Relative Sparing of Executive Functions in the Early Phase of Schizophrenia
Abstract
Findings about the impairment of executive functions in schizophrenia are not conclusive. The authors hypothesized that the severity of the impairments in the abilities that comprise EF might be different. Forty patients were assessed with a comprehensive battery that included four measures of executive functions and were compared with 60 healthy subjects. Set shifting and response inhibition showed no significant between-group differences. Mental flexibility and concept formation were significantly worse in patients, but the effect sizes were small. Some executive functions might be relatively spared, at least in the early phase of schizophrenia. Studies on individual executive functions may yield more replicable findings.
Neurocognitive dysfunction has been established as a core feature of schizophrenia.1–2 Many studies have shown the presence of generalized cognitive dysfunction,3 and among other deficits, executive dysfunction,4–7 attentional impairment,8–9 memory deficits10–11 and psychomotor slowing12 are the most consistently reported findings. Evidence has already shown that healthy comparison subjects outperform patients on many cognitive tasks.3 However, an open question is the selectivity of the deficits, as compared to the global cognitive level, which has consistently been shown to be deficient in patients with schizophrenia.13–14
Most researchers agree that schizophrenia is not homogeneous in terms of the cognitive deficits associated with the illness,15–16 and debate on whether a specific cognitive function is significantly more impaired than other cognitive functions has not been resolved yet.
Although many studies have reported executive dysfunction,4–7 others have shown that the performance of patients with schizophrenia is not uniformly impaired on tests that measure the cognitive abilities termed executive functions.17–19 One reason for the controversial findings is the lack of a strict definition of these functions and the fact that none of the available neuropsychological tests is a pure measure of executive functioning. The concept, as defined in the current literature, is comprised of a combination of abilities like abstraction and planning, set shifting, mental flexibility, and response inhibition in pursuit of a long-term goal. One approach to this methodological problem could be to dissect the concept to simpler components.
Furthermore, since we are still far from delineating the exact neurobiological substrates for many cognitive functions, the question of whether a putative deficit can be explained by other impairments in information processing still needs to be addressed by proper inferential statistical methods. Some studies,19 for instance, included set shifting and response inhibition abilities among executive functions, whereas others3 were designed with the assumption that these abilities reflect attention and vigilance. Given that vigilance needs to be maintained throughout the performance of the tasks that tap these functions, it is inconceivable that these tasks would be unrelated to attentional abilities. General level of intelligence is another possible confounder. Although many other confounders such as age, gender, medication use, chronic hospitalization and symptom severity have been controlled by proper study design or statistical methods, the impact of general intelligence and attention has been neglected in many studies that reported significant executive dysfunction in patients with schizophrenia.
Given the complexity of the concept of executive functions, we were interested in delineating the performance of patients with schizophrenia on tasks that tap abilities like set shifting, response inhibition, category formation, and tendency to perseverate, rather than measuring “executive function” as one single cognitive ability. We hypothesized that these abilities would show different degrees of impairment if controlled for attention and general intelligence.
METHOD
Subjects
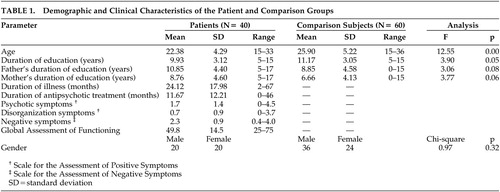
Data for the analysis were obtained through assessments of 40 patients who had DSM–IV20 schizophrenia, with a duration of illness that varied between 2 and 67 months and a mean of 24.12 (SD=17.89) months. Sixty healthy subjects recruited through newspaper advertisments served as comparison subjects. Consecutive patients who gave written consent were included. Patients with any other central nervous system (CNS) disorder, mental retardation, substance use disorder or history of head injury were excluded.
Assessment Tools
All patients received structured interviews with the Structured Clinical Interview for DSM–IV Axis I Disorders (SCID)21 in addition to the Scale for the Assessment of Positive Symptoms (SAPS)22 and Scale for the Assessment of Negative Symptoms (SANS).23 Patients were jointly interviewed and assessed by two psychiatrists, trained and experienced in the use of the SCID, SANS, and the SAPS. Consensus ratings were entered into the analysis. Neuropsychological tests were performed by a trained psychologist when the patients’ clinical condition permitted cooperation, therefore the cognitive assessment reflected a clinically stable phase of the illness with minimal positive symptoms (Table 1).
The neuropsychological tests used were as follows: Benton Revised Visual Retention Test (BRVRT),24 Cancellation Test,25 Face Recognition Test,26 Rey Auditory Verbal Learning Test (RAVLT),27 Stroop Color-Word Test,28 Trail Making Test (TMT),29 WAIS,30 Wechsler Memory Scale (WMS),31 and the Wisconsin Card Sorting Test (WCST).32
The demographic and clinical features of the sample are summarized in Table 1. Patients were significantly younger and less educated than comparison subjects.
