positive lorazepam challenge test. To our knowledge, there are no previous reports of the CDT in the evaluation of catatonia. This case broadens the clinical approach to addressing the cognitive disturbances observed in catatonia and offers proposed networks of dysfunction associated with this syndrome. Furthermore, we aimed to demonstrate that the CDT could be an integral part of the initial assessment of all cases of suspected catatonia, be used to obtain quantitative information regarding response to treatment, and be used as a form of face-valid data to facilitate communication with persons who are not familiar with catatonia.
Case Report
A man in his late 40s with a history of type 2 diabetes mellitus, cardiovascular disease, myocardial infarction, and a stage 2 sacral decubitus ulcer as well as a long-standing history of bipolar disorder presented with a change in mental status. The patient’s family reported that 2 days before his hospital admission, he became confused, demonstrated child-like behavior, and believed that he was dead. He repeated nonsensical phrases, such as “I have no eyes” and “my brain is yellow.” He had been psychiatrically stable for the past 5 years and employed as a manager of a fast food chain. He was treatment compliant with asenapine (10 mg/day), which he had been taking for bipolar disorder before his hospital admission. His clinical presentation on admission was different from his presentation in previous episodes of mania and psychosis, which last occurred more than 14 years ago. The patient had no history of alcohol abuse or illicit drug use. His family history was negative for psychiatric and neurologic conditions. He was admitted to the internal medicine service with a diagnosis of delirium due to multiple etiologies, and psychiatry was consulted for evaluation and management of the delirium.
On examination, the patient was initially found hiding under the sheets because the sound of a nearby helicopter had frightened him. He appeared to be confused but was very alert. He had episodes of unexplained laughter and excitement in response to some of the hospital staff. He exhibited verbigeration and repeated a variety of phrases about himself being dead. He then became mute and stared intensely with decreased blinking. The patient’s motor examination revealed waxy flexibility, automatic obedience, and stereotypies. His tone and reflexes were normal. His BFCRS score was 16. He was asked to draw a clock in this state (Figure 1A). The patient was diagnosed with catatonia, and all neuroleptics were discontinued. A lorazepam challenge with administration of 2 mg intravenously resulted in immediate response and temporary relief of the patient’s catatonic symptoms and signs. His BFCRS score decreased from 16 to 0 in less than 10 minutes. He was asked to draw a clock again and to set the time to “10 past eleven.” The patient’s improvement in his clock-drawing ability, which occurred concurrently with rapid resolution of his catatonia, is shown in Figure 1. His Cotard delusion also rapidly resolved, and he reported feeling as though he had died and “came back to life.” He correctly recalled all of the events leading up to his admission. The patient’s family, at his bedside, was impressed with the dramatic dissolution of his catatonia and improvement in his clock-drawing abilities.
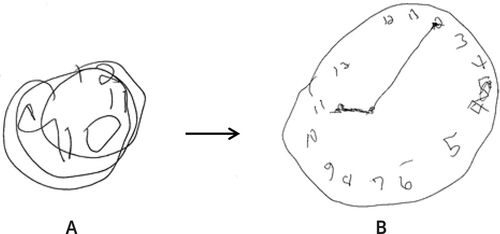
FIGURE 1. Cognitive Impairments and Resolution of Catatonic Symptom Severity as Demonstrated Using the Clock Drawing Testa
a Panel A shows a clock drawn before lorazepam challenge. Panel B shows a clock drawn 10 minutes after intravenous administration of lorazepam (2 mg).
Laboratory investigations revealed that the patient had mild iron deficiency anemia. His comprehensive metabolic panel, thyroid-stimulating hormone, vitamin B12, folate, ammonia, and creatine kinase levels were all within normal limits. His cardiac enzymes were normal, and an EKG showed a previous infarct. His antinuclear antibody level was elevated at 640 (reference, 0–80), and his antidsDNA antibody level was mildly elevated at 20 (reference <10). A lumbar puncture with limbic encephalitis panel (including anti-N-methyl-d-aspartate receptor antibodies) was unremarkable. A brain MRI showed mild nonspecific white matter disease consistent with chronic small vessel ischemia. An EEG was absent of any encephalopathy or epileptiform abnormalities. Over the next few days, the patient’s catatonia resolved with scheduled oral lorazepam (14 mg/day), but an underlying psychosis became apparent. He reported ideas of reference from the television and persecutory delusions. He was started on oral quetiapine, which was titrated to 600 mg daily. About a week later, his psychotic symptoms improved, and he was discharged to a partial hospitalization and intensive outpatient program.
