Understanding Psychiatric Disorders in Idiopathic and Inherited (Monogenic) Forms of Isolated and Combined Dystonia: A Systematic Review
Abstract
Objective:
The relationship between idiopathic and inherited (monogenic) forms of isolated and combined dystonia and psychiatric disorders remains unclear. In the present review, the authors aimed to provide increased clarity on this association through a systematic review of all controlled quantitative studies using a structured or semi-structured psychiatric interview to diagnose psychiatric disorders in individuals with these conditions.
Methods:
Three databases were searched to identify 20 eligible studies, with a total of 1,275 participants fulfilling inclusion criteria. Eligible articles were quality appraised and divided into four sections (idiopathic forms of dystonia [N=11], early-onset torsion dystonia [N=2], gene mutation positive myoclonus dystonia; DYT-SGCE [N=6], and rapid-onset dystonia-parkinsonism [N=1]).
Results:
For each study, results were grouped into subcategories (overall psychiatric comorbidity, anxiety disorders, mood disorders, substance misuse, and other [personality disorder and cognitive impairment]). For idiopathic dystonia, higher rates of psychiatric comorbidity, including mood and anxiety disorders, were noted when cases were compared with both healthy control subjects and control groups with a medical comorbidity. However, for major depressive disorder and obsessive-compulsive disorder (OCD) specifically, no differences were seen between groups. Study subjects with DYT-SGCE appeared to be at higher risk of psychiatric comorbidity, major depressive disorder, OCD, and alcohol dependence than control populations.
Conclusions:
Overall, the prevalence of psychiatric comorbidity appears to be increased in individuals with idiopathic and inherited (monogenic) forms of isolated and combined dystonia compared with control subjects. This finding is not consistent for all comparisons, and further research is required to understand the nature of these associations and the underlying causative etiologies.
Dystonia describes a number of hyperkinetic movement disorders presenting with intermittent or sustained patterned, twisting, or tremulous muscle contractions that can result in abnormal postures. In 2013, Albanese et al. (1) proposed a new classification system for dystonia structured around two distinct axes. The first describes the clinical characteristics of an individual patient, including age at onset, body distribution, temporal pattern, and associated features (e.g., comorbid movement or neurological manifestations). The second axis classifies dystonia according to etiology, with subsections for dystonia occurring secondary to pathology of the nervous system, inherited dystonia (autosomal dominant, autosomal recessive, X-linked recessive, and mitochondrial), acquired dystonia (secondary to perinatal complications, vascular injury, neoplasm, trauma, toxins including drugs, infections, and psychogenic causes), and idiopathic dystonia (divided into sporadic and familial forms). In this systematic review, we utilized the second axis and aimed to explore the current and lifetime prevalence of psychiatric diagnoses occurring in idiopathic and inherited (monogenic) forms of isolated and combined dystonia.
The underlying cause of many forms of dystonia remains unclear. While electrophysiological studies and functional neuroimaging have suggested that abnormalities in a number of neural networks located throughout the cerebral cortex, cerebellum, thalamus, midbrain, and brainstem are involved (2, 3), it is likely that significant etiological heterogeneity exists between the different forms of dystonia, with variations altering the extent of involvement in various brain regions and networks and the aberrant communication between them (3). Two previous reviews have been completed on this subject. In 2011, Kuyper et al. (4) published on nonmotor phenomena in primary dystonia. The search employed was not systematic, did not use a replicable strategy, and focused mainly on mood disorders. No attempts were made to robustly critique the methodology of primary data studies, and equal importance was attributed to all results, regardless of study design or statistical significance. In 2017, Jahanshahi et al. (5) explored the cognitive features of idiopathic and early-onset torsion dystonia (DYT-TOR1A), including presentation both before and after deep brain stimulation. No changes in cognition were noted secondary to this intervention.
The relationship between idiopathic and inherited forms of dystonia and mental illness remains uncertain. The objective of this systematic review is to provide an up-to-date appraisal of all controlled quantitative studies completed using structured and/or semi-structured interview techniques to explore the current and lifetime prevalence rates of psychiatric diagnoses occurring in subjects with idiopathic and inherited forms of dystonia. Specifically, we aim to improve upon previous publications through coverage of all forms of idiopathic and inherited (monogenic) isolated or combined dystonia included in the published literature, strict search and entry criteria for primary data studies, and inclusion of a standardized critique of the methodology of all primary data studies meeting inclusion criteria.
Methods
Search Strategy and Selection Criteria
Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines informed the strategy for this systematic review (6). We searched PsychINFO, Medline, and Embase for articles published from January 1, 1806, 1946, and 1974, respectively, to October 27, 2020, with the terms: “(dystonia OR spasmodic torticollis OR blepharospasm OR spasmodic dysphonia OR writer’s cramp OR dyt1 OR dyt2 OR dyt3 OR dyt4 OR dyt5 OR dyt6 OR dyt7 OR dyt8 OR dyt9 OR dyt10 OR dyt11 OR dyt12 OR dyt13 OR dyt14 OR dyt15 OR dyt16 OR dyt17 OR dyt18 OR dyt20 OR dyt24 OR dyt25 OR dyt28 OR DYT-TOR1A OR DYT-THAP1 OR DYT-ANO3 OR DYT-GNAL OR DYT-KMT2B OR DYT-GCH1 OR DYT-TH OR DYT-SPR OR DYT-TAF1 OR DYT-PRKRA OR DYT-ATP1A3 OR DYT-SGCE OR PxMD-PNKD OR PxMD-PRRT2 OR PxMD-SLC2A1 OR PxMD-ECHS1) AND (structured clinical interview OR SCID OR psy screen OR schedules for clinical assessment in neuropsychiatry OR SCAN OR MINI OR diagnostic interview schedule OR DIS OR schedule for affective disorders and schizophrenia OR SADS OR composite international diagnostic interview OR CIDI OR revised clinical interview schedule OR CIS).” Terms were designed to capture all forms of inherited dystonia identified to date, using both the now outdated DYT nomenclature and the new naming system for the nomenclature of genetic movement disorders outlined by the International Parkinson’s Disease and Movement Disorder Society Task Force (7). To ensure reproducibility, the initial database search was completed by two authors independently (M.L. and V.L.) and the results collated.