We recognized that novel antipsychotics are difficult to categorize and quantify in clinical studies, since their effects, therapeutic or adverse, involve multiple receptor occupancies.33–34 The traditional classifications of chlorpromazine equivalents, defined in terms of D2 occupancy, do not apply readily to novel agents. Therefore, we preferred to list the antipsychotic medications and the mean doses (mg) that the patients had been stabilized on at the time of the assessment: Olanzapine 13.9 ([SD=5.8] (N=13), risperidone 5.3 ([SD=3.4] N=13), trifluoperazine 13.3 ([SD=5.8] N=3), pimozide 4.0 ([SD=0.0] N=2), quetiapine 300.0 ([SD=141.4] N=2), haloperidol 15 mg (N=1), zuclopenthixol decanoate 200 mg/3 weeks (N=1), fluphenazine decanoate 25 mg/3 weeks (N=1). Four patients were off medications at the time of assessment. Nine patients were on the anticholinergic biperiden with a mean daily dose of 4.7 (SD=2.0) mg.
Statistical Analyses
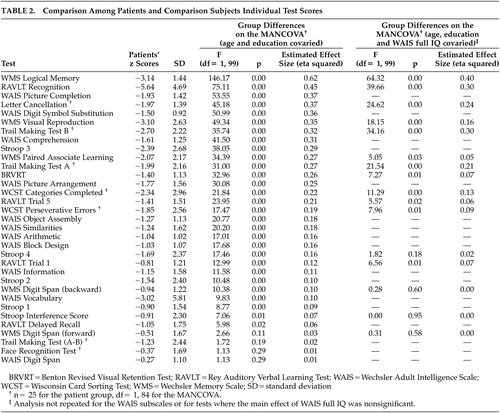
Neuropsychological test scores were transformed into standard z scores using the means and SDs of the normal comparison group. Scores were reversed when applicable so that higher scores always indicated better performance. Group differences were analyzed in a multivariate analysis of variance (MANCOVA) model covarying for age and education level. Bonferronni correction was used to adjust the level of significance of all analyses. Eta-squared values (effect size estimates) were calculated to measure the magnitude of difference between comparison subjects and patients on individual tests.
Data were further analyzed to control for the possible effects of attention and general intelligence. Freedom from distractibility, which has been validated as a separate factor on the WAIS-R in patients with schizophrenia,14 was used as a measure of attention. This factor is comprised of three subtests: Digit Symbol Substitution, Arithmetic, and Digit Span. For the general intelligence level, the full IQ score on the WAIS was used.
For the test scores with a significant main effect of either attention or general intelligence, the analysis of variance was repeated, covarying for the relevant variable as well as for age and education.
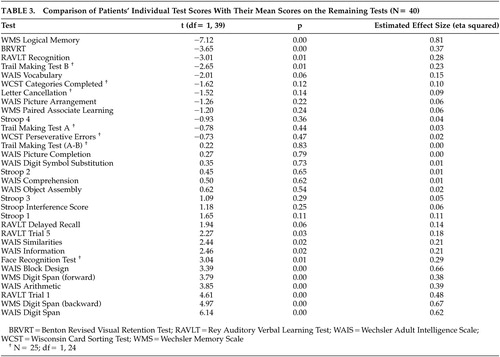
Patients’ performance on the individual tests was also compared with the mean of their scores on the remaining tests in the profile. This procedure allowed the determination of selectively more pronounced deficits. Bonferroni correction was used to adjust the level of significance of the contrast analysis. Eta-squared values (effect size estimates) were calculated to measure the magnitude of difference.
All statistical analyses were performed using the SPSS 10.0 program.
RESULTS
The initial MANCOVA where age and education were covaried demonstrated that the patients’ performance was significantly worse than and 1–5 SD’s below the performance of healthy comparison subjects on all tests, with the exception of Digit Span on the WMS and the WAIS, Face Recognition Test, and the A–B Difference on the Trail Making Test (Table 2).
We examined the effect of attention by repeating the analysis of variance with the freedom from distractibility score as an additional covariate. Although the main effect was significant for letter cancellation and WMS Visual Reproduction, group differences remained significant with large effect size estimates for both tests (F=29.70, p=0.00, eta squared=0.27 for letter cancellation, and F=36.84, p=0.00, eta squared=0.28 for WMS Visual Reproduction).
On the other hand, the main effect of the general intelligence level was significant for many tests. Group differences and the magnitude of the difference for each test are shown in Table 2. Verbal declarative memory (WMS Logical Memory and RAVLT Recognition) and general psychomotor speed (Letter Cancellation Test and Trail Making Test) were the only domains where group differences remained significant after controlling for the general level of intelligence. It is particularly noteworthy that either no significant group differences were found or the magnitude of group differences was small for the abilities of set shifting, response inhibition, concept formation and mental flexibility, as measured by the Trail Making Test A–B difference, Stroop Interference score and the Categories Achieved and Perseverative Errors on the WCST, respectively.
