Cavum Septum Pellucidum and Its Increased Prevalence in Schizophrenia: A Neuroembryological Classification
Abstract
Thirty-two female (mean age=52.9 years [SD=9.2]) patients with a diagnosis of residual schizophrenia and 19 female (mean age=51.1 years [SD=12.7]) control subjects were studied through cerebral Magnetic Resonance Imaging. Along the entire surface of the septum pellucidum, 1-mm coronal slices were performed in all subjects. The authors classified the cavum septum pellucidum into three types based on embryological development. The prevalence of a cavum was significantly higher in the patients with schizophrenia (Chi square 6.112. p < 0.05). No other significant associations with previously described morphological brain changes were found. Although this result was found in previous reports (DeGreef et al., 1992; DeLisi et al., 1993), our discussion focused on the neurodevelopmental theory of the septum pellucidum and its possible association with schizophrenia.
Schizophrenia is probably the most challenging neuropsychiatric disease to both neuroanatomists and neuropathologists. Controversy is mainly concerned with whether schizophrenia is a mind or a brain disease.3 In support of the last hypothesis, many morphological changes have been associated with this mental disease, and the presence of a cavum septum pellucidum (CSP) is one marker that has been consistently found in imaging studies4,5 and neuropathological reports.6,7 The CSP is part of normal development and persists shortly after birth, hence it is present in all preemies and persists in approximately 10% of adult individuals. The compartment is located between the leaflets of the Septum Pellucidum (SP) that separates the ventricular frontal horns. It is located below the anterior part of the corpus callosum, above the fornix. Although it is filled with fluid, it is usually isolated from the ventricular system.8 The cavum contents may originate from either the ependymal secretion of the leaflets or from a communication with the third ventricle.9 Embryologically, the CSP is found in the caudal portion of the interhemispheric fissure. During the 5th month of the embryonic period, the corpus callosum begins to close superiorly and the forceps minor grows to the frontal lobules, while the fornix is relatively fixed in its original position.13 The corpus callosum forms from anterior to posterior with the exception of the rostrum, which connects the genu and the lamina terminalis. Hence, the leaflets of the SP are pulled towards the lamina terminalis, closing the cavum from the posterior fornix to the rostrum of the corpus callosum, due to the frontalization of fibers in the genu portion by the 7th month.8
DeGreef et al.1 originally described the increased prevalence of the CSP in schizophrenia. Many researchers have since made similar conclusions.2,10,11 It is our intention to report our experience in this particular entity, and discuss our theory regarding its embryological origin.
PATIENTS AND METHODS
Subjects
Thirty-two female schizophrenic patients from the National Psychiatric Hospital Braulio Moyano, Buenos Aires, Argentina, were enrolled in our protocol study. Patients presented with a mean age of 52.9 years, SD=9.2, range 37–68 years. Each patient underwent a structured interview and all subjects had a diagnosis of residual schizophrenia,12 with a mean time of evolution of 28 years (SD=9.8). All were Caucasian, right-handed, and had a high school diploma. All were under neuroleptic treatment during the study, but none had received previous electroconvulsive therapy. No subject had associated neurological disease or received corticosteroid therapy 30 days prior to the study. We excluded patients with any of the following: schizophreniform disorder; schizoaffective disorder; schizophrenia, subchronic type; psychoses not specified; and schizotypal personality disorder.
Nineteen female control subjects were included in this study. Control subjects presented a mean age of 51.1 years (SD=12.7, range 25–68). All were healthy subjects, right-handed and Caucasian, with either a high school diploma or Bachelor's degree. Each control subject underwent a structured interview and was excluded if there was any family history of schizophrenia. No subject had a previous history of alcohol and/or drug addiction. After description of the study to the subjects, written informed consent was obtained.
