The Patient Experience of Recovery Following Anti-NMDA Receptor Encephalitis: A Qualitative Content Analysis
Abstract
Objective:
The authors examined patients’ perceptions of the factors affecting their recovery from anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis, which is a rare, severe immune-mediated neurological disorder.
Methods:
Seven patients completed semistructured interviews exploring their experience of recovery. Participants were interviewed between 7 and 41 months after the initiation of treatment. Interviews were transcribed and subjected to qualitative content analysis.
Results:
Facilitators of recovery included the presence of a support system and treatment-related factors. Barriers to recovery included perceived psychiatric stigma, insufficient illness education, and lifestyle disruptions to accommodate ongoing treatment. Adverse physical, psychological, and neurocognitive sequelae of anti-NMDAR encephalitis continued to affect participants’ daily functioning. Most participants described strategies to manage neurocognitive deficits, fatigue, and anxiety.
Conclusions:
Anti-NMDAR encephalitis contributes to persistent burden on patients, their families, and health services after the resolution of acute symptoms. Physical, psychological, and cognitive changes contribute to long-term disease morbidity. To optimize recovery and reduce disability, further attention must be directed toward illness education, reducing stigma, and role disruption. Longer-term disability support may benefit those who do not fully recover.
Anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis is a severe but treatable autoimmune-mediated disorder of the CNS (1, 2). There is a female predominance, typically younger age distribution, and an association with tumors, mostly ovarian teratomas (1, 3). More than 90% of patients with anti-NMDAR encephalitis present acutely with psychiatric and neurocognitive symptoms, such as agitation, psychosis, and confusion, and within 1–2 weeks exhibit neurological deterioration, with movement disorders, seizures, and autonomic dysfunction (2, 3). The severity and range of symptoms often necessitate polypharmacy, intensive care support, and multidisciplinary team involvement (4–8). Although immunosuppression and tumor removal, where appropriate, are reported to result in complete to near-complete neurological recovery in the majority of patients (7, 9), longer-term, persistent disability may be underestimated.
There are increasing reports of persistent psychosocial, psychiatric, and cognitive impairments following treatment for anti-NMDAR encephalitis (4, 8, 10–13). Hospital admissions for this disorder are often protracted over many months, with long rehabilitative phases (4, 5, 7). Neurocognitive deficits in processing speed, episodic memory, and executive functioning are thought to be particularly common (11–14). Recovering patients report disruption to employment and education, as well as reduction in independence and confidence and loss of community connection (4, 13). Additionally, an estimated 12%− 25% of patients experience a relapsing and remitting disease course that is most often represented by psychiatric and cognitive symptoms (7, 15–17). These psychosocial, psychiatric, and cognitive impairments are minimally captured by screening tools such as the modified Rankin Scale (mRS) (18, 19), which have been used to quantitatively describe the outcomes for anti-NMDAR encephalitis (7, 17, 20).
Qualitative research techniques provide an opportunity to understand the experiences of people affected by rare disorders, as well as to inform the benefits and gaps in care for this patient population (21, 22). In the present study, we aimed to develop an understanding of the patient experience of recovery following resolution of the acute phase of anti-NMDAR encephalitis. Qualitative analysis of semistructured patient interviews was undertaken to develop a broad-based understanding of patients’ perspectives and lived experience (23). We used an interview schedule that was focused on participants’ experiences of recovery and the accommodations that they made in their lives following the onset of the condition.
Methods
Here, we report on the qualitative component of a mixed-methods study examining prognostic factors following anti-NMDAR encephalitis in seven patients (females, N=6; males, N=1). Ethical clearance was provided by the Human Research Ethics Committees of the Royal Brisbane and Women’s Hospital and the University of Queensland, and participation followed provision of informed consent. Semistructured interviews (24) were used to examine participant experiences using a framework-analysis approach (25–27). A companion quantitative analysis examining the cognitive and social functioning of patients in this same cohort is presented elsewhere (13).