Many forms of complex dystonia, including those occurring secondary to neurodegeneration, structural lesions, and acquired pathology, were intentionally excluded from the search criteria for this systematic review, as they already have widely accepted significant psychiatric presentations. The purpose of this review is to explore acquired and inherited (monogenic) isolated or combined dystonia, where the relationship with psychopathology remains unclear.
In order to maximize the sensitivity of the search, terms describing mental state were intentionally not included. Instead, two authors (M.L. and V.L.) completed a two-step literature search; when a title or abstract appeared to fulfill the inclusion criteria, the full text of the article was reviewed to assess eligibility. Any disputes were settled by consultation with the senior author (H.R.). Additionally, the references for all publications meeting the inclusion criteria were reviewed for further eligible studies.
Studies were eligible for inclusion if they reported primary data from human populations with a diagnosis of idiopathic or inherited dystonia according to standardized diagnostic criteria, were published in a peer-reviewed journal, and used a validated psychiatric structured or semi-structured interview based on a standardized psychiatric diagnostic manual to determine psychiatric diagnosis. Both adult and pediatric populations were included, with no age limit or language restrictions.
Studies that did not include a control group were excluded. Studies that compared cases to a representative sample of the population were included, though the methodological choice to use normative data instead of a matched control group is highlighted in the study critique. One study was excluded despite having a control group, on the grounds that authors excluded all control subjects with a psychiatric diagnosis. Studies published in the form of conference abstracts, posters, or oral presentations were excluded because they were not subject to the peer-review process (8).
Each article was quality appraised using the validated Quality Assessment Tool for Quantitative Studies developed by the Effective Public Health Practice Project (9). This allowed a standardized review of each study in eight areas: selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity, and analysis. To ensure reproducibility, each article was appraised by two authors independently (V.L. and M.L.), with disputes resolved by consultation with the senior author (H.R.).
Study Analysis
The 20 eligible studies (Figure 1) were grouped into idiopathic dystonia (N=11) or inherited forms of dystonia, which comprise DYT-TOR1A (N=2), epsilon-sarcoglycan (SGCE) gene mutation positive myoclonus dystonia (DYT-SGCE) (N=6), and rapid-onset dystonia-parkinsonism (DYT-ATP1A3; N=1). Each study was reviewed by two authors independently (V.L. and M.L.) using a standardized template that included participants, interventions, comparisons, outcomes, funding sources, and all results relating to psychiatric diagnoses. These results were then grouped under the following subcategories: overall psychiatric comorbidity, anxiety disorders, mood disorders, substance misuse, and other (personality disorder and cognitive functioning). Where studies specified diagnoses, they are marked as current or lifetime.
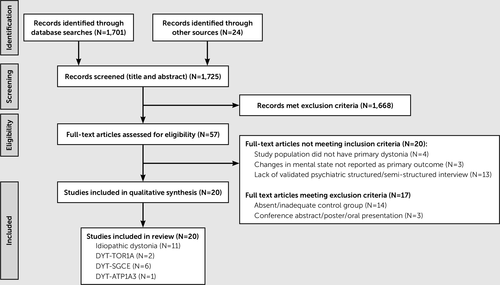
a DYT-TOR1A=early-onset torsion dystonia; DYT-ATP1A3=rapid-onset dystonia-parkinsonism; DYT-SGCE=SGCE gene mutation positive myoclonus dystonia.
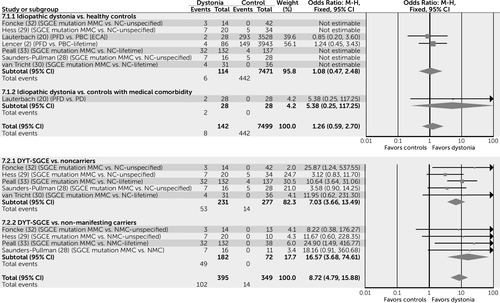
In order to facilitate the interpretation of key results, the four most commonly reported categories of psychiatric diagnosis are displayed as forest plots, created using Review Manager, version 5.3 (10). These include overall psychiatric comorbidity (Figure 2), mood disorders (Figure 3), anxiety disorders (Figure 4), and alcohol dependence (Figure 5). These are incorporated only to provide a visual representation of key results using simple pooling of data.
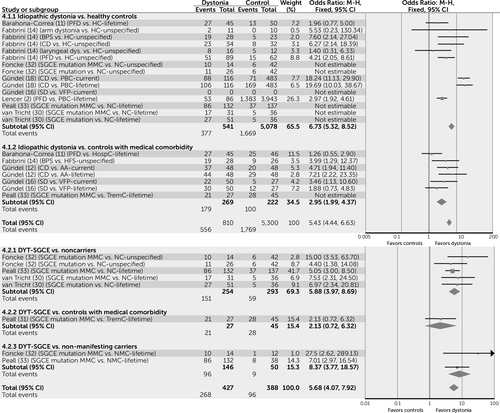
a AA=alopecia areata; BPS=blepharospasm; CD=cervical dystonia; HC=healthy control; HFS=hemifacial spasm; HospC=hospital control subjects; M-H=Mantel-Haenszel method; MMC=motor manifesting carriers; NC=noncarrier; PBC=population-based controls; PFD=primary focal dystonia; SD=spasmodic dysphonia; TremC=tremor control subjects; VFP=vocal fold paralysis.
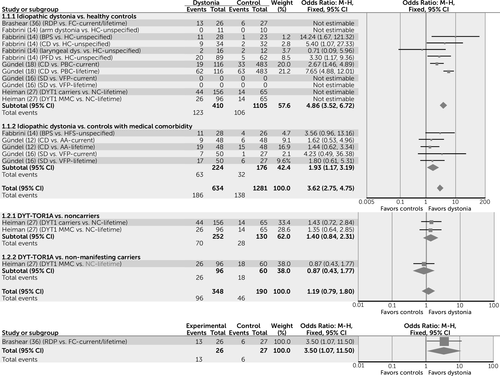
a AA=alopecia areata; BPS=blepharospasm; CD=cervical dystonia; FC=familial controls; HC=healthy control; HFS=hemifacial spasm; M-H=Mantel-Haenszel method; MMC=motor manifesting carriers; NC=noncarrier; PBC=population-based controls; PFD=primary focal dystonia; RDP=rapid-onset dystonia-parkinsonism; SD=spasmodic dysphonia; VFP=vocal fold paralysis.
