Effectiveness of ECT in Patients With Parkinsonism
Abstract
Electroconvulsive therapy (ECT) has been used to treat the psychiatric complications of Parkinson's disease. Concurrent improvement of parkinsonian motor symptoms has been noted. This retrospective study compared the outcomes of 25 patients with parkinsonism receiving ECT for psychiatric indications with outcomes of 25 patients (matched for age and gender) without neurological disease also receiving ECT for psychiatric indications. Significant improvement in psychiatric symptoms was noted following ECT for both groups. No differences in efficacy of ECT were found between the two groups. Fourteen of the 25 patients with Parkinson's symptoms were noted to have at least transient improvement in motor function at discharge. ECT is an effective treatment for patients with parkinsonism and psychiatric comorbidity.
Parkinson's disease affects 1% of the population over age 60;1 the motor symptoms result predominantly from the degeneration of dopaminergic cells of the substantia nigra within the midbrain. Although the focus of this disease is on the motor system, psychiatric morbidity is common. For example, depression occurs in nearly 40%.2 Anxiety can also be disabling; one study reported that 28% of patients with Parkinson's disease had a formal anxiety disorder and another 40% had anxiety symptoms but no formal diagnosis.3 Psychosis develops with advanced disease in a significant minority of patients. Dementia eventually occurs in approximately 30%, and subtle neuropsychological deficits without associated dementia occur in up to 40% of patients with Parkinson's disease.2
The introduction of levodopa 25 years ago revolutionized the symptomatic treatment of the motor manifestations of this condition. Dopamine replenishment with carbidopa/levodopa (Sinemet) remains the foundation of therapy. Unfortunately, despite optimal levodopa therapy, progressive disability typically develops, involving motor fluctuations and levodopa-refractory symptoms. Adjunctive therapy with other medications, including the dopamine agonists bromocriptine and pergolide, anticholinergic agents, or the selective monoamine oxidase B inhibitor selegiline, are only partially beneficial in treating the motor symptoms of advancing disease.4 Despite these available medical therapies, disability increases with time.
The possible beneficial effects of electroconvulsive therapy (ECT) on the psychiatric complications as well as the motor symptoms of Parkinson's disease have been observed since 1947.5 More than 40 reports have been published since these first observations were made. Faber and Trimble6 reviewed studies reporting on the use of ECT in Parkinson's disease through 1991. Between 1975 and 1991, there have been 21 reports describing ECT in a total of 44 Parkinson's patients with psychiatric comorbidity including depression (with and without psychosis), mania, and drug-induced psychosis. After 3 to 15 treatments, 31 were clearly improved and 13 were unchanged with regard to psychiatric and/or neurologic symptoms; deterioration was not reported. In 1991, we reported the effects of ECT in patients with Parkinson's disease and psychiatric comorbidity (depression, psychosis, mania).7 After 3 to 9 ECT treatments, 9 of 11 patients received at least some benefit; however, 7 of 11 patients experienced post-ECT confusion.
Electroconvulsive therapy has also been used as treatment for the motor manifestations of Parkinson's disease in patients without psychiatric comorbidity. Faber and Trimble found 6 reports describing a total of 34 psychiatrically unimpaired Parkinson's patients receiving ECT for treatment of severe “on-off” symptomatology. Twenty-two of the 34 patients experienced some improvement in their motor symptoms. Of the six studies, only Ward et al.8 reported no benefit in motor function from ECT.
In the only prospective randomized, double-blinded trial, Anderson et al.9 noted improvement of motor symptoms in 9 of 11 patients, lasting hours to months; however, 5 of 11 had transient post-ECT confusion, necessitating a reduction in number of treatments in 3 of the 5. Pridmore and Pollard,10 at 30-month follow-up of 14 patients receiving ECT in an open prospective trial to assess the benefit of ECT on physical symptoms of Parkinson's disease, found that three-fourths of patients continued to have some sustained benefit from 2 weeks to 35 months, and more than half said they would elect to have further courses of ECT if their symptoms worsened.
This literature raises a number of questions: 1) which psychiatric problems in Parkinson's patients respond to ECT; 2) what is the impact of ECT on both the motor abnormalities and cognitive decline associated with ECT in this patient population; and 3) what are potential complications from ECT in this patient population? This study attempts to address these questions.
