Anxiety in Parkinson’s Disease: A Systematic Review of Neuroimaging Studies
Abstract
Objective:
The mechanisms and neuronal networks associated with anxiety in Parkinson’s disease (PD) are incompletely understood. One of the best tools for investigating both component function and neuronal networks associated with psychiatric symptoms is functional MRI (fMRI). Unlike structural scans, functional scans, whether task-based or resting-state, are more likely to be clinically relevant and sensitive to changes related to treatment. The investigators provide a comprehensive review of and present results for imaging studies of anxiety in PD.
Methods:
A systematic review of the literature on fMRI and anxiety in PD was conducted, and the quality of all included studies was simultaneously assessed. Eighteen studies were included: 15 studies assessed anxiety directly, and three evaluated emotional processing. Imaging methodology and behavioral assessments varied across studies, preventing direct comparison of results in most cases.
Results:
There was a convergence in findings across methods, implicating involvement of the amygdala, caudate, and putamen in association with anxiety in PD. For both task-based activation and resting-state connectivity, dopamine medication status was associated with differences in activation and behavioral function.
Conclusions:
Although there is little consensus in the current fMRI literature studying anxiety in PD, these results suggest an overlap between structures classically involved in the brain’s fear circuit (particularly the amygdala) and the alterations in the nigro-striatal system (e.g., the caudate and putamen and on-off dopamine findings) related to PD and its dopaminergic treatments.
Anxiety is one of the most prevalent neuropsychiatric symptoms in Parkinson’s disease (PD), occurring in at least 30% of patients (1). The presence of anxiety is one of the main determinants of quality of life among patients with PD (2) and one of the top unmet needs as a result of under-recognition and limited effective treatments (3, 4). Clinical ascertainment of anxiety in PD is complicated by atypical presentations; the most common atypical presentation consists of panic-like episodes associated with on- and off-medication fluctuations, which is thought to have a dopaminergic basis (4, 5). In addition, comorbidity between depression and anxiety is so ubiquitous in PD that it is debated whether they are dissociable (6). Finally, misattribution of other nonmotor symptoms, such as autonomic dysfunction, which can be experienced as anxiety by some (e.g., heart palpitations, increased tremor) because of increased autonomic tone, further complicates accurate diagnosis (7).
As a result, anxiety is often underdiagnosed; up to 20% of patients with clinically significant anxiety symptoms do not meet DSM-5 diagnostic criteria (1, 3, 4, 6). In addition, effective treatment for anxiety in PD is limited. Currently, there are no randomized, placebo-controlled clinical trials of medications for the treatment of anxiety in PD to guide evidence-based practice (3, 8). Thus, there is a clear need to advance knowledge of the mechanistic underpinnings of anxiety in PD to facilitate the development of biomarkers for improved diagnosis and tracking of therapeutic response.
Clinically, anxiety is often comorbid with depression, and the two symptoms are difficult to dissociate. The ability to distinguish and mechanistically separate these symptoms would allow for more specific treatments. Therefore, to be useful for diagnosis and monitoring treatment response any potential biomarker would have to be specific to anxiety, even if there were mechanistic overlap between the two symptoms. The genetics of PD and anxiety are not well understood and would not function well as markers of treatment response. Similarly, structural markers would be unlikely to show acute change with treatment and would not inform functional networks associated with symptoms. Task-based and resting-state connectivity functional MRI (fMRI) may be useful for identifying networks associated with anxiety and response to treatment that may be dissociable from depression. However, to date, few fMRI studies have examined anxiety in PD, and there has been little consistency in the methodology used to assess anxiety or image the brain, making it difficult to draw conclusions about their findings. The aim of the present review was to summarize the findings of the currently available fMRI studies on anxiety in PD.
Methods
Search Strategy and Study Selection
The initial literature search was conducted in July 2019 and updated in August 2020, using Medical Subject Headings terms related to PD, anxiety, emotional functioning, and imaging methods (for further details, see Table S1 in the online supplement). After removing duplicates, the titles and abstracts of 723 articles were screened by two reviewers (K.P. and C.S.). Inclusion criteria for the full-text screening were the study participants included patients with PD; the study used neuroimaging methods; and at least one outcome of the study focused on anxiety, depression, or emotional processing (i.e., processing of emotions such as fear and anger). Articles were excluded if they were not available in English or if they did not present original research.
To provide a comprehensive review of studies reporting results from imaging studies of anxiety in PD, we included studies that used different instruments and data collection methods to assess anxiety. Because of the limited number of studies on this topic and the variety of scales and neuroimaging methods used, we were unable to conduct a meta-analysis on the reported data; instead, we provide a qualitative synthesis of the literature.
After initial screening of the title and abstract, 660 articles were excluded, leaving 64 articles for full-text evaluation (Figure 1). Two reviewers examined the full-text articles to confirm whether they met inclusion criteria (K.P. and F.N.). A third reviewer (G.P.) served as an adjudicator when a disagreement occurred about inclusion in the full-text evaluation. After full-text screening, 19 articles remained. An additional article was included after a manual search using the terms “emotional valence,” “Parkinson’s disease,” and “fMRI” to ensure all functional studies associated with processing of emotion were evaluated.