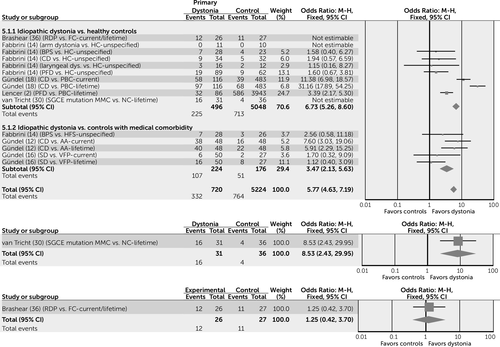
a AA=alopecia areata; BPS=blepharospasm; CD=cervical dystonia; FC=familial controls; HC=healthy control; HFS=hemifacial spasm; M-H=Mantel-Haenszel method; MMC=motor manifesting carriers; NC=noncarrier; PBC=population-based controls; PFD=primary focal dystonia; RDP=rapid-onset dystonia-parkinsonism; SD=spasmodic dysphonia; VFP=vocal fold paralysis.
a ECA=Epidemiologic Catchment Area Study; MMC=motor manifesting carriers; NC=noncarrier; PBC=population-based controls; PFD=primary focal dystonia.
Results
Our objective was to examine the prevalence of current and lifetime psychiatric diagnoses in patients of any age with idiopathic and inherited (monogenic) isolated/ combined forms of dystonia.
Idiopathic Dystonia
Eleven studies collected data on 544 patients with idiopathic forms of dystonia (2, 11–20). Specifically, these included cervical dystonia (N=350), blepharospasm (N=102), spasmodic dysphonia (N=50), laryngeal dystonia (N=16), writer’s cramp (N=15), and arm dystonia (N=11). The control groups comprised 263 control subjects with a medical comorbidity (hemifacial spasm, N=101; Parkinson’s disease [PD], N=56; alopecia areata, N=48; vocal fold paralysis , N=27; carpal tunnel syndrome, N=17; cervical spondyloarthropathy/cervical spinal root compression, N=14) and 115 healthy control subjects. The healthy control group included unspecified healthy subjects (N=62), friends of cases (N=30), and healthy subjects from other medical specialties (N=23). In addition to this, four studies also compared cases to a representative sample of population-based control subjects (2, 12, 18, 20). Studies are reviewed according to publication date.
Broocks et al. (19) compared 13 study subjects with blepharospasm to 13 hemifacial spasm control subjects. No differences in the prevalence of psychiatric diagnoses were identified, which may in part be attributable to the small sample size and the eligibility criterion that all subjects were required to have received botulinum A toxin, which may have temporarily reduced potential triggers for psychiatric illness, including pain and disfigurement. Additionally, all subjects prescribed antidepressant or neuroleptic medications in the preceding 6 months were excluded, which may have further reduced the prevalence of psychiatric comorbidity in this sample.
Gündel at al. (18) compared 116 cervical dystonia cases recruited from a botulinum toxin clinic to 483 population-based control subjects taken from the Munich Follow-Up Study. A higher prevalence rate of both current and lifetime psychiatric diagnoses was noted in cervical dystonia cases, particularly with regard to anxiety and mood disorders. The main limitation of this study is that normative population data were used in lieu of a well-matched control group. It is worth noting that to be eligible for inclusion, cases were required to have had no previous exposure to neuroleptic medications and to have undergone treatment with botulinum toxin. This makes the results all the more striking, as both of these criteria may have contributed to a relative bias that may have acted to reduce the prevalence of psychiatric diagnoses in the case group.
A second study by Gündel et al. (12) compared cases with cervical dystonia to matched control subjects with alopecia areata and a representative sample of the German general population. Cervical dystonia case subjects were more likely than alopecia areata control subjects to have a psychiatric diagnosis, particularly an anxiety disorder. These trends remained when cases were compared with population-based control subjects, where an additional increase in mood disorders was also noted. To control for the impact of disfigurement on the prevalence of psychiatric diagnoses, participants with cervical dystonia and alopecia areata were matched for body image dissatisfaction. Unfortunately, the value of this matching was limited, as the visual analog scale used was highly subjective, unvalidated, and not specific to primary dystonia. Furthermore, no attempts were made to control for pain, which commonly presents in cervical dystonia and may have had a secondary impact on mental state (21).
Lauterbach et al. (17) compared 28 patients with cervical dystonia to 28 control subjects with PD. Generalized anxiety disorder (GAD) was more common in the cervical dystonia group: over half of subjects with anxiety also reported a comorbid mood disorder. Conversely, the PD group had a higher lifetime prevalence of panic disorder. The gender disparity (71.4% of female cervical dystonia case subjects compared with 35.7% of PD control subjects) between groups may have contributed to the higher levels of GAD reported in the cervical dystonia group, as this condition more commonly manifests in females (22). Higher rates of anticholinergic drug use in the PD group may have predisposed these individuals to panic disorder through an increased incidence of related side effects. Additionally, the higher average age in the PD control group (13.29 years older) may have increased the opportunity for control subjects to accumulate a psychiatric diagnosis over a longer lifetime. It is worth noting that none of the PD control subjects fulfilled the diagnostic criteria for GAD, which is unlikely to be representative of this population; prevalence data suggest that 5.3%−40% of subjects with PD have an anxiety disorder (23).
A second study by Lauterbach et al. (20) on the same population calculated odds ratios for the lifetime prevalence of psychiatric conditions in cervical dystonia/PD subjects compared with 3,528 control subjects drawn from the National Institutes of Health Epidemiologic Catchment Area (ECA) Study (24). Subsequent comparison of the two groups demonstrated significantly higher lifetime prevalence rates of social phobia, GAD, major depressive disorder, and bipolar disorder in the cervical dystonia group, whereas PD control subjects reported higher rates of phobic disorders and simple phobia. Presumably the ECA study control subjects were used to compensate for the poor matching of subject groups seen in the previous Lauterbach study. This approach has limitations, as the ECA data were collected more than 20 years prior and classified subjects using broad age brackets only (24).