Participants
A convenience sample comprised of patients with known CSF anti-NMDAR antibody-positive encephalitis was recruited via Queensland-based physicians in 2015. Inclusion criteria were ages 15–50 years, previous or current diagnosis of anti-NMDAR encephalitis, fluency in the English language, absence of previous significant head injuries unrelated to encephalitis, and absence of uncorrected hearing and visual impairments. All eligible individuals agreed to participate in the study.
Interview Process
The interviews comprised the initial 30–45 minutes of an approximately 4-hour neuropsychological assessment battery administered in a nonclinical university setting. All participants received an $80 (Australian dollars) gift card as reimbursement for expenses incurred associated with their participation.
The semistructured interviews were conducted by a psychologist (G.M.), who was at the time a provisionally registered psychologist and had not been involved in the delivery of care for the participants. The interview schedule was developed by a psychologist (G.M.) and a psychiatrist (J.S.) and focused on two questions: “Please tell me about your recovery from encephalitis,” and “How have you adjusted to accommodate your recovery?” (see also the online supplement). After the interview, participants were asked a single closed question: “How would you rate your recovery on a scale of 1–10 (1=worst, 10=best)?” All interviews were audio-recorded, transcribed, and then deidentified by a psychologist (G.M.). The same psychologist maintained field notes during the interviews to assist transcript interpretation. No adaptions to the interview schedule occurred as the study progressed.
Analytic Strategy
Descriptive data about participants, including demographic and treatment information, are reported to provide contextual information about the study sample. NVivo qualitative analysis software (28) was used to facilitate the analysis of interview transcripts. The framework method of qualitative analysis was chosen because it provides a flexible structure to support concurrent data collection and analysis (26). This approach allowed predetermined research questions to deductively focus the interview, while maintaining flexibility to explore matters arising during data collection (inductively). Experts adhered to and carried out the following stages outlined by Gale et al. (25): verbatim transcription of interviews (G.M.); familiarization with each interview as a whole (G.M. and S.P.); deductive coding on the basis of the research questions (pertaining to recovery, symptoms, impairments, and accommodations) and inductive coding of subordinate themes (S.P.); joint development of a working analytic framework (G.M. and S.P.); application of the analytic framework to the transcripts (S.P.); charting data in the framework matrix (G.M. and S.P.); and interpreting the data (G.M., N.W., S.P.), including the identification of relationships between themes (G.M., N.W., and S.P.).
In presenting the qualitative data, quantitative information about the frequency of codes is reported. This reflects a deviation from a purist approach to framework analysis and qualitative analysis generally (26). However, we adhered to conventions associated with this deviation (29). In reporting the results, extracts from transcripts best illustrating key themes were chosen and agreed upon by the authors. Quantifiers were used to indicate the number of participants referring to a given coding element as follows: none (N=0), some (N=1–3), most (N=4–6), and all (N=7).
Results
Seven patients were identified (Table 1), with a mean age of 26.4 years (SD=8.54 [range, 16–37 years]), an estimated premorbid IQ of 98.42 (SD=8.26), and an average duration of formal education of 13.5 years (SD=1.97). All except one of the seven participants were female. Four participants (patient 2, patient 3, patient 6, and patient 7) received immunotherapy within 1 month of symptom onset and had not experienced a subsequent relapse. For the remaining three participants (patient 1, patient 4, and patient 5), the estimated time from symptom onset to treatment with immunotherapy ranged from 6 to 20 years. During this treatment delay period, each of these three participants experienced psychiatric admissions and treatment, including ECT. The perceived facilitators of and impediments to recovery during acute and subacute hospital care are summarized in Table 2.