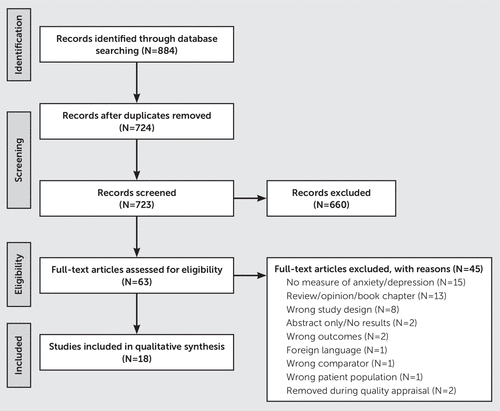
Data Extraction and Quality Appraisal of Included Studies
Two reviewers (F.N. and K.P.) used a quality appraisal checklist to independently evaluate the quality of the 20 articles included after the full-text assessment. We adapted the Newcastle-Ottawa Scale (9) to create a quality assessment checklist for neuroimaging studies. The appraisal resulted in the inclusion of 18 articles and the exclusion of two articles. These two articles were excluded because the examination of anxiety in one study pertained only to freezing of gait; the other study focused exclusively on depression and did not address anxiety. Initially, we listed depression as an inclusion criterion because of the frequency of comorbid depression and anxiety. However, we ultimately decided to exclude this article because of its focus on depression in patients with PD alone. Both reviewers (K.P. and F.N.) extracted demographic, psychiatric, and neuroimaging results from the 18 articles that remained after the quality appraisal.
Data Synthesis
Using the Cochrane procedure for narrative summary, two reviewers (K.P. and F.N.) reviewed each study and developed a preliminary composition of the results (10). A combination of inductive and deductive reasoning was applied for the analysis. We employed deductive synthesis to divide articles based on the type of neuroimaging methodology applied: functional neuroimaging with task, functional neuroimaging during resting, or structural neuroimaging. Then, within each group, an inductive synthesis was applied to identify patterns of topics discussed. The data from these studies were then synthesized to describe common conclusions across different studies (Table 1).
| Study | Type | Analysis | Result | Anxiety assessment | Quality appraisal scoreb |
|---|---|---|---|---|---|
| Bell et al. (26) | Functional task | Presented an affective go/no-go paradigm using emotional words to 13 PD patients and 12 HCs. Compared positive and negative valence between groups. | Significant interaction between group and valence in response to words of varying emotional valence in the ventral striatum and amygdala. | None | 4 |
| Right amygdala showed decreased activation for positive target, increased activation for negative words for HCs and increased activation for positive words, and decreased activation for negative words in PD patients. | |||||
| Dan et al. (45) | Functional task | HCs (N=32) compared with PD patients (N=25) in medication off- or on-medication state. Patients completed an emotional faces-matching task during fMRI. | PD patients showed reduced accuracy for facial stimuli in the off-medication state; no difference in accuracy between PD patients in the on-medication state and HCs. | None | 4 |
| PD patients in the off- and on-medication states had an increased BOLD response to negative emotional faces in the bilateral calcarine sulci and lingual gyri compared with HCs. | |||||
| PD patients in the off-medication state had an increased BOLD response to negative emotional faces in the bilateral posterior cingulate/retrosplenial cortex compared with PD patients in the on-medication state. | |||||
| Fleury et al. (23) | Functional task | Emotional Stroop task using emotional faces: Comparison of PD patients in the off- and on-medication states (N: PD=10; HC=12). Contrast: congruent versus incongruent trials | Significant effect of congruency when viewing negative faces in HC and PD patients in the on-medication state, but no congruency effect was found in PD patients in the off-medication state. | State-Trait Anxiety Inventory | 4 |
| In the off-medication state, PD patients showed decreased activation in the right pregenual anterior cingulate cortex, right pre- and postcentral gyri, and right thalamus. | |||||
| There were no differences between PD patients in the on-medication state and HCs. | |||||
| Heller et al. (28) | Functional task | Ekman faces task (behavioral) and emotional categorization using videos (fMRI): comparison of PD (N=25) and HCs (N=31). | Male PD participants were significantly worse than PD female participants and healthy male participants in recognizing disgust and anger on the Ekman faces categorization task. | BAI | 5 |
| Neural correlates of emotion processing: sad, angry, happy in male PD participants (N=12) versus male HCs (N=17). | PD patients exhibited decreased activation in the bilateral insula, putamen, hippocampus, fusiform gyrus, Heschl’s gyrus, right superior temporal gyrus, and left thalamus when processing negative emotions when compared with male HCs. | ||||
| Moonen et al. (31) | Functional task | Passively viewing emotional images (IAPS): Categorization of images as human/nonhuman (N: PD=17; HC=17) in arousal. Compared low- and high-arousal pictures collapsed across positive and negative emotions. Postscan affective rating was used as a regressor, and depression, anxiety, apathy, disease duration, motor severity, and use of medication were used as covariates. | No behavioral differences between groups in their valence and arousal ratings. | Hamilton Anxiety Rating Scale and Parkinson Anxiety Scale | 5 |
| ROI analysis comparing high- and low-arousal pictures showed differential activation in the left putamen for HCs and right dmPFC in PD patients. No significant effects for the left amygdala and left ventrolateral PFC ROIs. | |||||
| PD patients did show a negative correlation between the dmPFC activity and anxiety and apathy scores when viewing highly arousing emotional images. | |||||
| Schienle et al.(24) | Functional task | Emotional scenes eliciting disgust, fear, and neutral emotions were presented (N: PD=17; HCs=22). Compared pictures producing fear and disgust with neutral pictures and with each other. | No significant differences were observed between PD and control groups in affective ratings. | State-Trait Anxiety Inventory | 5 |
| ROI analysis revealed greater activations in bilateral amygdala in HCs when viewing fear-eliciting pictures compared with neutral pictures (fear > neutral). | |||||
| No significant activations were found in the amygdala in the PD group. | |||||
| HCs had greater activations in the right pallidum than the PD group for the contrast fear > neutral. | |||||
| Tessitore et al. (29) | Functional task | Used emotional face-matching task compared nine PD patients in drug off- and on-medication states with nine HCs while viewing emotional faces compared with the control task. | No significant differences were observed in performance on the emotional faces task between HCs and PD patients in the on- and off-medication states. | None | 3 |
| Activation in the bilateral amygdala while viewing the emotional faces compared with the control task in HCs and PD patients in the on-medication state but not in PD patients in the off-medication state. | |||||