Gündel et al. (16) completed a third study comparing 50 subjects with spasmodic dysphonia to a control sample with vocal fold paralysis (N=27) resulting from a postoperative lesion of the laryngeal nerve. A higher proportion of subjects with spasmodic dysphonia reported a current psychiatric diagnosis compared with vocal fold paralysis control subjects (44% [N=22/50] versus 18.5% [N=5/27]), and this psychiatric comorbidity was significantly associated with increased clinical severity of voice impairment (odds ratio [OR]=41.7, p=0.003). No other differences in the prevalence of psychiatric illness between groups were noted. One limitation of this study is that despite the severity of voice impairment being a significant factor in the development of psychiatric comorbidity, groups were not matched for this variable (higher levels in cases).
Lencer et al. (2) compared 86 patients with primary focal dystonia (cervical dystonia, N=70) and blepharospasm (N=16) to a control sample from the Northern German general population (N=3,943). Higher rates of social phobia, agoraphobia, panic disorder, OCD, major depressive disorder, alcohol abuse, and drug dependence were seen in primary focal dystonia cases. Posttraumatic stress disorder (PTSD) was the only condition noted to be more prevalent in control subjects. The choice of an unmatched normative population control sample limits the value of these findings, particularly as the data were drawn from a study published in 2000 (25), which primarily focused on substance misuse in 4,075 individuals. No information was provided on how the 3,943 control subjects included in the analysis were selected. Additionally, no information was provided on the 20% of the case sample who refused to participate, raising the possibility of selection bias.
Dias et al. (15) compared 22 cases with blepharospasm to 29 control subjects with hemifacial spasm. Higher current prevalence rates were seen in blepharospasm cases for specific phobia, social phobia, GAD, and OCD. Hemifacial spasm control subjects had marginally higher prevalence rates of other anxiety disorders (including hypochondria, body dysmorphic disorder, panic disorder, and PTSD), mood disorders (including major depressive disorder and dysthymia), and abuse of or dependence on alcohol). None of the differences between groups were significant.
Fabrinni et al. (14) compared four individual groups with focal dystonia (cervical dystonia [N=34], blepharospasm [N=28], laryngeal dystonia [N=16], and arm dystonia [N=11]) to age-matched control subjects (healthy subjects [N=62] and hemifacial spasm [N=26]). Psychiatric diagnosis was found to be more prevalent in cases, with significant results obtained for cervical dystonia and blepharospasm. The prevalence rates of anxiety and mood disorders were again higher in cases, particularly with regard to OCD. The observations of this study are limited by a paucity of information on the control group; no information was given on how subjects were recruited or matched. In particular, it is not clear why only the blepharospasm group was compared with the hemifacial spasm control subjects.
Barahona-Corrêa et al. (11) compared 45 cases with primary focal dystonia (cervical dystonia [N=15], blepharospasm [N=15], and writer’s cramp [N=15]) to 76 control subjects (hemifacial spasm [N=15], cervical spondyloarthropathy/cervical spinal root compression [N=14], carpal tunnel syndrome [N=17], and healthy volunteers [N=30]). The overall prevalence of psychiatric disorders was the same between groups. However, agoraphobia, social phobia, OCD, and psychosis were more common in primary focal dystonia cases, whereas GAD, major depressive disorder, dysthymia, heroin abuse in remission, and somatization were more prevalent in control subjects. The higher proportion of female control subjects may have skewed the results in favor of this group reporting increased levels of GAD, major depressive disorder, and dysthymia.
Finally, Mula et al. (13) compared 19 cases with focal dystonia (cervical dystonia [N=11] and blepharospasm [N=8]) to matched control subjects (hemifacial spasm [N=18] and healthy control subjects [N=23]). This study reported very limited results pertaining to the prevalence of psychiatric diagnoses. Both case subjects and hemifacial spasm control subjects reported higher levels of psychiatric comorbidity than the healthy control group. Although groups were matched, only six males were included in the whole study, reducing the external validity of results.
Inherited (Monogenic) Forms of Isolated and Combined Dystonia (DYT-TOR1A; formerly DYT1)
Heiman et al. (26, 27) completed two studies in 2004 and 2007 comparing 221 subjects (motor manifesting carriers [MMCs] of DYT-TOR1A GAG deletion, N=96; non-manifesting carriers [NMCs], N=60), and noncarriers [NCs], N=65). The 2004 study noted a trend that carriers of the mutation had higher lifetime prevalence rates of affective disorders compared with NC control subjects. A similar trend was noted when the MMC and NMC groups were individually compared with the NC group. No associations were seen between the severity of dystonia and the risk of depression (27).
The 2007 study by Heiman et al. specifically explored the association of OCD with the TOR1A gene (26). Only seven participants met the Composite International Diagnostic Interview (CIDI) criteria for OCD (MMC=3, NMC=1, and NC=3). The study reported no significant results. These findings are prone to response bias, as only half (53%) of eligible subjects participated. Rather than using face-to-face interviews, trained interviewers administered the CIDI via telephone. No information is given on how this training took place or the qualifications and experience of these interviewers. The methods describe attempts to reach each participant at least seven times; interviewers were blinded to both genotype and the study hypothesis.
SGCE gene mutation positive myoclonus dystonia (DYT-SGCE, formerly DYT11)
Six studies explored the prevalence of psychiatric diagnoses in populations with DYT-SGCE (28–33). Patients with an SGCE mutation were assessed for the presence of motor symptoms and split into a symptomatic group (MMC, N=240) and an asymptomatic group (NMC, N=82). Studies compared these groups to NCs without the mutation (N=293). It is worth noting that studies refer to all subjects with the SGCE gene as carriers (MMC and NMC) and subjects without any motor symptoms as asymptomatic (NMC and NC). In addition to these groups, van Tricht et al. (30) included data on 20 subjects with a myoclonus dystonia phenotype who were found not to have the SGCE mutation, whereas Peall et al. (31) compared SGCE mutation positive carriers to control subjects with an alcohol responsive tremor (N=45). Additionally, three studies included an additional analysis with population-based control subjects (28, 29, 33).