| Immunomodulation therapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age at diagnosis (years) | Sex | Self-reported recoveryb | Tumor identified | Surgery | Months since initiation | Months since completion | Modified Rankin Scalec | Psychiatric admission | ECT |
| P1 | 37 | Female | 5 | No | No | 41 | — | 3 | Yes | Yes |
| P2 | 19 | Female | 6 | No | No | 31 | 31 | 1 | No | No |
| P3 | 28 | Female | 7.5 | Yes | Yes | 12 | 9 | 2 | No | No |
| P4 | 19 | Male | 8 | Possible | Yes | 14 | — | 2 | Yes | Yes |
| P5 | 36 | Female | 7 | No | No | 19 | — | 1 | Yes | Yes |
| P6 | 30 | Female | 8 | No | No | 38 | 35 | 1 | No | No |
| P7 | 16 | Female | 8 | Yes | Yes | 7 | 4 | 2 | No | No |
TABLE 1. Demographic and clinical characteristics of seven patients with between 7 and 41 months after initiation of treatmenta
| Recoveryb and focus | N | Factor | N | Illustrative transcript extract(s) |
|---|---|---|---|---|
| Facilitator | ||||
| Support | 5 | Presence of family and friends | 3 | You realize who your true friends are. They’re the ones who can actually look at you in a state in [the] hospital and just go, “She’s in there somewhere.” I’ve got a really good friend who came up and saw me in the hospital. Apparently, she was one of the only people that I actually responded to. (Patient 1) |
| My partner—he was great. He came to see me every day. When I got really sick, they let him stay, and he stayed with me in the hospital for like a week, sleeping on the chairs next to me. (Patient 3) | ||||
| Staff kindness | 2 | I don’t remember the doctors or nurses helping out with things like spelling or speech. They were all just really kind, and that helped. (Patient 6) | ||
| I could tell that they cared so much and that they looked after me. They’d sit down with me and talk to me; they’d braid my hair. They were amazing. I love them. (Patient 5) | ||||
| Treatment | 4 | Cognitive remediation | 2 | Doing the computer programs was really helpful. (Patient 1) |
| Relearning skills | 2 | Getting me to physically do things—something as simple as walking up the stairs. They did training with me for walking up and down stairs. (Patient 1) | ||
| Diagnosis and education | 1 | The doctor said, “No,” you have not got a mental illness. You have got an autoimmune, neurological disease. Don’t ever class yourself as mentally ill.” So that reassured me. (Patient 1) | ||
| Medication | 1 | My medication. (Patient 4)c | ||
| Surgery | 1 | I got my thymus cut out. It really hurt…for weeks and months I was in so much pain. They thought that was what the antibodies were getting made from…the thymus. I feel better. (Patient 4) | ||
| Impediment | ||||
| Hospital-based care | 3 | Disrupted schooling | 2 | I had to go to [the] hospital. I have to go to [the] hospital to do my IVIG. I missed a lot of school. (Patient 4) |
| Illness education (poor)d | 1 | Maybe a little bit more information about the illness would be really helpful. I have very little understanding of the illness. It’s so complex. (Patient 1) | ||
| Treated as “mentally ill” | 1 | It was hard when they said I was mentally ill, because I could feel in myself that I wasn’t just mentally ill—there was something else going on. (Patient 1) |
TABLE 2. Factors perceived by patients as facilitating and impeding recovery from anti-NMDAR-mediated encephalitis during hospital-based care (N=7)a
At the time of the interviews, participants ranged between 7 and 41 months postimmunotherapy commencement, with the initiation of this treatment coinciding with the anti-NMDAR encephalitis diagnosis. Most participants had part-time employment (N=4/7), which reflected a reduction in previous hours and duties. The remaining participants were part-time (N=2/7) or full-time (N=1/7) students. All participants described persisting symptoms following resolution of the acute phase of the illness, and a range of associated functional impairments were identified (Figure 1). Participants described various coping strategies to accommodate their ongoing symptoms and impairment (Table 3).