| Wabnegger et al. (25) | Functional task | Gender discrimination task while viewing emotional faces (N: PD=17; HC=22). Compared fear, anger, disgust, and sad pictures against neutral faces. Postscan affective ratings were collected. | Trend toward differences in sadness rating only behaviorally. | State-Trait Anxiety Inventory | 5 |
| Greater activation in the putamen and inferior frontal gyrus in HCs compared with PD patients while viewing sad faces. | |||||
| PD patients showed greater activation in parietal regions (secondary somatosensory cortex and inferior parietal cortex) during the performance of this task. These activations were correlated with intensity and accuracy ratings of the stimuli. | |||||
| The secondary somatosensory cortex activation was correlated with fear and disgust ratings. | |||||
| The activation in inferior parietal cortex was associated with anger ratings. | |||||
| Cheng et al. (36) | Functional rest | Voxel-wise comparison of cerebral blood flow for PD patients (N=20), PPS patients (N=16), and HCs (N=17). Correlations between cerebral blood flow and psychiatric measures. | PD patients had reduced cerebral blood flow in the cerebellum_crus2, left middle frontal gyrus, triangle inferior frontal gyrus, left frontal medial orbital gyrus, and left caudate nucleus compared with HCs. | HAM-A | 5 |
| Cerebral blood flow was positively correlated with scores on the HAM-A in PPS patients, but not PD patients. | |||||
| Dan et al. (30) | Functional rest | Examined unique contribution of depression, anxiety, and apathy using connectivity ROI to ROI analysis (N: PD=27; HCs=29) | Increased anxiety scores were associated with increased connectivity between the OFC, amygdala, hippocampus, and parahippocampus gyri, and decreased connectivity between the OFC and precentral gyri, postcentral gyri, paracentral lobule, and supplementary motor areas. | State-Trait Anxiety Inventory | 5 |
| Anxiety scores were negatively correlated with functional connectivity between the lateral middle-superior frontal gyrus and the amygdala, hippocampus, parahippocampal gyri, temporal pole, and OFC. | |||||
| Li et al. (35) | Functional rest | ReHo during rest in PD patients with left onset (N=30), PD patients with right onset (N=27), and HCs (N=32) while controlling for age and gray matter density. Correlations were used to evaluate the association between clinical variables and ReHo. | Patient groups did not differ from each other and showed higher ReHo in right temporal pole compared with HCs. | HAM-A | 5 |
| Right-onset patients had lower ReHo in the right precentral gyrus and right middle frontal gyrus compared with HCs. | |||||
| Anxiety and depression symptoms were significantly correlated with the right temporal pole ReHo in PD patients who had right-side motor-symptom onset. | |||||
| The left-onset patients did not show significant associations between ReHo and neuropsychological measures. | |||||
| Wang et al. (33) | Functional rest | Examined anxiety in PD patients using resting-state functional connectivity (PD patients with anxiety: N=18; PD control subjects: N=45; HCs: N=24). Correlated connectivity measures with HAM-A scores while controlling for age, sex, education, gray matter volume, and depression. | Anxious PD patients showed increased functional connectivity between the putamen and the caudate and decreased functional connectivity of the bilateral putamen with the right orbitofrontal gyrus and right cerebellum compared with PD control subjects. | HAM-A | 5 |
| PD patients (with anxiety and control subjects) showed positive correlations between HAM-A scores and connectivity between the right putamen and left caudate, a negative correlation with connectivity between the contralateral putamen and OFC, and a negative correlation with connectivity between the right putamen and the right cerebellum. | |||||
| Wang et al. (32) | Functional rest | Analyzed effect of anxiety by comparing anxious PD patients with PD control subjects and HCs (PD patients with anxiety: N=15; PD control subjects: N=33; HCs: N=19) while controlling for age, sex, education, gray matter volume, cognitive and depression scores. | PD patients with anxiety showed increased amplitude in low-frequency fluctuations in the right cerebellar posterior lobe (cerebellum_9) extending to the bilateral brainstem, and right orbitofrontal gyrus compared with PD patients without anxiety. | HAM-A | 5 |
| HAM-A scores were negatively correlated with the amplitude of low-frequency fluctuations in the right cerebellum. | |||||
| Zhang et al. (34) | Functional rest | Correlated anxiety and depression symptoms with the measures of temporal synchronization in PD patients (N=36). | The left amygdala was associated with anxiety severity, while the left parahippocampal gyrus was associated with depression severity. By using the left amygdala seed, the investigators found that the anxiety scores were positively correlated with connectivity between the amygdala and left angular gyrus, left superior parietal lobule, and left cuneus. | Zung Anxiety Scale | 5 |
| Anxiety scores were negatively correlated with connectivity between the amygdala seed and right inferior frontal gyrus and left superior temporal gyrus. | |||||
| Li et al. (37) | Structural | Fixel-based analysis was used to determine whether there were differences in fiber tracts between PD patients (N=98) and HCs (N=76). | PD patients, compared with HCs, had reduced fiber density in the corpus callosum and increased fiber density in the cortical spinal tract. | HAM-A | 5 |
| PD patients had increased fiber-bundle cross-section in the superior cerebellar peduncle. | |||||
| Fiber density in the corpus callosum was negatively correlated with HAM-A scores in PD patients. | |||||
| Oosterwijk et al. (37) | Structural | Structural covariance analysis with five bilateral regions of interest, including the basolateral and centromedial-superficial amygdala, dorsal caudate nucleus, dorsal-caudal putamen, and nucleus accumbens. Correlation of BAI with structural covariance between ROIs (N=115) | BAI scores were negatively correlated with structural covariance between the left striatal subregions and the contralateral caudate nucleus. | BAI | 5 |
| Anxiety severity was related to reduced structural covariance between the right dorsal caudate nucleus and ipsilateral ventrolateral prefrontal cortex and between the left nucleus accumbens and ipsilateral dorsolateral prefrontal cortex. | |||||
| BAI scores (total and affective) were not significantly correlated with structural covariance of the amygdala regions of interest. | |||||
