Repetitive Transcranial Magnetic Stimulation Treatment of Comorbid Posttraumatic Stress Disorder and Major Depression
Abstract
Twelve patients with comorbid posttraumatic stress disorder (PTSD) and major depression underwent repetitive transcranial magnetic stimulation (rTMS) to left frontal cortex as an open-label adjunct to current antidepressant medications. rTMS parameters were as follows: 90% of motor threshold, 1 Hz or 5 Hz, 6,000 stimuli over 10 days. Seventy-five percent of the patients had a clinically significant antidepressant response after rTMS, and 50% had sustained response at 2-month follow-up. Comparable improvements were seen in anxiety, hostility, and insomnia, but only minimal improvement in PTSD symptoms. Left frontal cortical rTMS may have promise for treating depression in PTSD, but there may be a dissociation between treating mood and treating core PTSD symptoms.
Posttraumatic stress disorder (PTSD) is a serious psychiatric illness with an estimated prevalence of 1% to 2% in the general U.S. population1 and 15% to 25% in Vietnam combat veterans.2 Studies have shown that PTSD in Vietnam combat veterans is associated with a wide range of social and psychological problems, including alcohol and drug abuse, suicidal ideation, depression, unemployment, marital and familial conflicts, suspiciousness, and a reduced social support system.3,4 Current treatments for PTSD include medications, cognitive-behavioral techniques, and group therapy. Antidepressants have been the most intensively studied medications for treatment of PTSD, but their efficacy is variable and they are probably not as effective for combat PTSD as for noncombat PTSD.5 For example, amitriptyline, a tricyclic antidepressant, was superior to placebo in reducing depression, anxiety, and PTSD symptoms in a controlled study of 46 veterans with combat PTSD.6 However, a comparable controlled study with desipramine showed no difference between active drug and placebo.7 Fluoxetine, a selective serotonin reuptake inhibitor, significantly reduced PTSD symptoms and improved depressed mood in patients with noncombat PTSD in a controlled study of 64 patients.5 However, patients with combat PTSD improved only in mood, not in avoidance symptoms. There are comparable studies showing only moderate efficacy for paroxetine and fluvoxamine,8–11 as well as for nefazodone,12–14 bupropion,15 and the monoamine oxidase inhibitor moclobemide.16 Sertraline, another SSRI, is the only medication that is FDA-approved for treatment of PTSD on the basis of a placebo-controlled, multicenter study with 186 subjects.17 This study demonstrated improvement in both mood and PTSD symptoms, but included very few combat PTSD subjects (6%). In an interesting “real world” study, Dow and Kline18studied 72 combat PTSD patients in a real-world clinical setting but with standard PTSD and mood measures. They found that the probability of clinical response to each individual trial of antidepressant was only 20%, considerably less than in the controlled studies referred to above. These results reflect the experience of many clinicians that combat PTSD patients often have limited symptom response to antidepressant medications.
Repetitive transcranial magnetic stimulation (rTMS) is a new technique that has been found to be useful for investigations of cortical and cognitive function and more recently as a potential treatment for neuropsychiatric illnesses. There are several uncontrolled and controlled studies in which rTMS treatment led to mood improvement in major depression.19–26 There are also two published open-label studies of rTMS in PTSD. McCann et al.27 studied two patients with noncombat PTSD; the stressors were rape and a shooting incident. The patients' PTSD symptoms had been highly refractory to medications, and one patient had not responded to a trial of left frontal rTMS. Both patients had baseline cortical hypermetabolism on [18F]fluorodeoxyglucose positron emission tomography (FDG-PET). They administered rTMS to right frontal cortex for 20 to 30 daily sessions at 1 Hz, 80% of motor threshold, administering 1,200 stimuli daily. After rTMS, PTSD symptoms decreased by about half and both global and right frontal cortical hypermetabolism decreased on FDG-PET. Grisaru et al.28 administered rTMS to 10 patients with PTSD, with a stimulation rate of 0.3 Hz at the maximum output of a Magstim single-pulse stimulator, administering 30 pulses bilaterally over motor cortex with a nonfocal coil. They found transient improvement in both self- and observer ratings of PTSD symptoms. The transient nature of these improvements may be due to very conservative rTMS parameters, with only 60 repetitions daily as opposed to 500 to 1,000 repetitions typically in rTMS studies of major depression.