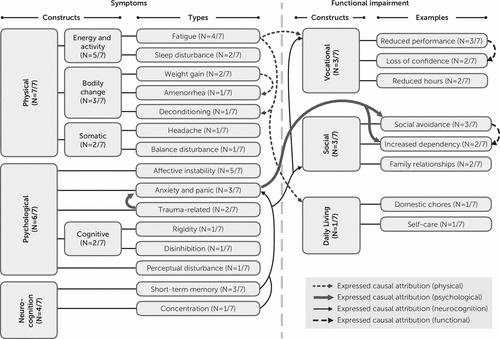
FIGURE 1. Symptoms associated with functional impairment and expressed causal attributions identified by patients following recovery from the acute phase of anti-NMDAR encephalitis (N=7)a
a Anti-NMDAR=anti-N-methyl-d-aspartate receptor.
| Symptom type and approach | Descriptor | N | Focus | Example | N |
|---|---|---|---|---|---|
| Memory and concentration impairment (N=6) | |||||
| Coping strategies | Meta-cognitive | 4 | Self | Writing lists and notes | 3 |
| Use of calendars and diaries | 2 | ||||
| Other | Allowing extra time for task completion | 1 | |||
| Accommodations | Employer/education provider flexibility | 3 | Other | Not working alone (reminders and reassurance) | 2 |
| Regular refresher courses | 1 | ||||
| Extensions for academic work | 1 | ||||
| Social support | 1 | Other | Reliance on reminders (domestic activities) | 1 | |
| Fatigue (N=6) | |||||
| Coping strategies | Not pushing oneself too hard | 4 | Self | Doing and expecting less | 4 |
| Taking rest time | 3 | ||||
| Maintaining activity (despite fatigue) | 2 | Self | Regular exercise | 2 | |
| Not taking breaks | 1 | ||||
| Maintaining a routine | 1 | Self | Getting enough sleep and regular meals | 1 | |
| Social support | 1 | Self | Encouraging activity | 1 | |
| Accommodations | Employer/education provider flexibility | 3 | Other | Reduced hours | 3 |
| Social support | 1 | Other | Flexibility to assist with fluctuating impairment | 1 | |
| Anxiety (N=5) | |||||
| Coping strategies | Distraction | 1 | Self | Music, pets | 1 |
| Accommodations | Social support | 3 | Other | Restricting interactions to familiar people | 2 |
| Physical presence of family | 2 | ||||
| Reassurance | 1 | ||||
| Reduced vocational demands | 3 | Other | Change to less demanding work/career | 3 | |
| Employer/education provider flexibility | 2 | Other | Reduced attendance | 2 | |
| Special consideration | 1 | ||||
| Affective instability (N=1) | |||||
| Coping strategies | Emotional regulation | 1 | Self | Self-distancing (deep breathing and counting to 10) | 1 |
| Perceptual disturbance (N=1) | |||||
| Accommodations | Social support | 1 | Other | Assistance with reality testing | 1 |
| Physical presence of family | 1 |
TABLE 3. Self-reported methods to cope with and accommodate residual symptoms following anti-NMDAR encephalitis (N=7)a
Most participants indicated that their recollections of the acute phase of illness, including hospital-based care, were limited by memory impairments. The experience of the acute phase was described by some participants as confusing and aversive. Diverse symptoms were recalled (see also the online supplement). Physical symptoms were most frequently reported, and for some participants, the emergence of these was the trigger for a diagnosis of encephalitis and a shift away from psychiatric care, as illustrated in the patient report below.
Some of the worst episodes were when I thought I was dying and said goodbye to my Dad….I remember all the clocks going backwards. I remember thinking I had to hold my breath as part of a game, and they had to put an oxygen mask on me…just things like that. I don’t talk about any of this with my family. (Patient 2)
I was in [the] hospital for about 6 months. I was treated initially for the psychotic symptoms because they didn’t know what was wrong with me, and they kept putting me in for ECT…when that didn’t work, and I got progressively worse and started showing physical symptoms. I became catatonic….I had no way of knowing where I was or what I was doing. I couldn’t dress myself; I couldn’t feed myself; I couldn’t do anything for myself. I didn’t know anybody was there or talking to me…then I did actually have an arrest [cardiac arrest] at one stage. [And that prompted doctors to look for another cause?] Yeah. (Patient 1)
Factors Facilitating and Impeding Recovery During Hospital-Based Care
Most participants described factors that they perceived as facilitating their recovery during hospital-based care (acute and subacute) (Table 3), including support and specific treatment interventions. The nature of this support reported by some participants included the physical presence of family and friends, despite the patient’s unusual or disturbing behavior, and acts of kindness from hospital staff. A range of interventional, educational, and rehabilitation treatments were reported as facilitating recovery, but there was a lack of consistency between participants regarding the nature of these.