| Vriend et al. (36) | Structural | Correlated structural volume with subscales of BAI: affective, thermoregulation, tremble, hypotension, and hyperventilation in PD patients (N=110). | Negative correlation between the BAI affective score and volume of the left amygdala. | BAI | 5 |
| Hypotension subscale was correlated with the left hippocampal volume. | |||||
| The hypertension subscale was associated with right amygdala volume. | |||||
| Wee et al. (46) | Structural | Longitudinal (18 months) examination of effect of medication on anxiety in PD patients (N=73) | Baseline anxiety was correlated with decreased gray matter volume in the bilateral precuneus and anterior cingulate cortex. | Hospital Anxiety and Depression Scale | 5 |
| At the 18-month follow-up visit, anxiety was correlated with decreased gray matter volume in the left precuneus and the anterior cingulate cortex after controlling for age, gender, education, and depression. |
TABLE 1 Synthesis of study results across different studies of anxiety in Parkinson’s disease (PD)a
Results
Participants
Sample sizes for the studies included in this review ranged from nine to 115. All studies enrolled nondemented participants who were diagnosed with idiopathic PD. Ten studies used United Kingdom PD Brain Bank Criteria (11), three studies relied on diagnosis by a movement disorders specialist, one study used Queen Square Brain Bank Diagnostic Criteria for PD (12), one study used the National Institute for Neurological Disorders criteria (13), one study used the Clinical Diagnostic Criteria for Parkinson’s Disease in China, and one study did not clearly specify how idiopathic PD patients were diagnosed. The average age of participants was 64.5 years (range: 55.2–71.7 years). Most samples were primarily male (64%). The average disease duration for PD was 6 years (range: 3.3–11.1 years). Most testing was performed in the on-medication state; however, five studies were conducted in the off state, and five studies were conducted in both the off- and on-medication states.
Measures
Several instruments were used to assess anxiety in the studies included in this review, and some studies included more than one measure. The most frequently used scales were the State-Trait Anxiety Inventory (14) (N=4), the Hamilton Depression Rating Scale (HAM-D) (15) (N=4), and the Beck Depression Inventory (BDI) (16) (N=4). Other scales that were used included the Geriatric Depression Rating Scale (17) (N=2), the Parkinson’s Disease Questionnaire-39 (18) (N=1), the Beck Anxiety Inventory (BAI) (19) (N=3), the Hamilton Anxiety Rating Scale (HAM-A) (20) (N=6), the Parkinson Anxiety Scale (21) (N=1), and the Zung Anxiety Scale (22) (N=1).
Quality Assessment
The overall quality of each of the studies was evaluated using the following criteria: anxiety was directly measured; the sample size was appropriate with a comparable number of participants in each group; appropriate control subjects were included in the study (i.e., matched for subject characteristics); appropriate neuroimaging control measures were employed in the study (e.g., volumetric studies controlled for intracranial volume); and the statistical approach was appropriate for the research question (5). Fourteen articles met all criteria for this quality assessment. The remaining four articles met at least three criteria and were included because they contributed to the purpose of this review. The scores for the quality appraisal can be found in Table 1.
Synthesis of Included Articles
The findings from the studies included in this systematic review are synthesized according to neuroimaging methodology: functional (task-based), functional (resting-state), and structural.
Functional studies: task-based.
Fleury et al. (23) examined 10 patients in the on and off states and compared them to 12 age-, sex-, and education-matched healthy control subjects (HCs). PD patients in this study had significantly higher BDI scores but did not show significant differences in the State-Trait Anxiety Inventory (STAI) scores compared with the control group. Participants in this study completed an emotional Stroop task using happy and fearful emotional faces with the words “happy” or “fear” written on them to create congruent (happy face with “happy”) and incongruent (happy face with “fear”) trials. Participants were asked to make judgments about the faces while ignoring the words. Examining the neural correlates of incongruent versus congruent trials while viewing the negative faces revealed no significant difference between patients with PD in the on state and HCs. However, in the off state, patients with PD showed decreased activation in the right pregenual anterior cingulate cortex (pgACC), right pre- and postcentral gyri, and right thalamus.
Schienle et al. (24) investigated emotional processing in 17 nondepressed PD patients in the medication off state only and 22 HCs. Patients with PD did not differ in their BDI scores compared with the control group, although their STAI scores were marginally higher. Participants were presented with emotional scenes that elicit disgust, fear, or neutral emotions with instructions to view the pictures in the scanner and provide affective ratings for the pictures after the scan. Region-of-interest analysis of the brain revealed greater activations in the bilateral amygdala in HCs when viewing fear-eliciting pictures compared with neutral pictures (fear > neutral). No significant activations were found in the amygdala in the PD group. A direct comparison between the PD and control groups revealed greater activations in the right pallidum in the control group for the contrast fear > neutral. The difference in amygdala activation did not survive a direct comparison between the PD and control groups.
Similarly, Wabnegger et al. (25) used blocks of emotional faces to study emotional processing in 17 patients with PD tested in the off state only and 22 age- and education-matched HCs. The clinical characteristics of the participants revealed no differences between PD patients and HCs in BDI scores, but PD patients showed marginally increased STAI scores. Participants viewed faces with disgust, sadness, anger, fear, and neutral expressions while performing a gender discrimination task. The authors reported greater activation in the putamen and inferior frontal gyrus (IFG) in HCs compared with PD patients while viewing sad faces, whereas patients with PD showed greater activation in parietal regions (secondary somatosensory cortex and inferior parietal cortex) during the performance of this task. The parietal activations in the PD group were correlated with intensity and accuracy ratings of the stimuli. Although the secondary somatosensory cortex activation was correlated with fear and disgust ratings, the activation in the inferior parietal cortex was associated with anger ratings.