METHODS
This retrospective chart review using our ECT database was approved by our institutional review board. Our ECT database includes all individuals receiving ECT at our institution from 1991 to the present. Demographics, diagnoses, pre- and post-treatment rating scores, treatment parameters, and complications from treatment were obtained from our ECT coordinator (T.P.) and the medical records.
Subjects
The study subjects were those patients who were referred for ECT at our institution from March 1991 to January 1994 with a diagnosis of Parkinson's disease. These were matched by age and gender with patients without neurologic disease (including dementia) who were referred for ECT during the same time period.
Procedure
Demographic information including age, gender, and education level was recorded. Comprehensive psychiatric, neurologic, and medical diagnoses were provided by appropriate board-certified specialists. Prior psychiatric history, including previous ECT trials, was noted.
The date of initial diagnosis of Parkinson's disease was recorded, as well as the date that levodopa therapy was first initiated. The current stage of parkinsonism was estimated by using the Hoehn and Yahr scale for motor disability,11 a 5-point scale that is commonly used to measure the stage of disease. The Hoehn and Yahr score was determined by using the information available to the authors at the time of the chart review. This included the neurological evaluation performed by a board-certified neurologist as well as any other information available in the records.
All medications, including psychotropics and antiparkinsonian agents and their dosages, were recorded at the time of admission. Also recorded were changes that were made 24–48 hours prior to ECT initiation and at the time of hospital dismissal.
Results of current diagnostic testing procedures, including EEG, CT, and MRI, were recorded when available.
As part of our routine, our ECT coordinator completed pre- and post-ECT assessment on each patient. The evaluation consisted of the Mini-Mental State Examination (MMSE, to assess baseline and post-ECT cognitive status),12 Global Assessment of Functioning Scale (GAF, to assess level of overall functioning),13 Brief Psychiatric Rating Scale (BPRS, to assess general psychiatric status),14 Hamilton Rating Scale for Anxiety (Ham-A),15 and Hamilton Rating Scale for Depression (Ham-D),16 usually 1 to 2 days before their first ECT treatment and 1 to 2 days after their last ECT treatment. Upon chart review, any information recorded by the primary service regarding the patient's motor status during the course of ECT was noted.
ECT was administered with a Thymatron machine. Characteristics of the ECT treatments, including machine type, electrode placement, and number of treatments, were recorded. The amount of energy delivered to each patient was individualized and was determined by the titration method of Sackeim.17 Unilateral ECT treatment involved an electrical stimulus dose at 2.5 times the determined seizure threshold, and bilateral treatments were delivered at 1.5 times the threshold. Patients were given three ECT treatments per week unless complications (delirium) necessitated postponement or discontinuation of treatment. Standard peri-ECT anesthetics were used, including oxygen, glycopyrrolate (average dose 0.2 mg), succinylcholine (40–60 mg), and pentothal (60–80 mg). Any additional medications at the time of anesthesia were noted.
Treatment-related complications, including the development of intertreatment delirium, were documented. “Interictal delirium,” as defined by Figiel et al.,18 refers to a delirium that develops during a course of ECT and persists on days that the patient does not receive ECT. This is differentiated from the transient confusional state that is commonly seen after the patient awakens from ECT. In our study, delirium resulted in postponement or termination of treatment.
Statistical Analysis
For each group, changes in the pre and post psychometric test scores were assessed by using the Wilcoxon signed-rank test. The Wilcoxon rank sum test was used to assess 1) whether length of hospitalization differed between the two groups; 2) whether the age or stage of parkinsonism differed between those Parkinson's patients whose symptoms appeared to have improved upon discharge and those whose symptoms remained unchanged; 3) and whether age, number of treatments, or pre-ECT depression or anxiety scores differed between those Parkinson's patients who developed intertreatment delirium and those who did not. Fisher's exact test was used to assess whether the two groups differed with respect to the proportion who had ECT complications as well as to assess which dichotomous factors may be associated with the development of ECT-induced delirium in Parkinson's patients.