Thus, rTMS (particularly administered to left frontal cortex) may mimic the effect of antidepressant medications in mood disorders. We hypothesized that left frontal rTMS might similarly mimic the effect of antidepressant medications in patients with combat PTSD and comorbid major depression. Because slow (1 Hz) rTMS is inherently safer than fast (5 Hz) rTMS, we chose to compare 1 Hz vs. 5 Hz rTMS in an open-label design. Although slow left frontal rTMS has not been found to improve mood significantly in patients with major depression,29 we present the first report of its effect on PTSD symptoms.
METHODS
Study Subjects
Patients were recruited from the Partial Hospitalization Program and Post Traumatic Stress Disorder Clinic of the Mental Health Service Line at the Department of Veterans Affairs (VA) Medical Center in Washington, DC. Patients were eligible for inclusion in the protocol if they met the following criteria: 1) diagnoses of posttraumatic stress disorder and major depression on the Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-C);30 2) taking antidepressant medication (of any class) at an unchanged dose for one month prior to rTMS; 3) had a Hamilton Rating Scale for Depression (Ham-D) score greater than 17 at baseline; 4) age 20 to 80 years; 5) competent to sign informed consent. Patients were excluded from the protocol if they met any of the following criteria: 1) metal in the head or scalp; 2) implantable devices including cardiac pacemakers and defibrillators; 3) seizure within the past year; 4) substance abuse within three months prior to rTMS; 5) acute medical illness; 6) investigators unable to determine motor threshold after attempting on two successive days; 7) epileptiform abnormalities on electroencephalogram (EEG). The protocol was approved by the Institutional Review Board of the hospital, and all patients signed an informed consent document prior to taking part in the protocol. The inclusion criteria define a group of patients with treatment-refractory depression and PTSD who remain depressed after a minimum of one month on antidepressant therapy.
Procedures
Patients underwent a physical examination, electrocardiogram, and laboratory studies (including complete blood count, liver function tests, and electrolytes) to rule out comorbid acute medical illness. An EEG was administered to rule out epileptiform abnormalities; if nonspecific abnormalities (such as diffuse slowing) were found, a computed tomographic scan or magnetic resonance imaging scan of the brain was performed to exclude structural brain lesions. The following clinical rating instruments were administered at baseline: SCID-C, Ham-D (21-item version),31 Profile of Mood States (POMS),32 University of Southern California Repeatable Episodic Memory Test (USC-REMT),33 and Mississippi Scale of Combat Severity (MISS).34 Patients with 50% or greater decrease in Ham-D score after rTMS treatment were considered to have had an antidepressant response.
Magnetic Stimulation Methods
Magnetic stimulation was administered with a Dantec Maglite stimulator (Dantec Corporation, Skovlunde, Denmark), using a figure-8 coil (MCB70 coil, inner radius=10 mm, outer radius=50 mm, 2×10 windings, winding height 6 mm). rTMS was administered on 10 consecutive weekdays. Left cortical motor threshold was determined on each day prior to rTMS by increasing the intensity of stimulation by 2.5% increments until a reproducible motor evoked potential (>50 μV) was obtained from the electromyogram measured with gold surface electrodes placed over the right abductor pollicis brevis (APB) muscle. The TMS coil was then moved 4 cm anterior parasagittally and 2 cm laterally, to maximize stimulation to the estimated location of left dorsofrontolateral cortex. In preliminary studies, we found that the most widely used location for left frontal rTMS (5 cm anterior to the site of maximal stimulation for right APB) gave patients significant discomfort from left temporalis muscle contraction; we moved to a slightly more posterior placement with much improved patient tolerance for the procedure. Given that most estimates of the affected region of cortex involve several cm in each dimension from the center of the coil,35,36 this change should not significantly alter the anatomic region of stimulation. rTMS was administered at 90% of motor threshold, 40 stimulations per minute, for 15 minutes daily. Stimulation rates were either 1 Hz (40 s stimulation, 20 s rest per minute) or 5 Hz (8 s stimulation, 52 s rest per minute).