Discussion
The above case is an important example of the utility of the CDT in evaluating catatonia and suggests that it could easily be used in conjunction with the lorazepam challenge test. Additionally, the patient’s clock drawing provided face-valid data demonstrating the severity of cognitive impairment during his catatonic episode and helped to reduce any stigma around this poorly understood1 and underdiagnosed syndrome.18 The patient’s family and non-neuropsychiatric physicians were able to appreciate the profound cognitive impairments exemplified in Figure 1A, which helped to aid in their understanding that this syndrome requires a diagnostic workup to evaluate for general medical etiologies.
Although common signs of catatonia include mutism, posturing, and rigidity,2,4 many patients with catatonia speak and move about and are capable of performing the tasks required for clock drawing. Our patient performed a freehand drawing, using a standard, blank letter-sized sheet of paper and a pen. “Ten past eleven” was chosen for the time setting instruction in both clocks presented in Figure 1. This requires placement of the hands in both visual fields (to identify hemilateral neglect or hemianopia) and places a greater demand on executive function mediated by the frontal lobes.9 The latter can be appreciated when the CDT shows a stimulus-bound response error—for example, a frontal “pull” occurs when the patient hears the time setting instructions and incorrectly draws the minute hand toward the stimulus “10” instead of recoding it to point toward “2”19. Conversely, it is worth noting that valuable information could be obtained even if the test result is normal and absent of any errors. The CDT correlates highly with other tests of cognitive functions, such as the Mini-Mental State Examination,9 and in some studies has shown very high sensitivity (0.92) and negative predictive value (0.95).20 Thus, a normal result can be used to rule out significant cognitive impairment with a fairly high degree of confidence.
The neuroanatomy of catatonia is not fully understood; however, the majority of neuroimaging studies have pointed to dysfunction in the frontal and parietal cortices.2,3 A neuropsychological study of 13 patients with catatonia found visual-spatial deficits related to attentional abilities and right parietal function, suggesting attentional motor and frontoparietal dysfunction.21 This impairment in executive planning and visuospatial construction is shown in Figure 1A. Similarly, these clock-drawing errors suggest a neuroanatomical relationship to dysfunctional networks in the frontal and parietal lobes.19 It is essential to note that the observable change seen in our patient’s performance on the CDT occurred in the context of lorazepam pharmacotherapy; a study examining lorazepam administration in patients with catatonia who were successfully treated reported a decrease in orbitofrontal and ventromedial prefrontal cortex hyperactivation, leading to a regularization of orbitofrontal activity.22 This would explain the most profound qualitative error, perseveration of the clock face, as shown in Figure 1A, which is classically attributed to frontal systems deficits.19 Although gross distortions on the CDT can be easily identified without using standardized scoring criteria, it is important to consider quantitative and qualitative scoring systems in order to optimally interpret the test. The Clock-Drawing Interpretation Scale (CDIS) is a well-known scoring system, with lower scores representing greater cognitive dysfunction.23 Improvement in cognition (CDIS score increase from 6 to 15) in our patient that occurred in parallel to the resolution of catatonic symptom severity (BFCRS score decrease from 16 to 0) is shown in Figure 1B. This correlation implies that greater catatonic severity is associated with greater cognitive dysfunction. Further studies are needed to determine whether the CDT would be sensitive to the clinical improvement in cognition with interventions such as lorazepam therapy and to analyze whether errors on the CDT are related to severity of catatonia.