RESULTS
Over this 32-month period, 30 patients diagnosed with Parkinson's disease were treated with ECT. Five of the 30 were excluded because pre and post rating scale data were incomplete. However, their psychiatric diagnoses and severity of parkinsonian symptoms were similar to the remaining 25. Of the remaining 25 patients, 5 were on antipsychotic medications prior to the diagnosis of Parkinson's symptoms, suggesting drug-induced parkinsonism. Review of these charts revealed that 3 of these 5 patients carried the diagnosis of Parkinson's disease as diagnosed by a neurologist and were being treated with levodopa. The study group of 25 patients (13 male) with parkinsonism were each matched for age and gender to a single patient from a group of patients without neurologic disease. There were 25 patients in the comparison group. Patients ranged in age from 60 to 89 years; median age in each group was 71 years. The median education level was 12 years in each group.
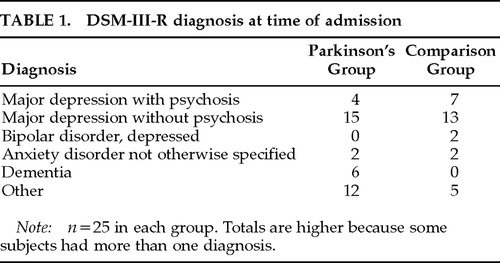
Psychiatric diagnoses made at the time of admission by board-certified staff psychiatrists using DSM-III-R criteria are found in Table 1. The majority of patients were diagnosed with major depression. Six of the patients in the Parkinson's group had a diagnosis of dementia, whereas no patients in the comparison group carried a diagnosis of a dementing illness (by design).
Medical diagnoses were similar between the two groups. The most common concurrent medical problem in these predominantly elderly patients was stable cardiovascular disease. In the Parkinson's group, 21 of 25 patients had a head scan, either CT (15 patients) or MRI (6 patients). Sixteen of these scans were remarkable for atrophy or nonspecific white matter changes. One patient had an old infarct. Fifteen of 25 patients in the comparison group underwent head scanning, either CT (11 patients) or MRI (4 patients); atrophy or nonspecific white matter changes were noted in 8. Electroencephalography, performed in 9 Parkinson's patients, revealed either nonspecific changes (6 patients) or no abnormalities. One of the 3 patients in the comparison group who had EEG showed nonspecific changes.
Hoehn and Yahr staging for the Parkinson's patients was as follows: Stage 1, n=4; Stage 2, n=4; Stage 3, n=10; Stage 4, n=2; Stage 5, n=4; and unknown, n=1.
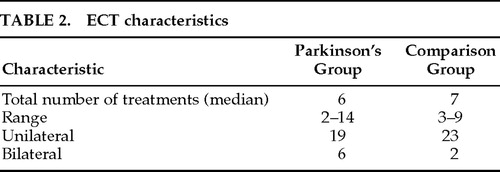
The parameters of administered ECT are described in Table 2. Parkinson's patients received a median of 6 treatments and comparison group received a median of 7 treatments. The majority of patients in both groups were given unilateral ECT.
There was a significant decrease in depression (Figure 1) and anxiety (Figure 2) for both the Parkinson's and comparison groups following ECT (P<0.0001;). Patients in both groups experienced a significant decrease in BPRS scores (P<0.0001; Figure 3). MMSE test scores (Figure 4) tended to be higher for both study patients (P=0.0877;) and the comparison group (P=0.0924). GAF scores (Figure 5) were significantly improved after ECT for both patient groups (P<0.0001).
Of the 25 Parkinson's patients, the status of Parkinson's symptoms at discharge improved subjectively in 14 (56%), remained unchanged in 10 (40%), and deteriorated in only 1 patient (4%). Those patients who appeared to have improvement in their Parkinson's symptoms were not found to differ from those who remained unchanged in terms of age (P=0.7922) or stage of Parkinson's disease (P=0.3972).
There were more complications following ECT for patients in the study group (56% of patients) than in the comparison group (12% of patients), with the most frequent being transient intertreatment delirium (P=0.0023). Fifty-two percent of the Parkinson's patients experienced intertreatment delirium necessitating postponement or termination of treatment, compared with only 20% of the comparison group patients (P=0.0378).