Some participants identified impediments to recovery, and these were all grouped under the focus of hospital care. Contributing factors reported by some participants were disruption to schooling associated with prolonged treatment, being (mis)treated as mentally ill, and inadequate illness education.
Persisting Symptoms and Functional Impairment
Persisting symptoms, associated functional impairment, and expressed causal attributions identified by participants following recovery from the acute phase of anti-NMDAR encephalitis are presented in Figure 1. All participants described persisting symptoms, and most described associated functional impairment following resolution of the acute phase. All participants had persisting physical symptoms, and most reported ongoing psychological and neurocognitive symptoms. Some participants reported impairment related to social and daily-living skills.
A range of causal attributions between symptoms and functional impairment were reported by participants. Fatigue was viewed as contributing to physical deconditioning, as well as vocational and daily-living impairment. Issues with short-term memory and concentration (neurocognition) were viewed causally as a driver for anxiety and panic, as well as impairment in social and vocational function. Traumatic experiences associated with the acute phase of the disorder were considered to increase anxiety and panic, which were in turn associated with social avoidance and increased dependency.
All participants reported self- and other-directed ways of coping with and accommodating residual symptoms (Table 3). It is important to note that all participants reported increased reliance on social support, which was used to accommodate a range of symptoms and functional impairments. For some participants, this social support was directed towards managing anxiety (including and specifically social anxiety) through physical presence and reassurance, as reported below.
Dad sleeps on my floor because his breathing puts me to sleep. When we’re in [the] hospital, it put[s] me to sleep. If I get really anxious, we’ll do that. We joke about it. For my birthday, he was like, “Oh, I’ll give you like 2 hours of my breathing.”
I just try and avoid situations where I’m not comfortable. I just don’t go. Socially, I’ll avoid situations where I don’t know people too. (Patient 2)
Quite often if I’m alone at home I’ll ring mum and ask her to come over. (Patient 1)
Participants described conflicting approaches for managing fatigue, with most reporting the importance of “not pushing yourself too hard” but some emphasizing maintaining activity (despite fatigue) and a routine.
Despite the presence of ongoing challenges, some participants reported learning, change, and personal growth arising through the illness experience:
I’m actually happier….I nearly died [when acutely unwell]. I couldn’t walk. I reverted back to a baby…[now] I just have this whole new appreciation [becomes tearful]. Little things just don’t bother me as much as they used to—the stories that I’ve heard about things that I did and the things that I said to people. If you all still love me despite that….it’s shown me who my real friends are. (Patient 3)
I get overwhelmed more easily, and money can be a stress. I like to remind myself and my husband that we’re here, we’re together, I could be dead. My friends thought I was going to die. It really does change your perspective….I’m trying to make sure that I don’t overload myself. I’ve decided it’s not worth it. (Patient 7)
Discussion
People who have recovered from the acute phase of anti-NMDAR encephalitis describe the experience as confusing and frightening. They often struggle to recall their symptoms and experiences while being severely ill. What was reported most prominently in our study sample was an incomplete recollection of distressing symptoms accompanied by positive recollections of support from family and friends and acts of kindness from hospital staff. All participants continued to experience persisting symptoms (predominantly physical), and most reported having to make accommodations for associated functional impairments after resolution of the acute phase and hospital-based care. Most participants employed a range of coping strategies, most commonly addressing memory and concentration impairments, fatigue, and anxiety. Overall, these findings suggest that the experience of anti-NMDAR encephalitis and its treatment have a profound and lasting effect on quality of life following resolution of the acute phase of illness.