Magnetic Resonance Imaging CSP Evaluation and Analysis
All scans were performed on a General Electric 1.5 Tesla scanner. T1-weighted images with acquisition parameters as follows: TE = 5 msec, TR = 35 msec, flip angle = 45, field of view = 24 cm, acquisition matrix = 256 × 256 × 124. These images were reformatted in the coronal plane, with 1-mm thickness, and consisted of 128 contiguous slices. The axis for coronal slices was parallel to the axis of the brainstem. Coronal sequences extended from the tip of the frontal pole to the tip of the occipital pole. Daily scanning and analysis was used to measure the scanner quality and stability with time.
During the 5th month of the embryonic period, the corpus callosum begins to close superiorly and the forceps minor grows to the frontal lobules, while the fornix is in its original position. The leaflets of the SP are pulled towards the lamina terminalis, closing the cavum from the posterior fornix to the rostrum of the corpus callosum, due to the frontalization of the corpus callosum by the 7th month.8,13
Based on this information, we classify the CSP into three embryological types (Figure 1).
Type I: CSP from the genu corpus callosum to 3 mm posteriorly, corresponding to the 7th month of embryonic life. This type dissapears last and is the most common to persist after birth.
Type II: CSP from the genu corpus callosum to 7 mm posteriorly, corresponding to the late 5th prenatal month. The exact length of the cavum during this period is determined by the time of development of the previous stage.
Type III: Complete CSP without cavum vergae. Corresponds to the early 5th month of embryonic life. Sporadically, it can be found after birth.
We did not attempt to classify CSP as normal or abnormal based on length, as reported by Nopoulus et al.11 Assessment of the CSP was determined from the corresponding films by at least three authors, who were blind to group membership. Measurements were performed on each scan by a trained research associate, without knowledge of the diagnostic status of the subjects. Total volumes of specific areas of interest and whole brain were computed by a method developed in our laboratory.14 The total area of the corpus callosum and four equal subdivisions of it were outlined on the most sagittal cut, marked by the longest view of the Sylvian aqueduct. Each section was an equal subdivision formed by a line joining the tips of the anterior and posterior poles. The temporal lobes were defined anteriorly by its pole and posteriorly by sections showing the external auditory canals. Measurements for lateral ventricles were taken from axial scans where frontal, ventricular body, and occipital horns were seen. The interater reliability for all reported regions was computed by using an interclass correlation coefficient (0.92 to 0.97).
We used Chi-square analysis between the study and control groups. When expected cell size was less than 5, a Fisher's exact test was performed. Spearman's rho (rs) was used to assess relationships between ratings of CSP and the volume of measured brain structures. A level of 0.05 was considered significant.
RESULTS
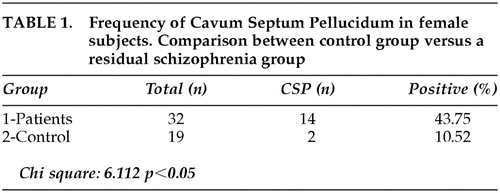
Table 1 shows the prevalence of CSP in both groups, with a statistically significant increased frequency in schizophrenia patients, as compared to control subjects. Fourteen of the 32 patients (43.75%) had ratings of Type I, Type II, or Type III of CSP (Table 2). One patient was rated with a CSP Type III while no control subjects were rated “3.” There was a clear tendency for schizophrenia patients to have higher abnormal ratings, although the difference when compared with each specific type was not statistically significant (Table 2). Cavum vergae and Cavum velum interpositum were not disclosed in any of the analyzed images. Correlational analysis using Spearman's rho was calculated between the severity ratings for the CSP and the sizes of ventricles, temporal lobes, area of corpus callosum and total brain volumes. Other variables such as age, educational level, length of disease, neuroleptic doses, and positive or negative symptoms were studied in relation to differences between absence or presence of cavum. No statistically significant correlations were found. Correlations between age and surface of corpus callosum, whether CSP was present or not, disclosed a significant decrease in the surface of the corpus callosum with age in the schizophrenia group (rs −0.46 p < 0.05). In the control group, the surface was not altered with age. The negative sign of the correlation in the schizophrenia group indicates that the area of the corpus callosum, contrary to what happens in the control group, decreases with the increment of age. These results are reported in detail elsewhere.14