The development of ECT-induced delirium was not found to be associated with age, other current medications (such as anticholinergics or anesthetic agents), number of treatments, electrode placement, pre-ECT depression or anxiety scales, admitting diagnosis of dementia, abnormal head imaging studies (which were not performed on all patients), or EEG findings prior to ECT (done on only 9 patients). However, 5 of 13 patients who experienced intertreatment delirium had levodopa instituted or increased prior to beginning ECT.
Other complications noted for the study group were as follows: 5 patients developed urinary retention, of whom 2 developed a urinary tract infection; 1 patient developed choreiform movements, which resolved when levodopa was decreased; and 2 patients fell (not sustaining a major injury). Two control group subjects developed urinary retention, and 1 fell. Length of hospital stay was significantly longer for the Parkinson's patients who underwent a course of ECT (P=0.0062).
DISCUSSION
To our knowledge, this is the largest clinical series published to date describing the responses to ECT in patients with parkinsonism and psychiatric disorders. Our data support the clinical observation that affective symptoms associated with parkinsonism improved immediately after ECT completion without worsening the underlying movement disorder. All forms of affective disorders appeared equally responsive to ECT. Anxiety symptoms, which are frequently comorbid with depression in Parkinson's patients,3 also appeared to respond to ECT, as evidenced by the improvement in Ham-A scores. We acknowledge that the clinical ratings scales were performed by our unblinded ECT nurse coordinator, which potentially could be a source of bias.
Most studies reviewing the effect of ECT on the motor manifestations of Parkinson's disease have found some benefit with ECT.6–8 Likewise, 14 of our 25 patients experienced at least some transient benefit in their parkinsonian symptoms. However, this result was confounded to some degree in that levodopa dosage was either started or increased in 5 of these patients immediately prior to ECT. Further, ratings of motor status during the course of ECT was strictly subjective and made by unblinded caretakers. The duration of motor improvement beyond the hospitalization was not assessed; thus, we cannot comment on whether the motor benefits were of longer-standing functional significance. The improvement in motor symptoms could have been a primary event due to alterations in basal ganglia neurochemistry or, alternatively, could have been secondary to an improved psychiatric state. A well-designed prospective study could address many of these areas and perhaps further delineate which of the motor symptoms are likely to respond to ECT.
Baseline cognitive impairment was present in the majority of our Parkinson's disease patients and was not further compromised by ECT, as assessed by the MMSE performed after the last ECT treatment. Many actually improved cognitively, presumably from the improvement in their severe psychiatric symptomatology. However, MMSE scores declined within 2 days of the last ECT treatment in 2 patients, both of whom had an intertreatment delirium. Presumably, this decline was the direct result of delirium and would be reversible as the delirium cleared.
A significant number of Parkinson's patients developed complications during their course of ECT. The most notable adverse event was intertreatment delirium developing in 13 of 25 patients. This resolved within several days in most cases. Figiel et al.19 found MRI or CT evidence of structural changes in the basal ganglia and subcortical white matter in patients who developed delirium after receiving ECT for treatment of psychiatric illnesses. For this reason, patients with Parkinson's disease, which is a basal ganglia neurodegenerative condition, may be more sensitive to ECT-induced delirium. We looked at the relationship between pre-ECT head imaging results and the development of post-ECT delirium but found no association; however, not all patients had these studies completed.
A proposed mechanism of the antiparkinsonian effect of ECT is that it increases the sensitivity of dopamine receptors, and, in fact, dopamine-induced delirium and psychosis are well described. In addition, the degenerative nigrostriatal system in Parkinson's disease results in a compensatory hypersensitivity of striatal dopamine receptors.20 Ten of the 13 delirious patients were on levodopa at admission or were started prior to ECT. In fact, Rasmussen and Abrams21 have suggested decreasing levodopa dosages prior to ECT to prevent emergent dyskinesias. This recommendation might also be extended to include delirium. This decision should be made on a case-by-case basis.
Although transient ECT-induced delirium created difficulties for the patient and caregivers, this problem resolved within several days in most patients. We recommend that patients being considered for ECT be informed of this potential complication.