Patients were randomized to 1 Hz or 5 Hz rTMS. The patients described in this study are a subset of a larger sample in an ongoing study of patients with major depression comparing 1 Hz and 5 Hz rates. rTMS was administered in an open-label fashion, with both patients and investigators aware of the treatment condition. Patients were maintained on unchanged antidepressant therapy during the rTMS treatments and for two months afterward; that is, rTMS was used as an adjunct to antidepressant treatment.
Follow-up
The POMS was administered after the first and fifth rTMS treatments, and the Ham-D, POMS, MISS, and USC-REMT were administered after the final (10th) rTMS treatment (“post-rTMS”) and at 1-month and 2-month follow-up.
Statistical Analysis
Statistical analysis was performed with WINKS 4.6 Evaluation software (Texasoft, Cedar Hill, TX). The effect of rTMS treatment on the clinical measures was assessed with a repeated-measures analysis of variance (ANOVA), using a significance level of P<0.05 and a post hoc Scheffé comparison. The influence of demographic variables on clinical measures was assessed with chi-square or Fisher's exact test for categorical variables and independent-sample t-test for continuous variables.
RESULTS
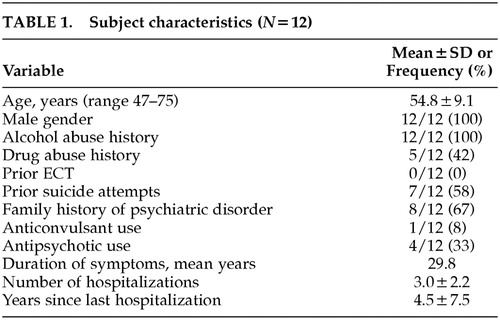
Fifteen outpatients gave signed informed consent and were enrolled in the protocol. Twelve patients completed the protocol. The characteristics of the study completers are outlined in Table 1. All patients had a history of alcohol abuse and a substantial number had a history of drug abuse as well, which are typical findings in combat PTSD populations. No patients had a current diagnosis of substance abuse. There were no serious adverse events, seizures, or development of new neurological deficits. One of the 15 patients enrolled dropped out because of marked tension headache after two rTMS treatments; it is notable that this individual had an initial diagnosis of cluster headaches and rTMS was delayed for several months until he reached a quiescent point of his headache cycle; the headache symptoms he developed with rTMS were not typical of his cluster headaches but instead resembled tension headache. The other two patient dropouts never received rTMS: one patient did not meet inclusion criteria because of mood improvement prior to rTMS, and in the other patient we were unable to determine motor threshold at up to 80% of maximal output of the TMS machine, and higher stimulus intensities would not have been tolerated.
The patients were receiving a wide variety of antidepressant medications, including amitriptyline, fluoxetine, sertraline, paroxetine, venlafaxine, bupropion, nefazodone, trazodone, doxepin, and mirtazapine. Seven of the 12 completers were on two antidepressant medications and one was on three antidepressant medications.
Six patients received 1 Hz and six received 5 Hz rTMS. There were no statistically significant differences between the two groups in the demographic variables listed in Table 1, nor in baseline clinical ratings (Ham-D, MISS, USC-REMT recall scores).
Clinical Efficacy
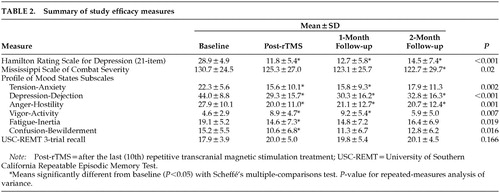
The results for the clinical measures are presented in Table 2. Ham-D decreased robustly, and the decrease was largely sustained at 2-month follow-up. Rates of antidepressant response, displayed in Table 3, show a 50% response rate at 2-month follow-up. Comparably sustained improvements were seen in the POMS subscales Tension-Anxiety, Depression-Dejection, and Anger-Hostility; the remaining POMS subscales improved transiently. Core combat PTSD symptoms as measured by the MISS showed a modest but statistically significant decrease (6%). Short-term recall as measured by the USC-REMT was unchanged after rTMS.