Saunders-Pullman et al. (28) compared 27 SGCE mutation carriers (MMC, N=16; and NMC, N=11) from three families to NCs (N=28), and population estimates. OCD was more common in carriers compared with NCs and also in MMCs compared with groups without motor signs. OCD occurred after motor phenomena in all MMC subjects and was unrelated to the severity of motor signs. Alcohol dependence was also more common in the MMC group than asymptomatic groups. The small numbers of individuals with psychiatric comorbidity and poor gender matching limit the value of these observations.
Hess et al. (29) compared 30 carriers from five families (MMC, N=20; and NMC, N=10) with NCs (N=34). No significant differences were noted in the prevalence of psychiatric disorders when the carrier groups were compared with the NC group. However, when the MMC group was compared with the groups with no motor signs (NMC and NC), higher rates of OCD and alcohol dependence were seen. Again, OCD was noted to occur after the onset of motor signs. We noted that the NMC group actually had a lower prevalence of most psychiatric disorders compared with population estimates drawn from the National Comorbidity Survey Replication (34). This may have skewed results in favor of the MMC group manifesting higher rates of OCD than asymptomatic participants.
Foncke et al. (32) reported on one large Dutch family (MMC, N=14; NMC, N=13; and NC, N=42) consisting of 38 mutation-negative family members and four married-in spouses. Subjects with an SGCE mutation were found to have significantly higher levels of all DSM-IV psychiatric diagnoses and a trend toward higher rates of anxiety and mood disorders, when compared with the NC group (35). Out of the 11 mutation carriers (MMC/NMC) found to have a psychiatric diagnosis, only one did not have motor signs. Groups were poorly matched for age and gender, with more males in the MMC group.
Peall et al. (31) compared carriers (MMC, N=27; and NMC, N=10) with control subjects (NC, N=16), and subjects with an alcohol responsive tremor (N=45) recruited from the United Kingdom and Ireland. Significantly higher rates of DSM-IV disorders were seen in the MMC group compared with the NMC group, but not in the MMC/NMC groups compared with tremor control subjects. MMC subjects had four times the risk of both social phobia and GAD and seven times the risk of OCD compared with tremor control subjects. This study used alcohol responsive tremor control subjects (matched for quality of life using the Short Form Health Survey) to examine the common association that is seen between DYT-SGCE and alcohol excess, which is thought to occur because of the therapeutic effect that alcohol mediates on motor symptoms. Interestingly, higher rates of alcohol dependence/abuse were seen in MMC subjects compared with both tremor control subjects and population estimates, suggesting there may possibly be an SGCE gene effect predisposing to alcohol-related comorbidity. This association is far from clear, as no significant differences were noted between NMC cases and control subjects.
Peall et al. (33) completed a second multicenter study comparing 132 MMC, 38 NMC, and 137 NC subjects recruited from multiple locations (the Netherlands, the United Kingdom, the United States, and Germany). The MMC group had seven times higher rates of DSM-IV psychiatric diagnoses than the NMC group and five times higher rates than the NC group. Specifically, MMC subjects reported higher rates of anxiety disorders (GAD, panic disorder, specific phobia, social phobia, and OCD) than asymptomatic groups (NMC and NC). Higher rates of major affective disorder were also seen in MMC subjects compared with NCs. With regard to alcohol consumption, a significant difference was seen between MMC and NMC groups; the NMC and NC patients had similar levels of alcohol intake.
van Tricht et al. (30) compared 51 subjects (MMC, N=31; non-mutation-carrying myoclonus dystonia, N=20) with 36 NCs (32 SGCE mutation-negative family members and four married-in spouses). Carriers reported a higher prevalence of psychiatric comorbidity compared with control subjects. Specifically, anxiety disorders were found to be 15 times more common in MMCs. It is worth noting that control subjects were younger than cases at the time of assessment, reducing the length of time over which they might have developed psychiatric symptoms. In addition, the classification of nonmutation-carrying myoclonus dystonia subjects may change over time, as new forms of the SGCE mutation are being recognized.
Rapid-onset dystonia-parkinsonism (DYT-ATP1A3, formerly DYT12)
Brashear et al. (36) compared 29 cases with the DYT-ATP1A3 mutation to 27 familial control subjects. Higher rates of mood disorders, substance misuse disorders, and psychosis were noted in cases. Results may have been influenced by poor gender matching and the exclusion of NMCs (N=3) from the statistical analysis. It is also worth noting the possible limitations of using familial controls, who might share many of the biopsychosocial factors that predispose case subjects to developing mental illness, including genetic risk and the stress of living with or caring for a loved one with a chronic condition.
Discussion
Principal Findings
Simple pooling of available data suggests that for those subjects with idiopathic forms of dystonia, risk of psychiatric comorbidity is increased by a factor of seven compared with healthy control subjects (odds ratio=6.74, 95% confidence interval [CI]=5.32–8.52) and a factor of three when compared with control subjects with a medical comorbidity (odds ratio=2.95, 95% CI=1.99–4.37) (Figure 2).
MMCs of the DYT-SGCE mutation are also more likely to experience psychiatric comorbidity than both NCs (odds ratio=5.88, 95% CI=3.97–8.69) and NMCs (odds ratio=8.37, 95% CI=3.77–18.57), suggesting that this association may be independent of gene status (Figure 2).
Associations are similar for mood disorders: Subjects with idiopathic forms of dystonia appear to have five times the risk of developing a mood disorder when compared with healthy control subjects (odds ratio=4.86, 95% CI=3.52–6.72) and two times the risk when compared with control subjects with a medical comorbidity (odds ratio=1.93, 95% CI=1.17–3.19) (Figure 3). Subjects with idiopathic dystonia appear to be more likely to have major depressive disorder than healthy control subjects (odds ratio=4.79, 95% CI=3.64–6.29), though this association is not found when idiopathic dystonia is compared with control subjects with a medical comorbidity (odds ratio=1.19, 95% CI=0.80–1.77), suggesting that in idiopathic dystonia, major depressive disorder may develop as a secondary nonspecific response to illness.