Patients with catatonia often describe experiencing intense emotions, mostly fear, in the presence of a flattened affect and impaired volition.24 This state of catatonia has been postulated to be an evolutionary “scared stiff” fear response.25 This phenomenon may be secondary to malfunction of the orbitofrontal cortex, which has reciprocal connections with the amygdala. 22 Decreased inhibition of affective stimuli results in uncontrolled and intense fearfulness.22 Indeed, surveys have found that up to 15% of catatonic patients thought that they were already dead or going to die.3 It is noteworthy that the patient in our case developed Cotard delusion as an initial manifestation of his catatonia, and this was the first clue to his family that this presentation was unlike his previous manic-psychotic episodes. Although the pathophysiology of Cotard delusion is not known, damage to the frontal lobes has similarly been attributed to its development.26 This serves as a reminder that even in cases of idiopathic psychiatric disorders, the presentation of a delusional misidentification syndrome, such as Cotard delusion, should raise suspicion for underlying neuropsychiatric conditions, including catatonia.26
1 : Catatonia is a systemic medical syndrome. Acta Psychiatr Scand 2016; 133:250–251Crossref, Medline, Google Scholar
2 : Catatonia. CNS Spectr 2016; 21:341–348Crossref, Medline, Google Scholar
3 : Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci 2009; 21:371–380Link, Google Scholar
4 : Catatonia, I: rating scale and standardized examination. Acta Psychiatr Scand 1996; 93:129–136Crossref, Medline, Google Scholar
5 : The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry 2009; 66:1173–1177Crossref, Medline, Google Scholar
6 : Catatonia, II: treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand 1996; 93:137–143Crossref, Medline, Google Scholar
7 : Refractory catatonia due to N-methyl-D-aspartate receptor encephalitis responsive to electroconvulsive therapy: the clinical use of the Clock Drawing Test. J ECT 2017; 33:223–224Crossref, Medline, Google Scholar
8 : The test of time: a history of clock drawing. Int J Geriatr Psychiatry 2018; 33:e22–e30Crossref, Medline, Google Scholar
9 : Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 2000; 15:548–561Crossref, Medline, Google Scholar
10 : Discrimination of dementia with Lewy bodies from Alzheimer disease and Parkinson disease using the Clock Drawing Test. Cogn Behav Neurol 2003; 16:85–92Crossref, Medline, Google Scholar
11 :Clock Drawing Test in acute stroke and its relationship with long-term functional and cognitive outcomes. Clin Neuropsychol 2018 (Epub ahead of print, July 9, 2018)Crossref, Medline, Google Scholar
12 : Getting clocked: screening for TBI-related cognitive impairment with the clock drawing test. Brain Inj 2017; 31:1501–1506Crossref, Medline, Google Scholar
13 : Clocking delirium: the value of the Clock Drawing Test with case illustrations. Am J Hosp Palliat Care 2008; 25:385–388Crossref, Medline, Google Scholar
14 : Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn 1992; 18:70–87Crossref, Medline, Google Scholar
15 : Screening for early cognitive impairment in multiple sclerosis patients using the clock drawing test. J Clin Neurosci 2002; 9:629–632Crossref, Medline, Google Scholar
16 : The use of the Clock Drawing Test in bipolar disorder with or without dementia of Alzheimer’s type. Arq Neuropsiquiatr 2014; 72:913–918Crossref, Medline, Google Scholar
17 : Clock Drawing Test in patients with schizophrenia. Psychiatry Res 2004; 121:229–238Crossref, Medline, Google Scholar
18 : Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci 2018; 30:145–151Google Scholar
19 : The Clock Drawing task: common errors and functional neuroanatomy. J Neuropsychiatry Clin Neurosci 2012; 24:260–265Link, Google Scholar
20 : Use of the Clock-Drawing Test in a hospice population. Palliat Med 2007; 21:559–565Crossref, Medline, Google Scholar
21 : Impairment in visual-spatial function in catatonia: a neuropsychological investigation. Schizophr Res 1999; 37:133–147Crossref, Medline, Google Scholar
22 : Lorazepam modulates orbitofrontal signal changes during emotional processing in catatonia. Hum Psychopharmacol 2010; 25:55–62Crossref, Medline, Google Scholar
23 : Development of scoring criteria for the Clock Drawing task in Alzheimer’s disease. J Am Geriatr Soc 1992; 40:1095–1099Crossref, Medline, Google Scholar
24 : What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci 2002; 25:555–577, discussion 578–604Crossref, Medline, Google Scholar
25 : “Scared stiff”: catatonia as an evolutionary-based fear response. Psychol Rev 2004; 111:984–1002Crossref, Medline, Google Scholar
26 : A neuropsychiatric analysis of the Cotard delusion. J Neuropsychiatry Clin Neurosci 2018; 30:58–65Link, Google Scholar