We found no significant differences in mood improvement between 1 Hz and 5 Hz rTMS (Table 3), nor any differences between these groups in MISS or USC-REMT scores.
Subjective Sleep Changes
Patients anecdotally reported improved sleep after rTMS. Although we did not use a formal sleep assessment, we analyzed the three insomnia items from the Ham-D (early, middle, and late insomnia). The sum of these three items decreased from 5.0 at baseline (out of a possible 6.0) to 2.3 post-rTMS, 2.7 at 1-month follow-up, and 2.3 at 2-month follow-up (P<0.001 on repeated-measures ANOVA).
Time Course of Subjective Improvement
The rapidity of mood improvement was roughly linear through the 10 treatments. The POMS subscale scores during the course of the 10 treatments are graphed in Figure 1. There is no apparent change after treatment #1. The POMS subscale scores after treatment #5 are lower but not statistically significantly different from baseline. The POMS subscale scores only become statistically significantly different from baseline at treatment #10.
DISCUSSION
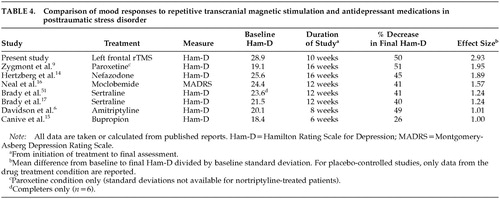
We found a clinically robust and sustained mood improvement after administering rTMS to patients with comorbid PTSD and major depression. Although we observed a slight relapse of depressive symptoms at 2-month follow-up, patients still remained substantially improved, showing a mean 50% reduction in Ham-D. The effect size and proportional reduction in depressive symptoms compare favorably with several controlled and uncontrolled studies of antidepressant medications in PTSD (Table 4). Core combat PTSD symptoms improved only minimally, and this was consistent with patients' subjective comments that intrusive memories were not diminished by rTMS. However, the Tension-Anxiety and Anger-Hostility subscales of the POMS decreased, suggesting that affective distress did decrease even if intrusive memories, avoidance, and hypervigilance did not. Left frontal rTMS thus appeared to be effective against the depressive, anxiety, and anger symptoms that are common in PTSD, but not against the core trauma symptoms. Five treatments yielded only a partial and statistically insignificant response and 10 treatments appear to be needed for full response, in a finding similar to that of Klein et al.26 Our patients' clinical profile is typical of VA PTSD clinic populations, with a high lifetime prevalence of substance abuse and chronic depressive symptoms.
Patients reported that their subjective experience of sleep was much improved. Because insomnia is one of the most common and distressing symptoms of PTSD,37 improving sleep is crucial to PTSD treatment. Our patients reported 54% fewer sleep complaints on the sum of the three insomnia items on the Ham-D. This magnitude of improvement is similar to the 30% to 50% decrease in sleep complaints seen in antidepressant trials of PTSD.8,9,12,14,16,38
These data lead to the question of why we saw such a robust improvement in mood but only modest improvement in core trauma symptoms. We chose to stimulate left prefrontal cortex on the basis of prior reports of mood improvement in major depression. This choice of laterality is loosely based on observations that cerebral perfusion and metabolism are often decreased in left prefrontal cortex in major depression,39,40 but comparable mood improvement has been observed after slow right prefrontal cortical rTMS.26 Similar ambiguity exists for the treatment of PTSD; evoked trauma memories are observed to increase cerebral perfusion in right limbic areas,41,42 which are not likely to be affected by left frontal stimulation.
Although our sample is too small to yield much statistical power, our preliminary finding that slow left frontal rTMS leads to mood improvements similar to those seen in fast left frontal rTMS is quite surprising. Fast rTMS (defined as ≥5 Hz) increases motor cortex excitability, and slow rTMS (1 Hz) decreases it.43,44 Because left frontal cortical metabolism has often been found to be decreased in major depression, one might expect fast left frontal rTMS to “normalize” metabolism and thus be more effective than slow left frontal rTMS in improving mood, and the data of Speer et al. support this.45 Our differing results may reflect the use of rTMS as an adjunct to antidepressant medications and the differences in patient populations.