In contrast to idiopathic dystonia, subjects with DYT-TOR1A appear to have a similar risk to control subjects (NC and NMC) of developing a mood disorder (odds ratio=1.19, 95% CI=0.79–1.80). Specifically, findings in the DYT-TOR1A population for major depressive disorder suggest that the risk is only marginally increased when MMCs are compared with NCs (odds ratio=1.85, 95% CI=1.08–3.17), but that this association is not found when MMCs are compared with NMCs (odds ratio=0.94, 95% CI=0.46–1.93). Overall, subjects with DYT-SGCE are more likely to have major depressive disorder when compared with both NCs (odds ratio=2.92, 95% CI=1.74–4.90) and NMCs (odds ratio=2.51, 95% CI=1.02–6.20), though not when compared with tremor control subjects (odds ratio=1, 95% CI=0.38–2.61).
Anxiety conditions also appear to be increased in subjects with idiopathic dystonia, compared with both healthy control subjects and control groups with a medical comorbidity (odds ratio=5.77, 95% CI=4.63–7.19) (Figure 4). The reasons for this observation are unknown; it may be that there is something specific to the experience of having an idiopathic form of dystonia that increases the risk of anxiety conditions in a way that other physical illnesses do not. The complex and ambiguous association between the human experience of neurotic symptoms and changes in muscle tone is well documented (33). This effect may be bidirectional, with the perceived increase in muscle tone experienced in dystonia compounding the subjective experience of mental tension and leading to the subsequent development of anxiety. Specifically, the risk of OCD appears to be higher when subjects are compared with healthy control subjects (odds ratio=3.46, 95% CI=1.49–8.00), but not when compared with control subjects with a medical comorbidity (odds ratio=2.14, 95% CI=0.78–5.85).
Data are lacking for the association between overall anxiety conditions and inherited forms of dystonia. However, with regard to OCD, subjects with DYT-SGCE appear to be at higher risk than all forms of control (NC, medical comorbidity and NMC; odds ratio=6.47, 95% CI=4.09–10.25). Although this suggests that the presence of motor signs rather than the SGCE gene is important in the development of OCD, this observation should be treated with caution, as it is based on only four out of a possible 131 NMC subjects who reported a diagnosis of OCD. No differences were noted in the prevalence of OCD in subjects with both DYT-TOR1A and DYT-ATP1A3, when compared with control populations.
No differences were noted in the risk of alcohol dependence between subjects with idiopathic dystonia and control subjects (odds ratio=1.2, 95% CI=0.59–2.70). Conversely, data pertaining to DYT-SGCE suggest that MMCs have nine times the risk of developing alcohol dependence compared with both NC and NMC control subjects (odds ratio=8.72, 95% CI=4.79–15.88) (Figure 5), suggesting that this association is likely to be due to the palliative effects of alcohol on motor symptoms rather than a direct effect of the SGCE gene.
Assessment of Quality and Limitations
These findings should be considered in conjunction with a number of limitations relating to the evidence from which they are drawn. As idiopathic and inherited forms of dystonia are rare, many of the studies not only reported on small numbers of sequentially sampled cases but also analyzed multiple types of primary dystonia in the same patient cohort. This has limited the ability of this review to examine the psychiatric associations of each individual condition and has led to subsequent subgroup analysis of results.
Because this study is a systematic review, the limitations pertaining to the overall effect estimates calculated and displayed in the forest plots need to be considered. These results were obtained using simple pooling of available data to provide an overall visual summary of results (Figures 2–5). This form of analysis can yield spurious findings, as data are combined irrespective of weighting for differences in study design, statistical models, and heterogeneity of study populations. Findings are limited by both small sample sizes and the limitations of publication bias, as smaller studies with negative findings are generally less likely to have been published and included in this review.
As only one study included analysis of subjects aged below 18 years of age, results cannot be generalized to pediatric populations (31). Furthermore, developed countries are overrepresented; only one study was completed on a population sampled from an upper-middle income country, Brazil (15). This is relevant, as the wealth of a nation has implications for the provision of mental health services and therefore the recognition and treatment of psychiatric illness (37).
Generally, study subjects were sampled from single locations; only two studies recruited from multiple centers (31, 33). This approach increased the risk of sampling bias by reducing the likelihood that cases were representative of their cohort population. For example, nine studies recruited from subpopulations in tertiary centers, which may have introduced selection bias (specifically, Berkson’s bias). However, the direction of this bias on the characteristics of the case population is unclear. It could be argued that tertiary patients are more likely to experience severe and disabling dystonia necessitating specialist intervention. Conversely, those with severe signs might be less able to travel to distant centers and therefore may have been underrepresented. The restrictions of each type of recruitment site (care registers, support groups, movement disorder outpatient clinics, botulinum toxin clinics, and inpatient units) should also be considered when interpreting results. For example, sampling dystonia support groups may have introduced membership bias, as subjects with higher levels of subjective distress and psychopathology might be more likely to seek support through membership. On the other hand, using botulinum toxin clinics as a site for recruitment may have falsely lowered the point prevalence of psychiatric illness; this treatment not only temporarily reduces associated pain and disfigurement but may also have additional effects on motor and psychiatric signs through a reduction in cortical somatosensory activity (12, 15).
To control for these confounding factors, matching between groups is essential. Despite this, only 17 of the studies provided data on the most basic variables of age (adequately matched, N=11) and gender (adequately matched, N=3). Unfortunately, as most studies made no attempt to match groups for disability or disfigurement, the effect size contributed by the secondary nonspecific effects of having a chronic illness on the prevalence rates of mental illness are less clear.