DISCUSSION
Our results showed a prevalence of CSP in chronic schizoprenia of 43.7% and 10.5% in control subjects, even though we studied a highly selected population of Caucasian, female patients with residual schizophrenia who were compared to normal subjects matched for sex and age. Several imaging studies have shown that the frequency of CSP in chronic schizophrenia is high, ranging from 15%,15 21%,1 36%,16 and 44.8%.2 Nonetheless, when the CSP was studied with different imaging techniques, as well as in different subtypes of schizophrenia, the prevalence varied consistently in patients and normal subjects. DeGreef et al.17 found 23% of CSP in first-episode patients versus a 2% in control subjects. Also in first-episode patients, Kwon et al.16 found a 25% of CSP in patients versus a 10% in controls. Others such as Fukuzako et al.10 disclosed a 47% presence of CSP in patients and 38% in controls, while Rajarethinam et al.18 found a 60% presence of septum anomaly in schizophrenics and 42% in normal subjects. On the other hand, Nopoulus et al.11 presented a meticulous imaging technique to report this anomaly and disclosed a 58% presence of CSP in patients and controls as well. Following this report, the same author19 defined abnormal enlargement of CSP as those greater than 6 mm in length and reported a 12.5% of enlarged CSP in childhood-onset schizophrenia. The results of previous studies seem highly variable in both patients and controls. The inconsistencies in incidences could be regarded, in part, for technical reasons (i.e., magnetic resonance imaging (MRI) acquisitions protocols). Variances in the characteristics of the samples related to race, sex, or age might not be available. Regarding the interpretation of our data, it is clear that a majority of patients with schizophrenia do not have a cavum, and some controls do, and the statistical association in our study could represent nothing more than epiphenomena.
It is our intention to make several hypotheses about the relationship between the CSP and other neuromorphological findings previously described in several studies. First, the possibility that the primary atrophy found in these patients (e.g., smaller frontal lobes,20 smaller temporal lobes,21 reduced hemispheric volumes,7 increased ventricular volumes)22 could secondarily impact on the SP conforming a postembryonary cavum.
The strict anatomical relationship of the fornix with the septum leaflets is evident. The fornix has its origin in the subiculum, alveus of the hippocampus, and the hippocampal pyramidal cells. It belongs to the limbic system and is directly or indirectly connected to the thalamus, hypothalamus, and reticular formation. Disarray of hippocampal pyramidal cells,23 reduced parahippocampal white matter24 and volume gyrus,25 reduced volumes of hippocampus and amygdalas,26,27 and lower neuronal densities in the cingulate gyrus28 are limbic disturbances that have been noted in schizophrenics. Other qualitative abnormalities of the limbic system disclosed in schizophrenia included gliosis of the hippocampus6 and cellular changes in the ansa lenticularis29 and rostral enthorrinal region,30 among others. It is possible that these abnormalities anatomically impact the fornix and in the septum.
Third, the abnormalities of the corpus callosum and septum pelucidum have been previously described in schizophrenia.14,15 An involution of the corpus callosum, specifically of the genu due to less interconnection of frontal fibers, could lead to a secondary effect in the anterior septum developing the CSP.
Another possibility is that the CSP in schizophrenia is a persisting anomaly from the prenatal period and not a secondary developmental anomaly. One possible way to study this hypothesis is to perform MRIs of neonates and children with predisposing factors such as direct family members with schizophrenia. It is important to recognize that more systematic studies will require thousands of subjects and many years of follow-up. However, the finding of developmental anomalies supports the theory that increased susceptibility to schizophrenia may result from a prenatal disturbance in brain development.2,31
An interconnected web among all these factors could lead to a conformation of a CSP in patients with schizophrenia. The theory that anatomical structures such as the basal ganglia, thalamus, and hippocampus have possible implications in the mechanisms of cognition, consciousness, intelligence, and creativity32 is applicable in patients with schizophrenia. Empirical data that confirms or refutes these hypotheses are still lacking.