The major limitation of the study is its open-label design, in which neither the patients nor the raters were blinded to treatment condition. There is considerable debate in the literature about the magnitude of placebo (sham) response to rTMS. George et al.24 found an increase in Ham-D scores during the placebo phase of a crossover trial, suggesting no placebo effect. Similarly, Padberg et al.46 found Ham-D scores slightly increased in their placebo group. Klein et al.26 found a moderate placebo effect: a 23% decrease in Ham-D in their placebo group versus a 46% decrease in their active-rTMS group. Berman et al.47 found no placebo effect, albeit the mood improvement they observed in the active rTMS group was modest. In contrast, Loo et al.48 observed similar mood improvement in active and sham groups, but their sham condition may have caused significant cortical activation.49 Given these questions about placebo effect in rTMS, any conclusions drawn from the results we have presented are necessarily preliminary. However, the magnitude of mood improvement we observed is considerably more than is usually seen in the placebo arm of rTMS studies or antidepressant medication studies,17,50 suggesting that we have observed a true clinical effect.
In short, we observed substantial improvement in mood, anxiety, and sleep symptoms after adjunctive left frontal cortical rTMS in patients with comorbid PTSD and major depression. These encouraging results suggest that rTMS may be useful as an adjunctive treatment in this notably treatment-refractory population, and merit replication in a double-blind, sham-controlled design.
ACKNOWLEDGMENTS
This work was previously presented at the Society for Biological Psychiatry annual meeting, Chicago, IL, May 11–13, 2000.
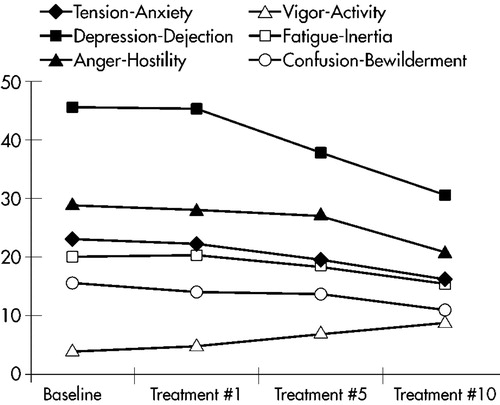
FIGURE 1. Rate of response measured with Profile of Mood States subscales.
 |
 |
 |
 |
1 Helzer JE, Robins LN, McEvoy L: Post-traumatic stress disorder in the general population. findings of the Epidemiologic Catchment Area survey. N Engl J Med 1987; 317:1630-1634Crossref, Medline, Google Scholar
2 Neylan TC, Marmar CR, Metzler TJ, et al: Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry 1998; 155:929-33Crossref, Medline, Google Scholar
3 Bende BC, Philpot J: Persistent post-traumatic stress disorder. BMJ 1994; 309:526-528Crossref, Medline, Google Scholar
4 Orsillo SM, Weathers FW, Litz BT, et al: Current and lifetime psychiatric disorders among veterans with war zone-related posttraumatic stress disorder. J Nerv Ment Dis 1996; 184:307-313Crossref, Medline, Google Scholar
5 Van der Kolk BA, Dreyfuss D, Michaels M, et al: Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry 1994; 55:517-522Medline, Google Scholar
6 Davidson J, Kudler H, Smith R, et al: Treatment of posttraumatic stress disorder with amitriptyline and placebo. Arch Gen Psychiatry 1990; 47:259-266Crossref, Medline, Google Scholar
7 Reist C, Kauffmann CD, Haier RJ, et al: A controlled trial of desipramine in 18 men with posttraumatic stress disorder. Am J Psychiatry 1989; 146:513-516Crossref, Medline, Google Scholar
8 DeBoer M, Op den Velde W, Falger PJR, et al: Fluvoxamine treatment for chronic PTSD. Psychother Psychosom 1992; 57:158-163Crossref, Medline, Google Scholar
9 Zygmont M, Prigerson HG, Houck PR, et al: A post hoc comparison of paroxetine and nortriptyline for symptoms of traumatic grief. J Clin Psychiatry 1998; 59:241-245Crossref, Medline, Google Scholar
10 Rothbaum BO, Ninan PT, Thomas L: Sertraline in the treatment of rape victims with posttraumatic stress disorder. J Trauma Stress 1996; 9:865-871Crossref, Medline, Google Scholar