Unfortunately, there are no validated psychiatric assessment tools for use in subjects with dystonia. To ensure the objective and standardized assessment of psychopathology, studies were required to use a structured psychiatric interview technique based on DSM or the International Classification of Diseases classification systems (38). The most commonly used diagnostic assessments were the Structured Clinical Interview for DSM (SCID), used in 12 studies (2, 12–14, 16–19, 30, 32, 33, 36,); the CIDI, used in eight studies (2, 12, 26–29, 33, 36); Mini International Neuropsychiatric Interview–Plus, used in five studies (11, 15, 30, 31, 33); and the Diagnostic Interview Schedule, used in two studies (18, 20). A number of studies also used a combination of assessor and self-rated psychiatric scales, including the brief self-reported Symptom Checklist–90–R, designed to broadly assess psychopathology (11–13, 18, 19, 39); the Beck Depression Inventory (14, 15, 20, 32, 40) and Montgomery Åsberg Depression Rating Scale (30–32, 41) to assess depression; and the Beck Anxiety Inventory (30, 32, 42), the Social Phobia Scale (12, 18, 43), and the Yale-Brown Obsessive Compulsive Scale (11, 13–15, 30–32, 36, 44) to assess anxiety and OCD. Significantly higher scores were noted among dystonia cases compared with control subjects for scales measuring depression (14, 30–32, 36), anxiety (30, 32), social phobia (12) and OCD (11, 19, 31) (specifically relating to increased compulsions involving control, cleaning, and ordering [19]).
Standardized assessments are advocated as the gold standard for psychiatric diagnostic interviewing (45) and have previously been described as the cornerstone of evidence-based assessment (46). These assessments have been reviewed extensively with regard to their degree of efficacy and reliability in obtaining psycho-diagnostic information and can be easily deployed by clinically inexperienced assessors (45).
Although there are many benefits to their use, the potential biases introduced through the use of these categorical systems are worthy of mention. The goal of psychiatric interviewing is to translate an individual’s subjective lived experience and objective observable appearance into an insightful, actionable format that informs diagnostic classification and guides treatment decisions. The structured interview predefines what counts as important information by employing a format that involves asking the individual a series of set questions in sequence and rating their response, in order to determine the diagnosis. The lack of conversational flow and contextual flexibility may mean that important subtleties in an individual’s presentation are missed or misinterpreted. Building on the notion of Gestalt, oversimplification of psychopathology can lead to diagnostic inaccuracy, as the whole is often so much more than the sum of its parts (45). More specifically, the structured interviews discussed in this article are not specific to dystonia and therefore may have missed important subtleties and subthreshold phenomena in the complex psychopathology of subjects, which may be key to understanding the differences in presentation between cases and control subjects. There may also be a degree of selection bias, particularly in relation to control subjects; for example, the small numbers of NMCs may not be representative of the group as a whole. The effects of these biases may be further compounded by the decision of some studies to use potentially clinically inexperienced assessors, including trained interviewers (12, 18, 28, 36), telephone interviewers (26, 27), and research assistants (32).
Although this article is able to offer information on the prevalence of current and lifetime psychiatric diagnoses in patients of any age with idiopathic and inherited (monogenic) isolated or combined forms of dystonia, the methods employed in the research to date do not yield the information required to describe differences in the characteristics of psychiatric presentation between case and control populations. This would be an interesting and valuable area to explore in future research, perhaps through the use of more detailed psychiatric assessments involving a phenomenologically oriented, semi-structured interview with a conversational flow completed by a reliability-trained psychiatrist with a broad base of clinical experience (45). As data collected on lifetime psychiatric symptomatology are prone to recall bias, this approach could be combined with a review of medical records to substantiate and support patient interviews.
Conclusions
Overall, the prevalence of psychiatric comorbidity appears to be increased in subjects with idiopathic and inherited (monogenic) forms of isolated and combined dystonia compared with control subjects, although this finding is not consistent for all comparisons. For idiopathic forms of dystonia, higher rates of psychiatric comorbidity, including mood and anxiety disorders, were noted when cases were compared with both healthy control subjects and control groups with a medical comorbidity. However, for major depressive disorder and OCD specifically, no differences in risk were noted between cases and control subjects. Subjects with DYT-SGCE appear to be at higher risk of psychiatric comorbidity, major depressive disorder, OCD, and alcohol dependence than control populations. Unfortunately, there are insufficient data available on the association between mental illness and DYT-TOR1A/ DYT-ATP1A3 to provide a summary of findings for these conditions.
The limitations of the existing evidence base should be taken into consideration when reviewing these findings and planning further studies. Future research would benefit from larger sample sizes of specific forms of dystonia (perhaps achieved through multicenter collaboration) and recruitment of control subjects well-matched for age, gender, and additional factors that might influence the development of psychopathology, including pain, disability, and disfigurement. Existing studies have included eligibility criteria that may have biased results with regard to psychiatric diagnosis (for example, requiring subjects to have received botulinum toxin and excluding subjects who have previously been prescribed antidepressants and neuroleptic medication) (18, 19). Future trials should consider eligibility criteria carefully, using a psychiatric perspective, to reduce potential bias. Modifications to the psychiatric assessment process could be applied to introduce an additional qualitative component facilitating deeper exploration of the characteristics of psychiatric disorders in idiopathic and inherited (monogenic) isolated or combined forms of dystonia.
Additional research should build upon our understanding of the development of psychopathology in idiopathic and inherited (monogenic) isolated or combined forms of dystonia, including a more detailed analysis of the nature of this association in different forms of dystonia, the characteristics of typical presentations, and the underlying causative etiologies. The development of a standardized, validated psychiatric structured interview for dystonia subjects would be extremely valuable. We suggest that based on these findings, the assessment of subjects with idiopathic or inherited forms of dystonia should include a comprehensive review of their mental state and substance misuse, in order to ensure an efficacious and holistic approach to management.
1. : Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013; 28:863–873Crossref, Medline, Google Scholar
2. : Primary focal dystonia: evidence for distinct neuropsychiatric and personality profiles. J Neurol Neurosurg Psychiatry 2009; 80:1176–1179Crossref, Medline, Google Scholar
3. : The functional neuroanatomy of dystonia. Neurobiol Dis 2011; 42:185–201Crossref, Medline, Google Scholar
4. : Nonmotor manifestations of dystonia: a systematic review. Mov Disord 2011; 26:1206–1217Crossref, Medline, Google Scholar
5. : The cognitive features of idiopathic and DYT1 dystonia. Mov Disord 2017; 32:1348–1355Crossref, Medline, Google Scholar
6. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097Crossref, Medline, Google Scholar
7. : Nomenclature of genetic movement disorders: recommendations of the International Parkinson and Movement Disorder Society Task Force. Mov Disord 2016; 31:436–457Crossref, Medline, Google Scholar
8. : Secondary social anxiety in hyperkinesias. Mov Disord 2008; 23:641–645Crossref, Medline, Google Scholar
9. Quality Assessment Tool for Quantitative Studies. Hamilton, Ontario, Canada, National Collaborating Centre for Methods and Tools. https://www.nccmt.ca/knowledge-repositories/search/14Google Scholar
10. Review Manager (RevMan). London, Cochrane Training, 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revmanGoogle Scholar
11. : Obsessive-compulsive symptoms in primary focal dystonia: a controlled study. Mov Disord 2011; 26:2274–2278Crossref, Medline, Google Scholar
12. : High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis 2003; 191:465–473Crossref, Medline, Google Scholar
13. : Obsessive-compulsive-spectrum symptoms in patients with focal dystonia, hemifacial spasm, and healthy subjects. J Neuropsychiatry Clin Neurosci 2012; 24:81–86Link, Google Scholar
14. : Psychiatric disorders in adult-onset focal dystonia: a case-control study. Mov Disord 2010; 25:459–465Crossref, Medline, Google Scholar
15. : Frequency of psychiatric disorders in blepharospasm does not differ from hemifacial spasm. Acta Neuropsychiatr 2010; 22:223–227Crossref, Medline, Google Scholar
16. : Psychiatric comorbidity in patients with spasmodic dysphonia: a controlled study. J Neurol Neurosurg Psychiatry 2007; 78:1398–1400Crossref, Medline, Google Scholar
17. : Correlates of generalized anxiety and panic attacks in dystonia and Parkinson disease. Cogn Behav Neurol 2003; 16:225–233Crossref, Medline, Google Scholar
18. : Social phobia in spasmodic torticollis. J Neurol Neurosurg Psychiatry 2001; 71:499–504Crossref, Medline, Google Scholar
19. : Higher prevalence of obsessive-compulsive symptoms in patients with blepharospasm than in patients with hemifacial spasm. Am J Psychiatry 1998; 155:555–557Crossref, Medline, Google Scholar
20. : Differential DSM-III psychiatric disorder prevalence profiles in dystonia and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2004; 16:29–36Link, Google Scholar
21. : Pain in focal dystonias: a focused review to address an important component of the disease. Parkinsonism Relat Disord 2018; 54:17–24Crossref, Medline, Google Scholar
22. : Gender differences in generalized anxiety disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J Clin Psychiatry 2008; 69:1606–1616Crossref, Medline, Google Scholar
23. : Parkinson’s disease and anxiety. Postgrad Med J 2001; 77:89–93Crossref, Medline, Google Scholar
24. : The Epidemiologic Catchment Area Program of the National Institute of Mental Health. Public Health Rep 1981; 96:319–325Medline, Google Scholar
25. : Prevalence of alcohol consumption, abuse and dependence in a country with high per capita consumption: findings from the German TACOS study. Soc Psychiatry Psychiatr Epidemiol 2000; 35:539–547Crossref, Medline, Google Scholar
26. : Obsessive-compulsive disorder is not a clinical manifestation of the DYT1 dystonia gene. Am J Med Genet B Neuropsychiatr Genet 2007; 144B:361–364Crossref, Medline, Google Scholar
27. : Increased risk for recurrent major depression in DYT1 dystonia mutation carriers. Neurology 2004; 63:631–637Crossref, Medline, Google Scholar
28. : Myoclonus dystonia: possible association with obsessive-compulsive disorder and alcohol dependence. Neurology 2002; 58:242–245Crossref, Medline, Google Scholar
29. : Myoclonus-dystonia, obsessive-compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology 2007; 68:522–524Crossref, Medline, Google Scholar
30. : Cognition and psychopathology in myoclonus-dystonia. J Neurol Neurosurg Psychiatry 2012; 83:814–820Crossref, Medline, Google Scholar
31. : SGCE mutations cause psychiatric disorders: clinical and genetic characterization. Brain 2013; 136:294–303Crossref, Medline, Google Scholar
32. : Is psychopathology part of the phenot2pic spectrum of myoclonus-dystonia? a study of a large Dutch M-D family. Cogn Behav Neurol 2009; 22:127–133Crossref, Medline, Google Scholar
33. : Psychiatric disorders, myoclonus dystonia and SGCE: an international study. Ann Clin Transl Neurol 2015; 3:4–11Crossref, Medline, Google Scholar
34. : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602Crossref, Medline, Google Scholar
35. :
36. : Psychiatric disorders in rapid-onset dystonia-parkinsonism. Neurology 2012; 79:1168–1173Crossref, Medline, Google Scholar
37. : Mental healthcare in Brazil: modest advances and major challenges. Adv Psychiatr Treat 2014; 20:113–115Crossref, Google Scholar
38.
39. : Symptom Checklist-90-Revised (SCL-90-R). London, Pearson Assessment, 1994Google Scholar
40. : Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev 1988; 8:77–100Crossref, Google Scholar
41. : The self-reported Montgomery-Asberg Depression Rating Scale is a useful evaluative tool in major depressive disorder. BMC Psychiatry 2009; 9:26Crossref, Medline, Google Scholar
42. : Is the Beck Anxiety Inventory a good tool to assess the severity of anxiety? a primary care study in the Netherlands Study of Depression and Anxiety (NESDA). BMC Fam Pract 2011; 12:66Crossref, Medline, Google Scholar
43. : Psychometric properties of the Liebowitz Social Anxiety Scale (LSAS) in a longitudinal study of African Americans with anxiety disorders. J Anxiety Disord 2011; 25:722–726Crossref, Medline, Google Scholar
44. : The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
45. : The psychiatric interview: validity, structure, and subjectivity. Eur Arch Psychiatry Clin Neurosci 2013; 263:353–364Crossref, Medline, Google Scholar
46. : Determinants and functions of standardized assessment use among school mental health clinicians: a mixed methods evaluation. Adm Policy Ment Health Ment Health Serv Res 2016; 43:122–134Crossref, Medline, Google Scholar