Conclusion
Our studies disclosed an increased prevalence of CSP in female patients with residual schizophrenia, compared with a control group matched by age and gender. We believe that a more anatomo-embryological-based classification of the CSP, such as the one presented here, would be more appropriate to distinguish this particular anomaly in schizophrenia patients. It is our opinion that the presence of a CSP in this disease is merely a developmental anomaly and is not adequate, per se, to produce schizophrenia, although it is related to other structural abnormalities that could not be established in the present study.
ACKNOWLEDGMENTS
The authors thank Mrs. Sue Ware for English assistance.
 |
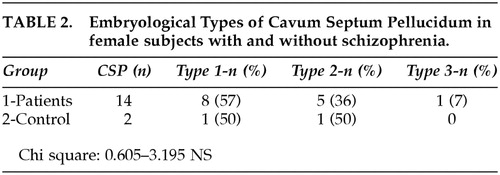 |
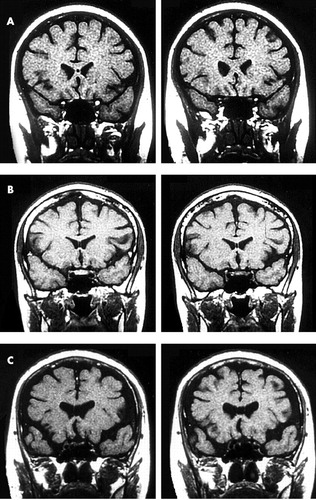
FIGURE 1. (a) Type I CSP—corresponds to the late embryological period during the seventh month of prenatal life (b) Type II CSP—corresponds to the mid embryological period during the late fifth month of prenatal life (c) Type III CSP—corresponds to the early embryological period during the early fifth month of prenatal life
1 DeGreef G, Bogerts B, Falkai P, et al: Increased prevalence of the Cavum Septum Pellucidum in magnetic resonance scans and post-morten brains of schizophrenic patients. Psychiatry Res 1992; 45:1–13Crossref, Medline, Google Scholar
2 DeLisi LE, Hoff AL, Kushner M, et al: Increased prevalence of cavum septum pellucidum in schizophrenia. Psychiatry Res 1993; 50:193–199Crossref, Medline, Google Scholar
3 Weinberger DR: Schizophrenia as a neurodevelopmental disorder, in Schizophrenia, 1st edition, edited by Hirsh SR, Weinberger DR. Oxford: Blackwell Sci Ltd, 1995, pp 293–323Google Scholar
4 Andreasen NC, Nasrallah HA, Dunn V, et al: Structural abnormalities in the frontal system in schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry 1986; 43:136–144Crossref, Medline, Google Scholar
5 Kawasaki Y, Maeda Y, Urata K, et al: A quantitative magnetic resonance imaging study of patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 1993; 242:195–200Crossref, Google Scholar
6 Nieto D, Escobar A: Major psychoses, in Pathology of the Nervous System, 1st edition, edited by Minkler J. New York: McGraw-Hill, 1972, pp 2654–2665Google Scholar
7 Pakkenberg B: Post-mortem study of chronic schizophrenic brains. Br J Psychiatry 1987; 151:744–752Crossref, Medline, Google Scholar
8 Larroche JC, Baudey J: Cavum septum pellucidi, Cavum vergae, Cavum veli interpositi. Cavités de la ligne médiane. Etude anatomique et pneuroencéphalographique dans la période néo-natale. Biol Neonate 1961; 3:193–236Crossref, Google Scholar
9 Liss L, Mervis L: The ependymal lining of the Cavum septum pellucidi: a histological and histochemical study. J Neuropathol Exp Neurol 1964; 23:355–367Crossref, Medline, Google Scholar
10 Fukuzako T, Fukuzako H, Kodama S, et al: Cavum septum Pellucidum in schizophrenia: a magnetic resonance imaging study. Psychiatry Clin Neurosci 1996; 50:125–128Crossref, Medline, Google Scholar
11 Nopoulus P, Swayze V, Flaum M, et al: Cavum septum pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biol Psychiatry 1997; 41:1102–1108Crossref, Medline, Google Scholar
12 American Psychiatric Association DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders. 3rd ed., revised. Washington, DC, American Psychiatric Press, 1987.Google Scholar