There has been limited research exploring the longer-term outcomes of anti-NMDAR encephalitis (7, 13). One study described a favorable outcome as an mRS score of 0–2 and concluded that most patients respond by 18 months following treatment initiation (7). In our sample, the mean mRS score was <2, and the mean follow-up duration was 21.1 months; however, most participants reported considerable ongoing impairment. The subjective report of our participants is consistent with persisting neurocognitive deficits previously described (13). Using assessments designed for other conditions potentially underestimates the ongoing disability accompanying anti-NMDAR encephalitis.
Participants described both facilitators and impediments to their recovery. Support from family and friends, kindness by treating clinicians, and the provision of effective interventions were all valued by participants. In contrast, disruption to education, misdiagnosis of the autoimmune illness as a primary psychiatric disorder, and lack of education about anti-NMDAR encephalitis were reported as barriers to recovery. The effect of stigmatizing attitudes about idiopathic mental disorders and neuropsychiatric presentations characterized by marked behavioral disturbance is worthy of further consideration. The contribution of stigma is suggested by participants’ expression of relief in response to no longer being thought of as mentally ill, and the value of family and friends who were willing to remain present in their lives despite their disturbed thinking and behavior.
Improved patient outcomes following anti-NMDAR encephalitis are likely to be supported by early diagnosis and treatment (9, 13), combined with the presence of supportive friends, family, and treating clinicians. The ubiquitous presence of persistent impairments in this sample suggests that disability support may be needed for many patients who have recovered from the acute illness phase. Positive outcomes have been reported in a small case series following the provision of long-term rehabilitation that included behavioral interventions, psychoeducation, and input from a multidisciplinary team (4). In addition, support networks of peers recovering from anti-NMDAR encephalitis may be beneficial. Identifying the best approach to optimize recovery after resolution of the acute phase and tailoring interventions to the individual’s needs should be a priority for clinicians involved in the management of this disorder.
Limitations
Assembling large sample sizes is a challenge in studies of rare disorders (21, 22). Our cross-sectional sampling approach limits the ability to understand the progression of recovery over time and the transferability of findings to other contexts. Additionally, while there was a general coherence between transcripts, the small number of participants in this study limits consideration of thematic saturation. Furthermore, the exploratory qualitative design limits consideration of the contribution of specific factors, such as undertreatment of the disorder, iatrogenic effects of medications, and other interventions for residual impairments. Data generation was participant led and omitted the perspectives of other key stakeholders (e.g., care givers and staff). Our findings are likely to be prioritized on the basis of what was most salient to participants at the time of the interview. Some phenotypes commonly associated with anti-NMDAR encephalitis, such as catatonia (30), may have been underreported by participants. Specific details about the nature of posthospital support and follow-up were not actively explored in the interview schedule. The provision of memory cues through a more structured interview or checklist approach may have generated a greater breadth of reported ongoing symptoms, impairment, treatment, and support. Additionally, available quantitative information about coding frequency should not be used to draw inferences about the prevalence of experiences beyond this study sample (29).
Conclusions
People recovering from anti-NMDAR encephalitis describe persisting symptoms and associated functional impairments after resolution of the acute phase of the disorder. Developing a better understanding of these impairments will guide the development of focused rehabilitation. Additionally, there is a clear need to identify more nuanced quantitative outcome measures for anti-NMDAR encephalitis. The dramatic improvement in neuropsychiatric symptoms following acute-phase treatment may overshadow acknowledgment of persisting impairments. Accurate assessment of these variables, coupled with appropriate psychosocial support, will enable a more complete recovery from anti-NMDAR encephalitis.