11 Marshall RP, Jorm AF, Grayson DA, et al: Posttraumatic stress disorder and other predictors of health care consumption by Vietnam veterans. Psychiatr Serv 1998; 49:1609-1611Crossref, Medline, Google Scholar
12 Hidalgo R, Hertzberg MZ, Mellman T, et al: Nefazodone in post-traumatic stress disorder: results from six open-label trials. Int Clin Psychopharmacology 1999; 14:61-68Crossref, Medline, Google Scholar
13 Davidson J, Weisler R, Malik M, et al: Treatment of posttraumatic stress disorder with nefazodone. Int Clin Psychopharmacol 1998; 13:111-113Crossref, Medline, Google Scholar
14 Hertzberg MA, Feldman JE, Beckham JC, et al: Open trial of nefazodone for combat-related posttraumatic stress disorder. J Clin Psychiatry 1998; 59:460-464Crossref, Medline, Google Scholar
15 Canive JM, Clark RD, Calais LA, et al: Bupropion treatment in veterans with posttraumatic stress disorder: an open study. J Clin Psychopharmacol 1998; 18:379-383Crossref, Medline, Google Scholar
16 Neal LA, Shapland W, Fox C: An open trial of moclobemide in the treatment of post-traumatic stress disorder. Int Clin Psychopharmacol 1997; 12:231-237Crossref, Medline, Google Scholar
17 Brady K, Pearlstein T, Asnis GM, et al: Efficacy and safety of sertraline treatment of posttraumatic stress disorder. JAMA 2000; 283:1837-1844Crossref, Medline, Google Scholar
18 Dow B, Kline N: Antidepressant treatment of posttraumatic stress disorder and major depression in veterans. Ann Clin Psychiatry 1997; 9:1-5Crossref, Medline, Google Scholar
19 Epstein CM, Figiel GS, McDonald WM, et al: Rapid rate transcranial magnetic stimulation in young and middle-aged refractory depressed patients. Psychiatric Annals 1998; 28:36-39Crossref, Google Scholar
20 Gellar V, Grisaru N, Abarbanel JM, et al: Slow magnetic stimulation of prefrontal cortex in depression and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:105-110Crossref, Medline, Google Scholar
21 George SM, Wasserman, EM, Williams WA, et al: Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6:1853-1856Crossref, Medline, Google Scholar
22 Kolbinger HM, Hoflich G, Hufnagel A, et al: Transcranial magnetic stimulation in the treatment of major depression: a pilot study. Human Psychopharmacology 1995; 10:305-310Crossref, Google Scholar
23 Pascual-Leone A, Catala MD, Pascual APL: Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology 1996; 46:499-502Crossref, Medline, Google Scholar
24 George MS, Wassermann EM, Kimbrell TA, et al: Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997; 154:1752-1756Crossref, Medline, Google Scholar
25 Figiel GS, Epstein C, McDonald WM, et al: The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci 1998; 10:20-25Link, Google Scholar
26 Klein E, Kreinin I, Chistyako Q, et al: Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression. Arch Gen Psychiatry 1999; 56:315-320Crossref, Medline, Google Scholar
27 McCann UD, Kimbrell TA, Morgan CM, et al: Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gen Psychiatry 1998; 55:276-278Crossref, Medline, Google Scholar
28 Grisaru N, Amir M, Cohen H, et al: Effect of transcranial magnetic stimulation in posttraumatic stress disorder. Biol Psychiatry 1998; 44:52-55Crossref, Medline, Google Scholar
29 Post RM, Speer AM: Speculations on the future of rTMS and related therapeutic modalities, in Transcranial Magnetic Stimulation in Psychiatry, edited by George MS, Belmaker RH. Washington, DC, American Psychiatric Publishing, 2000, pp 269-288Google Scholar