Bell et al. (26) examined correlates of emotional valence processing using an affective go/no-go task in 13 nondepressed PD patients assessed in the on state and 12 age-matched HCs. The task involved presentation of positive, negative, and neutral words from the Affective Norms for English Words (27) and the Cambridge Neuropsychological Test Automated Battery affective go/no-go task. Participants were asked to respond to the target valence while withholding response to distractors. Although the participants did not exhibit any differences in reaction time, the authors identified a significant interaction between group and valence in response to words of varying emotional valence in the bilateral ventral striatum and right amygdala regions of interest. After excluding two participants with a history of depression, only the right amygdala finding remained significant. Further exploration of the activation in the right amygdala showed a typical neural response in HCs—decreased activation for the positive target, increased activation for negative words, and an atypical response for PD patients; and increased activation for positive words and decreased activation for negative words, suggesting impaired emotional processing and cognitive control in PD.
Three studies used the Ekman emotional faces as stimuli to assess the neural basis of emotional processing in PD. Heller et al. (28) examined gender differences in PD patients in emotion recognition. They recruited 25 patients with PD tested in the on state and 31 HCs (males: PD group, N=12; HC group, N=17). Clinical characteristics of the participants indicated significantly higher depression and anxiety scores in PD patients compared with HCs as measured by BDI and BAI. Participants viewed the Ekman emotional faces with instructions to name the emotion that best described the facial expression. The fMRI task involved participants watching videos of actors performing emotional expressions with the instruction to categorize the emotion. The fMRI task did not show any difference in categorizing emotions between the two groups. Significant differences were observed in neural activation only in the comparison of PD male participants with healthy male participants—specifically, patients with PD exhibited decreased activation in the bilateral insula, putamen, hippocampus, fusiform gyrus, Heschl’s gyrus, right superior temporal gyrus, and left thalamus when processing negative emotions when compared with healthy male participants. No significant correlations between brain activation and depression, anxiety scores, or Ekman faces task performance were observed.
Tessitore et al. (29) used the emotional face-matching task to compare age-, education-, and gender-matched HCs to PD patients (N=18, nine per group) in both the on and off states. The task involved viewing three emotional faces (Ekman faces) and matching one of the faces to a target that expressed that same emotion (either angry or afraid). The control task consisted of three geometrical shapes and instructions to match the shape to the target. Although the authors did not report any measure of anxiety, they did observe activation in bilateral amygdala while viewing the emotional faces compared with the control task in HCs and PD patients in the on state but not in patients with PD in the off state. This difference was not associated with a history of depression, as they did not observe any differences between patients with or without depression regardless of their dopamine medication state. Authors suggested that this response could indicate increased neuronal activity in the amygdala as a result of dopamine gating of amygdala inputs.
Dan et al. (30) investigated the effect of dopaminergic medication on emotion recognition in PD patients. They administered the emotional face-matching task to 25 patients with PD (in both the on- and off-medication states) and 32 HCs. Compared with HCs, PD patients in the off and on states had an increased blood-oxygen-level-dependent (BOLD) response while viewing negative emotional faces compared with shapes in the bilateral calcarine sulci and lingual gyri (while controlling for cognitive functions such as attention, working memory, and executive function). For the same contrast (faces versus shapes using covariates), patients with PD in the off-medication state had an increased BOLD response in the bilateral posterior cingulate/retrosplenial cortex compared with PD patients in the on state. The authors suggested that the increased BOLD response in visual areas in PD patients may be due to altered top-down modulation from prefrontal/subcortical areas because of damage to their dopaminergic pathway.
Lastly, Moonen et al. (31) examined emotional processing using negative, positive, and neutral images from the International Affective Picture System in 17 patients with PD in the on state and 17 age-, gender-, and education-matched HCs. The task involved passively viewing positive, negative, and neutral images followed by a prompt screen asking participants to categorize the image as human or nonhuman. After the scan, participants rated these images for valence and arousal. Participants showed significant differences in clinical characteristics with PD patients showing significantly higher scores on the Hamilton Anxiety Rating Scale, HAM-D, and Parkinson Anxiety Scale (PAS). Region-of-interest analysis comparing high- and low-arousal pictures (collapsed across negative and positive pictures) showed differential activation in the left putamen in control subjects and the right dorsomedial prefrontal cortex (dmPFC) in PD patients. There were no significant effects for the left amygdala and left ventrolateral prefrontal cortex regions of interest. The results remained unchanged after controlling for the symptoms of apathy, depression, anxiety, and use of antidepressants. Patients with PD did show a negative correlation between the dmPFC activity and anxiety and apathy scores (PAS and Lille Apathy Rating Scale) when viewing highly arousing emotional images.
These studies show an overall altered emotional processing in PD patients, which is more pronounced for male participants. Although there are mixed results for behavioral measures of emotional processing, the neural correlates of emotional processing in patients with PD shows decreased activations in amygdala, putamen, thalamus, pgACC, somatosensory cortices, and prefrontal cortices such as IFG and increased activations in visual areas.
Functional studies: resting-state.
To account for the comorbidity most of the resting-state fMRI studies included in this review evaluated the effect of both depression and anxiety on functional connectivity (FC) in the brain. These studies have implicated limbic, striatal, and prefrontal regions with increased anxiety in PD. Wang et al. (32) explored the effect of anxiety in PD using 15 PD patients with anxiety in the off state and comparing them with 33 PD patients without anxiety and 19 HCs. The PD patients were categorized as anxious or nonanxious cases based on the HAM-A cut-off score of 12. Group differences in age, frontal assessment battery, and HAM-D scores were controlled by using these variables as covariates. In this study, PD patients with anxiety showed increased amplitude in low-frequency fluctuations in the right cerebellar posterior lobe extending to bilateral brainstem, and right orbitofrontal gyrus compared with PD patients without anxiety. In addition, HAM-A scores were negatively correlated with the amplitude of low-frequency fluctuations in the right cerebellum.