CONCLUSION
Electroconvulsive therapy is an appropriate treatment option for certain patients with psychiatric disorders (especially depression and, to a lesser degree, anxiety and psychoses) complicating parkinsonism. ECT has the additional benefit of frequently improving the patient's motor status, at least transiently. Cognitive improvement frequently occurs, most likely as the direct result of improvement in psychiatric problems. Patients with parkinsonism who are undergoing a course of ECT may be at increased risk of complications including transient intertreatment delirium, but appropriate precautions may decrease the incidence.
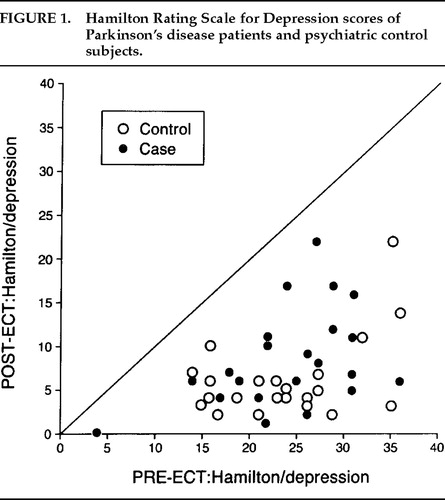
FIGURE 1. Hamilton Rating Scale for Depression scores of Parkinson's disease patients and psychiatric control subjects.
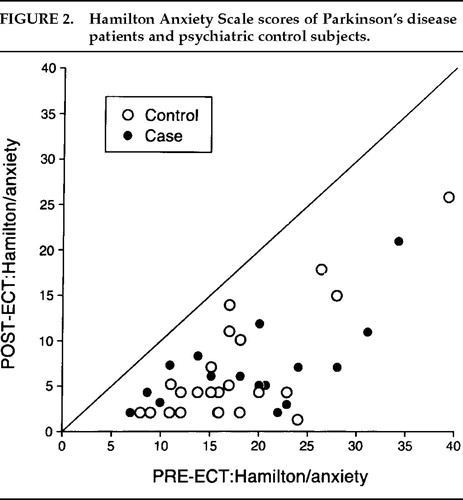
FIGURE 2. Hamilton Anxiety Scale scores of Parkinson's disease patients and psychiatric control subjects.
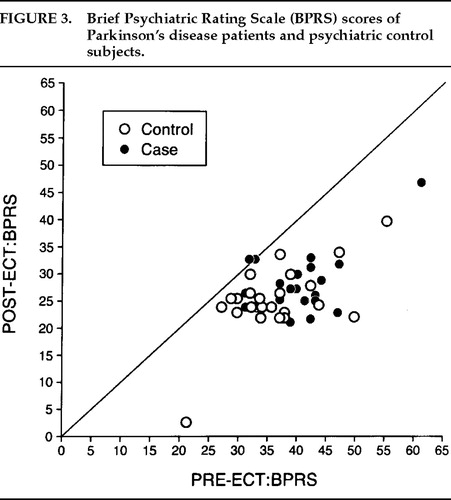
FIGURE 3. Brief Psychiatric Rating Scale (BPRS) scores of Parkinson's disease patients and psychiatric control subjects.
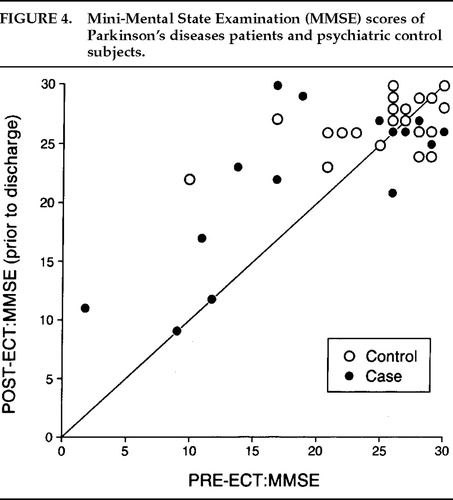
FIGURE 4. Mini-Mental State Examination (MMSE) scores of Parkinson's disease patients and psychiatric control subjects.