13 Sarnat HB: Substrates of maturation in the nervous system, in Cerebral Dysgenesis, Embryology and Clinical Expression, 1st edition, edited by Sarnat HB. New York, Oxford University Press, 1992, pp 78–82Google Scholar
14 Merlo AB, Albanese EF, Galarza M, et al: Morphological alterations of corpus callosum in schizophrenia. Medicina (Buenos Aires) 1997; 57:566–570Medline, Google Scholar
15 Scott TF, Price TR, George MS, et al: Midline Cerebral malformations and Schizophrenia. J Neuropsychiatry Clin Neurosci 1993; 5:287–293Link, Google Scholar
16 Kwon JS, Shenton ME, Hirayasu Y, et al: MRI study of Cavum Septum Pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am J Psychiatry 1998; 155:509–515Crossref, Medline, Google Scholar
17 DeGreef G, Lantos G, Bogerts B, et al: Abnormalities of the Septum Pellucidum on MR scans in first-episode schizophrenic patients. AJNR Am J Neuroradiol 1992; 13:835–840Medline, Google Scholar
18 Rajarethinam R, Miedler J, DeQuardo J, et al: Prevalence of Cavum septum Pellucidum in schizophrenia studied with MRI. Schizophrenia Res 2001; 48:201–205Crossref, Medline, Google Scholar
19 Nopoulus PC, Giedd JN, Andreasen NC, et al: Frequency and severity on enlarged Cavum septi pellucidi in childhood-onset schizophrenia. Am J Psychiatry 1998; 155:1074–1079Crossref, Medline, Google Scholar
20 Andreasen NC, O'Leary DS, Flaum M: Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. Lancet 1997; 349:1730–1734Crossref, Medline, Google Scholar
21 Suddath RL, Christie GW, Torrey EF, et al: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Eng J Med 1990; 322:789–794Crossref, Medline, Google Scholar
22 Nasrallah HA, Olsen SC, McCalley-Whiters M, et al: Cerebral ventricular enlargement in schizophrenia: a preliminary follow up study. Arch Gen Psychiatry 1986; 43:157–159Crossref, Medline, Google Scholar
23 Jeste DV, Lohr JB: Hippocampal pathologic findings in schizophrenia. Arch Gen Psychiatry 1989; 46:1019–1024Crossref, Medline, Google Scholar
24 Colter N, Battar S, Crow TJ, et al: White matter reduction in the parahippocampal gyrus of patients with schizophrenia. Arch Gen Psychiatry 1987; 44:1023Crossref, Medline, Google Scholar
25 Shenton ME, Kikinis R, Jolenz FA, et al: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Eng J Med 1992; 327:604–612Crossref, Medline, Google Scholar
26 Bogerts B, Ashtari M, Degreef G, et al: Reduced temporal limbic structures volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1993; 35:236–246Google Scholar
27 Bogerts B, Meertz E, Schonfeld-Bausch R: Basal ganglia and limbic system pathology in schizophrenia. Arch Gen Psychiatry 1985; 42:784–791Crossref, Medline, Google Scholar
28 Benes FM: An analysis of the arrangement of neurons in the cingulated cortex of schizophrenic patients. Arch Gen Psychiatry 1987; 44:31–35Crossref, Medline, Google Scholar
29 Averback P: Lesions of the nucleus ansae peduncularis in neuropsychiatric disease. Arch Neurology 1981; 38:230–235Crossref, Medline, Google Scholar
30 Jakob J, Beckmann H: Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transmission 1986; 65:303–326Crossref, Medline, Google Scholar
31 Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
32 Cotterill RJM: Cooperation of the basal ganglia, cerebellum, sensory cerebrum and hippocampus: possible implications for cognition, consciousness, intelligence and creativity. Progress in Neurobiology 2001; 64:1–33Crossref, Medline, Google Scholar