30 First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version. Washington, DC, American Psychiatric Press, 1997Google Scholar
31 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-65Crossref, Medline, Google Scholar
32 Lorr M, McNair DM: Manual, Profile of Mood States, Unipolar Form. San Diego, CA, Education and Individual Testing Service, 1984Google Scholar
33 Parker ES, Eaton EM, Whipple SC, et al: University of Southern California Repeatable Episodic Memory Test. J Clin Exp Neuropsychol 1995; 17:926-936Crossref, Medline, Google Scholar
34 Keane TM, Caddell JM, Taylor KL: Mississippi scale for combat-related posttraumatic stress disorder: three studies in reliability and validity. J Consult Clin Psychol 1988; 56:85-90Crossref, Medline, Google Scholar
35 Bohning DE: Introduction and overview of TMS physics, in Transcranial Magnetic Stimulation in Neuropsychiatry, edited by George MS, Belmaker RH. Washington, DC, American Psychiatric Publishing, 2000, pp 13-44Google Scholar
36 Roth BJ, Saypol JM, Hallett M, et al: A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroencephalogr Clin Neurophysiol 1991; 81:47-56Crossref, Medline, Google Scholar
37 Ross RJ, Ball WA, Sullivan KA, et al: Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989; 146:697-707Crossref, Medline, Google Scholar
38 Zisook S, Chentsova-Dutton YE, Smith-Vaniz A, et al: Nefazodone in patients with treatment-refractory posttraumatic stress disorder. J Clin Psychiatry 2000; 61:203-208Crossref, Medline, Google Scholar
39 Goodwin GM: Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol 1997; 11:115-22Crossref, Medline, Google Scholar
40 Soares JC, Mann JJ: The functional neuroanatomy of mood disorders. J Psychiatr Res 1997; 31:393-432Crossref, Medline, Google Scholar
41 Rauch SL, Van der Kolk BA, Eisler RE, et al: A symptom provocation study of posttraumatic stress disorder using PET and script-driven imagery. Arch Gen Psychiatry 1996; 53:380-387Crossref, Medline, Google Scholar
42 Shin LM, Kosslyn SM, McNally RJ, et al: Visual imagery and perception in posttraumatic stress disorder. Arch Gen Psychiatry 1997; 54:233-241Crossref, Medline, Google Scholar
43 Chen R, Classen J, Gerloff C, et al: Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997; 48:1398-1403Crossref, Medline, Google Scholar
44 Berardelli A, Inghilleri M, Rothwell JC, et al: Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res 1998; 122:79-84Crossref, Medline, Google Scholar
45 Speer AM, Kimbrell TA, Wassermann EM, et al: Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 2000; 48:1133-1141Crossref, Medline, Google Scholar
46 Padberg F, Zwanzger P, Thoma H, et al: Repetitive transcranial magnetic stimulation in pharmacotherapy-refractory major depression: comparative study of fast, slow, and sham rTMS. Psychiatry Res 1999; 88:163-171Crossref, Medline, Google Scholar
47 Berman RM, Narasimhan M, Sanacora G, et al: A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000; 47:332-337Crossref, Medline, Google Scholar
48 Loo C, Mitchell P, Sachdev P, et al: Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of major depression. Am J Psychiatry 1999; 156:946-948Crossref, Medline, Google Scholar
49 Loo CK, Taylor JL, Gandevia SC, et al: Transcranial magnetic stimulation in controlled treatment studies: are some “sham” forms active? Biol Psychiatry 2000; 47:325-331Crossref, Medline, Google Scholar
50 Stokes PE: Fluoxetine: a five-year review. Clin Ther 1993; 15:216-243Medline, Google Scholar
51 Brady KT, Sonne SC, Roberts JM: Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence. J Clin Psychiatry 1995; 56:502-505Medline, Google Scholar