In earlier work, Wang et al. (33) used a similar approach to examine 18 PD patients with anxiety and 45 PD patients without anxiety in the off state and 24 HCs. Differences between groups in gender, age, and HAM-D scores were controlled in further analysis. Compared with the nonanxious PD group, PD patients with anxiety showed increased FC between the putamen and the caudate and decreased FC of the bilateral putamen with the right orbitofrontal gyrus and right cerebellum. Furthermore, correlation of HAM-A scores with FC differences in PD patients with and without anxiety showed a positive correlation with connectivity between the right putamen and left caudate, a negative correlation with connectivity between the contralateral putamen and orbitofrontal cortex (OFC), and a negative correlation with connectivity between the right putamen and right cerebellum.
Zhang et al. (34) looked at anxiety and depressive symptoms in 36 patients with PD in the on state using the Zung Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale. They showed that the left amygdala was associated with anxiety severity while the left parahippocampal gyrus was associated with depression severity (34). By using the left amygdala cluster as seed region for connectivity analysis, they found that the SAS score was positively correlated with FC between the amygdala and left angular gyrus, left superior parietal lobule, and left cuneus. SAS scores were negatively correlated with FC between the amygdala seed and right IFG and left superior temporal gyrus.
Dan et al. (30) aimed to explore the unique contribution of comorbid depression, anxiety, and apathy by examining the effect of each of these neuropsychiatric conditions while controlling for others. The authors investigated FC in 27 PD patients in the on state compared with 29 HCs. The patient and control groups had significant differences in STAI and BDI scores, though their Starkstein Apathy scores did not significantly differ. Increased anxiety scores were associated with increased connectivity between OFC, amygdala, hippocampus, and parahippocampal gyri, and decreased connectivity between the OFC and precentral gyri, postcentral gyri, paracentral lobule, and supplementary motor areas. Additionally, anxiety scores were negatively correlated with FC between the lateral middle-superior frontal gyrus and the amygdala, hippocampus, parahippocampal gyri, temporal pole, and OFC.
Li et al. (35) investigated the effect of asymmetric motor symptoms using resting-state fMRI with regional homogeneity as a measure. Regional homogeneity is the summarized FC of a node with its nearest neighbors, where higher values indicate centrality of the node in the network. They recruited 30 patients with initial onset of motor symptoms on the left side, 27 patients with initial onset on the right side, and 32 HCs. Age and gray matter density were used as covariates in the imaging analysis. All testing (fMRI and clinical) was conducted in the off state. Both left- and right-onset patients had higher regional homogeneity in right temporal pole compared with healthy adults. Additionally, right-onset patients had lower regional homogeneity in the right precentral gyrus and right middle frontal gyrus. The higher regional homogeneity in the right temporal pole was correlated with anxiety and depression symptoms in right-onset PD patients, which the authors implied could be a compensatory mechanism, as temporal pole has been implicated in regulation of emotion.
While examining differences between PD and Parkinsonism-plus syndrome (PPS), Cheng et al. (36) compared cerebral blood flow using arterial spin labeling in 20 PD patients, 16 PPS patients, and 17 HCs (36, 37). Compared with the HC group, both PD and PPS patients had reduced cerebral blood flow in the following regions: the right cerebellum, left middle frontal gyrus, triangle IFG, left frontal medial orbital gyrus, and left caudate nucleus. Interestingly, cerebral blood flow of the right cerebellum was positively correlated with scores on the HAM-A in patients with PPS but not in patients with PD.
In summary, in PD patients with depression or anxiety, increased FC has been reported between the amygdala and the OFC, putamen, and caudate; decreased FC has been reported between the OFC and dorsolateral prefrontal cortex, and OFC and sensorimotor cortices.
In addition to the functional studies, four structural studies examined structural correlates of anxiety. All these studies were conducted in the on state and are summarized in Table 1. These studies examined structural differences in PD patients with anxiety; while mixed, they showed some consistency with the functional studies above in that several reported changes in the amygdala, anterior cingulate, and caudate with increased anxiety.
Discussion
In this systematic review, we identified 18 studies that used a variety of fMRI methods to investigate anxiety and emotional regulation in patients with PD. The heterogeneity of methods resulted in little convergence in the reported findings between studies. Most studies focused on functional outcomes: eight studies used task-based approaches, six were during resting-states, and four reported structural changes. Fifteen of the 18 studies directly measured anxiety but included five different anxiety scales, making comparisons of behavioral outcomes across studies difficult (38). The imaging findings were mixed and inconsistent even when the same methods were employed; however, some convergence in findings suggests an association between anxiety in PD and functional changes in the amygdala, caudate, and putamen.
Although a few consistent findings were reported, our review shows that dopamine medication status at the time of image acquisition is an important consideration for both task-based activation and resting-state connectivity. In the task-based studies, depending on the brain regions involved, the on or off status appears to be associated with differences in activation. For instance, decreased activation in the pregenual anterior cingulate cortex was observed with an emotional Stroop task in PD patients off but not on medication, compared with control subjects (39). Similarly, Tessitore et al. (29) found activation in the bilateral amygdala in both control subjects and PD patients in the on state, but not in patients in the off state, in response to the Ekman faces task. However, the directionality of the association between medication status and activation is not consistent, with both the on and off state associated with decreased activation or deactivation depending on the study and brain region involved. In addition to activation changes, Dan et al. (30) observed that PD patients in the off state had increased BOLD response during an emotional face-task in the bilateral posterior cingulate/retrosplenial cortex compared with PD patients in the on state. Although it is not possible to discern a reliable pattern of activation change associated with the on state versus off state from the existing literature as a result of differences in methods, there is a clear indication that medication status must be considered when conducting functional neuroimaging studies in PD.