1 : NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: the 2016 Cotzias Lecture. Neurology 2016; 87:2471–2482Crossref, Medline, Google Scholar
2 : An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol 2019; 18:1045–1057Crossref, Medline, Google Scholar
3 : Refining the psychiatric syndrome of anti‐N‐methyl‐d‐aspartate receptor encephalitis. Acta Psychiatrica Scandinavica 2018; 138:401–408Medline, Google Scholar
4 : Long term rehabilitation management and outcome of anti-NMDA receptor encephalitis: case reports. NeuroRehabilitation 2014; 35:863–875Crossref, Medline, Google Scholar
5 : Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10:63–74Crossref, Medline, Google Scholar
6 : Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev 2011; 7:189–193Crossref, Medline, Google Scholar
7 : Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165Crossref, Medline, Google Scholar
8 : Psychiatric management of anti-NMDAR encephalitis: a cohort analysis. Psychol Med (Epub ahead of print, November 19, 2019)Google Scholar
9 : The prevalence and treatment outcomes of antineuronal antibody-positive patients admitted with first episode of psychosis. BJPsych Open 2018; 4:69–74Crossref, Medline, Google Scholar
10 : Rehabilitation following anti-NMDA encephalitis. Brain Inj 2015; 29:785–788Crossref, Medline, Google Scholar
11 : Neuropsychological characterization of three adolescent females with anti-NMDA receptor encephalitis in the acute, post-acute, and chronic phases: an inter-institutional case series. Clin Neuropsychol 2017; 31:268–288Crossref, Medline, Google Scholar
12 : Cognitive outcomes following anti-N-methyl-D-aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol 2018; 40:234–252Crossref, Medline, Google Scholar
13 : Cognitive and social functioning deficits after anti-N-methyl-D-aspartate receptor encephalitis: an exploratory case series. J Int Neuropsychol Soc 2016; 22:828–838Crossref, Medline, Google Scholar
14 : Anti-N-methyl-D-aspartate receptor encephalitis: a review and neuropsychological case study. Clin Neuropsychol 2016; 30:150–163Crossref, Medline, Google Scholar
15 : Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 2009; 66:11–18Crossref, Medline, Google Scholar
16 : Analysis of relapses in anti-NMDAR encephalitis. Neurology 2011; 77:996–999Crossref, Medline, Google Scholar
17 : N-methyl-D-aspartate antibody encephalitis: Temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133:1655–1667Crossref, Medline, Google Scholar
18 : The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54:1044–1054Google Scholar
19 : Evolution of the modified Rankin Scale and its use in future stroke trials 2017; 48:2007–2012Google Scholar
20 : A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology 2019; 92:e244–e252Crossref, Medline, Google Scholar
21 : Qualitative research approaches in rare diseases: acid sphingomyelinase deficiency (ASMD) symptoms and impact as reported by patients and caregivers. Value Health 2016; 19:A388Crossref, Google Scholar
22 : Living with and treating rare diseases: experiences of patients and professional health care providers. Qual Health Res 2015; 25:636–651Crossref, Medline, Google Scholar
23 : Qualitative research in mental health and mental illness, in Handbook of Qualitative Health Research for Evidence-Based Practice. Edited by Olson K, Young RA, Schultz IZ. New York, Springer, 2016, pp 203–223Crossref, Google Scholar
24 : The qualitative research interview. Med Educ 2006; 40:314–321Crossref, Medline, Google Scholar
25 : Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Res Methodol 2013; 13:117Crossref, Medline, Google Scholar
26 : Qualitative Data Analysis for Applied Policy Research: Analyzing Qualitative Data. London, Routledge, 2002, pp 187–208Google Scholar
27 : Framework analysis: a qualitative methodology for applied policy research. J Admin Gov 2009; 4:72–79Google Scholar
28 QSR International: NVivo qualitative data analysis software, version 12 plus. Doncaster, Australia, QSR International, 2018Google Scholar
29 : Reporting quantitative information in qualitative research: guidance for authors and reviewers. Addiction 2014; 109:175–176Crossref, Medline, Google Scholar
30 : Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci 2019; 73:574–580Crossref, Medline, Google Scholar