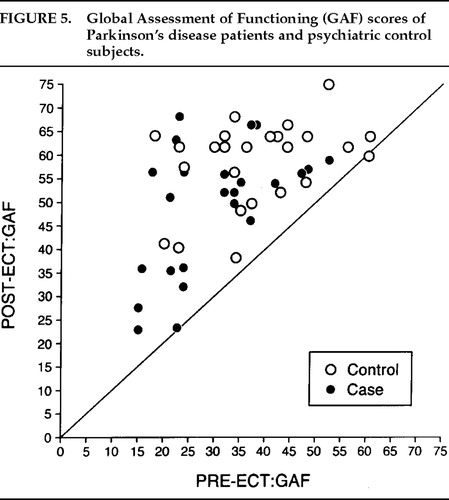
FIGURE 5. Global Assessment of Functioning (GAF) scores of Parkinson's disease patients and psychiatric control subjects.
 |
 |
1. Kurland LT: Epidemiology: incidence, geographic distribution, and genetic considerations, in Pathogenesis and Treatment of Parkinsonism, edited by Field W. Springfield, IL, CC Thomas, 1958, pp 5–43Google Scholar
2. Cummings J: Depression and Parkinson's disease: clinical review. Am J Psychiatry 1992; 149:443–445Crossref, Medline, Google Scholar
3. Menza M, Robertson-Hoffman DE, Bonapace AS, et al: Parkinson's disease and anxiety: comorbidity with depression. Biol Psychiatry 1993; 34:465–470Crossref, Medline, Google Scholar
4. Ahlskog JE: Parkinson's disease: update on pharmacologic options to slow progression and treat symptoms. Hospital Formulary 1992; 27:146–163Google Scholar
5. Gallinek A: Electroconvulsive therapy in geriatrics. New York State Journal of Medicine 1947; 47:1233–1241Medline, Google Scholar
6. Faber R, Trimble M: Electroconvulsive therapy Parkinson's disease and other movement disorders. Mov Disord 1991; 6:293–303Crossref, Medline, Google Scholar
7. Oh J, Rummans T, O'Connor MK, et al: Cognitive impairment after ECT in patients with Parkinson's disease and psychiatric illness (letter). Am J Psychiatry 1992; 149:271Medline, Google Scholar
8. Ward C, Stern GM, Pratt RTC, et al: Electroconvulsive therapy in parkinsonian patients with the “on-off” syndrome. J Neural Transm 1980; 49:133–135Crossref, Medline, Google Scholar
9. Anderson K, Balldin J, Gottpries CG, et al: A double-blind evaluation of electroconvulsive therapy in Parkinson's disease with “on-off” phenomena. Acta Neurol Scand 1987; 76:191–199Crossref, Medline, Google Scholar
10. Pridmore S, Pollard C: Electroconvulsive therapy in Parkinson's disease: 30-month follow-up. J Neurol Neurosurg Psychiatry 1996; 61:693–700Crossref, Google Scholar
11. Hoehn MM, Yahr MD: Parkinsonism: onset, progression, and mortality. Neurology 1967; 17:427–442Crossref, Medline, Google Scholar
12. Folstein MF, Folstein SE, Hugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
13. Endicott J, Spitzer RL, Fleiss JL, et al: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1979; 33:766–771Crossref, Google Scholar
14. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
15. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
16. Hamilton M: Development of a rating for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
17. Sackeim H, Prudic J, Devanand D, et al: Effects of electroconvulsive therapy. N Engl J Med 1993; 328:839–846Crossref, Medline, Google Scholar
18. Figiel GS, Hassen MA, Zorumski C, et al: ECT-induced delirium in depressed patients with Parkinson's disease. J Neuropsychiatry Clin Neurosci 1991; 3:405–411Link, Google Scholar
19. Figiel GS, Coffey CE, Djang WT, et al: Brain magnetic resonance imaging findings in ECT-induced delirium. J Neuropsychiatry Clin Neurosci 1990; 2:53–58Link, Google Scholar
20. Factor SA, Molho ES, Podskalny GD, et al: Parkinson's disease: drug-induced psychiatric states (review). Adv Neurol 1995; 65:115–138Medline, Google Scholar
21. Rasmussen K, Abrams R: Treatment of Parkinson's disease with electroconvulsive therapy. Psychiatr Clin North Am 1991; 14:925–933Crossref, Medline, Google Scholar