Although no clear mechanism underlying anxiety in PD was evident across studies, the amygdala, caudate, and putamen and their associated functional networks were most frequently reported as involved structures. Two resting-state studies (30, 34), one task-based study (29), and one structural study (40) found anxiety was correlated with reduced left amygdala volume and alterations in its network connectivity. However, the connectivity changes were increased in one study and negatively correlated with connectivity, and the structures involved with the amygdala network changes were not the same across those studies. Another task-based study using emotional words found the right amygdala (rather than the left) showed activation opposite that of HCs with decreased activation for negative words and increased activation for positive words in PD patients (28). Again, medication state changes seemed to yield the clearest dissociation, with a facial emotion recognition task failing to show activation in the bilateral amygdala in the of state but showing significant activation in controls and patients with PD in the on state (29). Yet, the relevance of this off state related failure to activate the amygdala network in PD remains unclear, as the aforementioned study did not include a measure of anxiety. Despite a lack of consensus, these findings suggest a role for the amygdala in anxiety in PD, particularly for anxiety with a dopaminergic basis, given that most alterations were a function of medication state.
Similarly, the caudate and putamen frequently showed alterations across studies, specifically in three of the task-based studies (25, 41, 42), one of the resting-state studies (32), and one of the structural neuroimaging studies (36). The task-based studies generally showed decreased activation in the putamen during tasks of emotional processing, although unfortunately none of these included correlations with anxiety (25, 41, 42). One resting-state study found that PD patients with anxiety had increased FC between the caudate and putamen and bilaterally decreased connectivity between the putamen and right orbital frontal gyrus and right cerebellum (32). Finally, BAI score was found to be negatively correlated with structural covariance between the right caudate nucleus and the contralateral striatal nuclei (43). The preponderance of findings in the putamen rather than the caudate parallels the pattern of dopaminergic dysfunction progressing from posterior putamen to the head of the caudate nucleus, which is relatively spared in most cases of early stages of PD (44, 45). However, dopaminergic denervation of the caudate in PD associated with depression and cognitive impairment reported in the literature is potentially consistent with the finding of increased anxiety associated with structural changes in the right caudate reported above (33). Anxiety in PD associated with dysfunction in the caudate and putamen in PD appears to parallel the progression of dopaminergic denervation in these structures (36). When combined with the findings from medication state changes in anxiety and emotional processing reported above, these results suggest that dopamine-based interventions may have a role in the treatment of anxiety disorders in PD.
The remaining literature reviewed here reported a variety of additional brain regions implicated in anxiety in PD, but with little consensus. The task-based functional imaging studies showed PD patients, in general, have an impaired response in the neural circuit associated with emotional processing. Specifically, some authors have observed an impaired amygdala response in PD patients compared with HCs based on medication state. These findings suggest that PD patients in a hypodopaminergic state may experience increased amygdala activity due to dopamine gating of amygdala inputs (29, 46, 47). In the resting-state studies, increased FC was observed between limbic regions, the OFC, and putamen and caudate. Alternately, decreased FC was observed between OFC and dorsolateral prefrontal cortex, and OFC and sensorimotor cortices. Ultimately, there was not enough consistency between these studies to confidently determine whether an association with PD anxiety and these networks or structures was present, as none of the studies reproduced previously reported findings. One exception was two resting-state studies published a year apart by the same author(32, 33). Both reported connectivity changes in the right orbital frontal gyrus and right cerebellum; however, these findings were not reported in other studies.
In the present review, we summarized the current literature, applying Cochrane’s robust methodology during the review process with more than one reviewer at each stage of the screening process to reduce bias. In addition to these strengths, several limitations should be discussed. First, we were unable to perform a meta-analysis as part of this systematic review due to the heterogeneity of anxiety assessments and the focus on imaging data. However, we provide a qualitative synthesis of the results. Furthermore, we included studies that assessed anxiety in a variety of ways, not all of which have been validated in the PD population. Most scales used in previous studies are not recommended by the Movement Disorders Society, except the PAS (38).
Conclusions
Currently, there is little consensus among MRI studies of anxiety in PD. Several issues likely contribute to the failure to find overlapping mechanisms across studies, including the use of different imaging methods, no standard for the assessment of anxiety, and the relatively small number of published studies. Despite these limitations, the amygdala, caudate, and putamen appear to play a role in PD anxiety across several studies, and there is a clear indication that dopaminergic medications alter patterns of task activation and resting-state networks. Future MRI studies in PD are needed to clarify the individual and network-based contributions of the amygdala, caudate, and putamen in PD patients identified as anxious using validated assessments while strictly accounting for medication state.
1. : Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2016; 31:1125–1133Crossref, Medline, Google Scholar
2. : Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Mov Disord 2010; 25:838–845Crossref, Medline, Google Scholar
3. : Report from a multidisciplinary meeting on anxiety as a non-motor manifestation of Parkinson’s disease. NPJ Parkinsons Dis 2019; 5:30Crossref, Medline, Google Scholar
4. : Anxiety and self-perceived health status in Parkinson’s disease. Parkinsonism Relat Disord 2011; 17:249–254Crossref, Medline, Google Scholar
5. : The clinical spectrum of anxiety in Parkinson’s disease. Mov Disord 2014; 29:967–975Crossref, Medline, Google Scholar
6. : Clinical markers of anxiety subtypes in Parkinson disease. J Geriatr Psychiatry Neurol 2018; 31:55–62Crossref, Medline, Google Scholar
7. : Autonomic failure, depression and anxiety in Parkinson’s disease. Br J Psychiatry 1995; 166:789–792Crossref, Medline, Google Scholar
8. : Update on treatments for nonmotor symptoms of Parkinson’s disease: an evidence-based medicine review. Mov Disord 2019; 34:180–198Crossref, Medline, Google Scholar
9. : The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ontario, Canada, Ottawa Hospital Research Institute, 2012Google Scholar
10. : Cochrane Consumers and Communication Review Group: data synthesis and analysis. London, Cochrane Consumers and Communication Review Group, 2013Google Scholar
11. : Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184Crossref, Medline, Google Scholar
12. : A population perspective on diagnostic criteria for Parkinson’s disease. Neurology 1997; 48:1277–1281Crossref, Medline, Google Scholar
13. : Diagnostic criteria for Parkinson disease. Arch Neurol 1999; 56:33–39Crossref, Medline, Google Scholar
14. : Manual for the State-Trait Anxiety Inventory. Menlo Park, Calif., Mind Garden, 1983Google Scholar
15. : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
16. : Beck Depression Inventory, 2nd ed. San Antonio, Tex., Psychological Corporation, 1996Google Scholar
17. : Screening tests for geriatric depression. Clin Gerontol 1982;1(1):37–43Crossref, Google Scholar
18. : The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997; 26(5):353–357Crossref, Medline, Google Scholar
19. : The Beck Anxiety Inventory. Psychol Assess 1993; 5:408–412Crossref, Google Scholar
20. : The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
21. : The Parkinson Anxiety Scale (PAS): development and validation of a new anxiety scale. Mov Disord 2014; 29:1035–1043Crossref, Medline, Google Scholar
22. : A rating instrument for anxiety disorders. Psychosomatics 1971;12(6):371–379Crossref, Medline, Google Scholar
23. : Dopaminergic modulation of emotional conflict in Parkinson’s disease. Front Aging Neurosci 2014; 6:164Crossref, Medline, Google Scholar
24. : Experience of negative emotions in Parkinson’s disease: an fMRI investigation. Neurosci Lett 2015; 609:142–146Crossref, Medline, Google Scholar
25. : Facial emotion recognition in Parkinson’s disease: an fMRI investigation. PLoS One 2015; 10:e0136110Crossref, Medline, Google Scholar
26. : Neural correlates of emotional valence processing in Parkinson’s disease: dysfunction in the subcortex. Brain Imaging Behav 2019; 13:189–199Crossref, Medline, Google Scholar
27. : Affective Norms for English Words (ANEW): Instruction Manual and Affective Ratings. Gainesville, Fla, Center for Research in Psychophysiology, University of Florida, 1999Google Scholar
28. : Impact of gender and genetics on emotion processing in Parkinson’s disease: a multimodal study. Neuroimage Clin 2018; 18:305–314Crossref, Medline, Google Scholar
29. : Dopamine modulates the response of the human amygdala: a study in Parkinson’s disease. J Neurosci 2002; 22:9099–9103Crossref, Medline, Google Scholar
30. : Separate neural representations of depression, anxiety and apathy in Parkinson’s disease. Sci Rep 2017; 7:12164Crossref, Medline, Google Scholar
31. : An fMRI study into emotional processing in Parkinson’s disease: does increased medial prefrontal activation compensate for striatal dysfunction? PLoS One 2017; 12:e0177085Crossref, Medline, Google Scholar
32. : Alterations of the amplitude of low-frequency fluctuations in anxiety in Parkinson’s disease. Neurosci Lett 2018; 668:19–23Crossref, Medline, Google Scholar
33. : Altered putamen functional connectivity is associated with anxiety disorder in Parkinson’s disease. Oncotarget 2017; 8:81377–81386Crossref, Medline, Google Scholar
34. : The relationship of anxious and depressive symptoms in Parkinson’s disease with voxel-based neuroanatomical and functional connectivity measures. J Affect Disord 2019; 245:580–588Crossref, Medline, Google Scholar
35. The relationship between side of onset and cerebral regional homogeneity in Parkinson’s disease: a resting-state fMRI study. Parkinson’s Dis 2020;2020:5146253Google Scholar
36. : Discriminative pattern of reduced cerebral blood flow in Parkinson’s disease and parkinsonism-plus syndrome: an ASL-MRI study. BMC Med Imaging 2020; 20:78Crossref, Medline, Google Scholar
37. : Fixel-based analysis reveals fiber-specific alterations during the progression of Parkinson’s disease. Neuroimage Clin 2020; 27:102355Crossref, Medline, Google Scholar
38. : Anxiety rating scales in Parkinson’s disease: a critical review updating recent literature. Int Psychogeriatr 2015; 27:1777–1784Crossref, Medline, Google Scholar
39. : A smaller amygdala is associated with anxiety in Parkinson’s disease: a combined FreeSurfer-VBM study. J Neurol Neurosurg Psychiatry 2016; 87:493–500Crossref, Medline, Google Scholar
40. : Anxiety in Parkinson’s disease is associated with reduced structural covariance of the striatum. J Affect Disord 2018; 240:113–120Crossref, Medline, Google Scholar
41. : Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 1991;114(5):2283–2301Crossref, Medline, Google Scholar
42. : Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease: pathophysiologic and clinical implications. N Engl J Med 1988; 318:876–880Crossref, Medline, Google Scholar
43. : Clinical implications of early caudate dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2019; 90:1098–1104Crossref, Medline, Google Scholar
44. : A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 1997; 25:192–216Crossref, Medline, Google Scholar
45. : Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology 2002; 27:1036–1040.Crossref, Medline, Google Scholar
46. : Impact of dopamine and cognitive impairment on neural reactivity to facial emotion in Parkinson’s disease. Eur Neuropsychopharmacol 2019; 29:1258–1272Crossref, Medline, Google Scholar
47. : Neural correlates of anxiety symptoms in mild Parkinson’s disease: a prospective longitudinal voxel-based morphometry study. J Neurol Sci 2016; 371:131–136Crossref, Medline, Google Scholar