Comparing the patients’ performance on each test with the mean of the remaining tests revealed significantly greater impairment in the WMS Logical Memory, BRVRT, RAVLT Recognition, and Trail Making Test B scores. The estimated effect sizes were large (>0.29) for the former two (Table 3).
DISCUSSION
This study opted for a conservative approach in analyzing group differences and took into account the effects of attention and general level of intelligence in addition to age and education. The magnitude as well as the significance of the group differences was observed.
The most important finding is a sparing of set shifting and response inhibition abilities in patients in the first 5 years of the illness, as measured by the Trail Making Test A–B difference and the Stroop Interference Score, respectively. Abilities like concept formation and mental flexibility, as measured by the number of categories achieved and perseverative errors on the WCST, respectively, were also relatively less impaired.
Although the Trail Making test B has been used in some studies as a measure of set shifting abilities, the A–B difference, which controls for the effect of general psychomotor speed, might be a more accurate measurement.35–36 Our results point to a significant group difference in the general psychomotor speed (Trail Making A) and attention (Trail Making B) in contrast to the nonsignificant difference in the set shifting ability (Trail Making A–B difference).
The small magnitude of between-group differences on the WCST along with preserved set shifting and response inhibition abilities point to a relative sparing of the cognitive abilities that have been called “executive functions.” These results are consistent with the findings of Hutton et al.37 who reported relatively unimpaired set shifting abilities in first-episode patients. Similarly, Goldstein et al.38 found that dense perseverative behavior is not common to all patients with schizophrenia. Comparing the discriminative power of the WCST and the WAIS, Dieci et al.17 demonstrated a lack of selectivity of the WCST performance in identifying individuals with schizophrenia from healthy comparison subjects.
On the other hand, studies that showed impaired WCST performance outnumber the ones that demonstrated a sparing of these functions. Our results therefore require explanation.
First, “executive functioning” remains a somewhat elusive construct. The classification of the four abilities we studied (set shifting, response inhibition, concept formation and mental flexibility) as “executive functions” is not based on a definite neurobiological substrate they all share. The cerebral localization of the cognitive abilities termed executive functions also remains elusive and controversial. Regions of the prefrontal cortex may play a special role in recruiting other brain areas in a series of distributed networks that handle different components of executive functions, depending on the processing demands of the specific task.39 Results from other studies also suggest that the cognitive abilities collectively termed as “executive functions” differ from one another in many aspects and may be better studied when measured separately. One example is the difference in their span of development: Pantelis et al.19 have recently suggested that cognitive functions developing earlier in life (e.g., set shifting abilities) are less likely to show deficits, whereas those that take longer to fully develop (e.g., declarative memory or executive function on the WCST) are more likely to be impaired. Preserved set shifting and response inhibition abilities in our group suggest that more strict definitions of executive functions might be useful in future studies.
Second, the degree of the impairment in set shifting abilities might be related to the chronicity of the illness. Hutton et al.,37 for instance, showed relative unimpairment of these abilities in first-episode patients, while Elliott et al.40 found that patients with moderately severe schizophrenia, like frontal lesion patients, showed a specific deficit in set shifting ability due to a tendency to perseverate. Our patient group with a mean illness duration of 2 years might be more similar to first-episode patients in terms of their set shifting abilities.
Third, any study on the relative impairments in cognitive functions might be biased because of a difference in the level of difficulty on tests that are assumed to measure different functions.41–42 It must be noted that this study is not exempt from such a possible problem. It is our belief, however, that controlling for attention and the general level of intelligence has partly compensated for a possible bias.
Group differences on verbal declarative memory and general psychomotor speed remained significant with large ES estimates, even after controlling for the general level of intelligence. In fact, the differences on the two tasks that reflect verbal declarative memory (recall on the WMS Logical Memory and recognition on the RAVLT) have the largest magnitudes of difference (Table 2). Performances on these two tasks were most impaired within the patient group compared to the mean of the remaining tests (Table 3). In their recent comprehensive review, Cirillo and Seidman43 have concluded that, along with attention and executive functions, verbal declarative memory is among the most impaired neurocognitive domains in schizophrenia. Our results are in line with these findings. However, as pointed out by Chapman and Chapman41 and Russell,42 the possibility that the tests used to measure verbal declarative memory might be more sensitive to any brain impairment cannot be ruled out. Therefore, the specificity of the tests for schizophrenia need to be studied before drawing conclusions about the presence of a differential deficit in verbal declarative memory in schizophrenia.
Significant psychomotor slowing in the patient group might be a consequence of the antipsychotic medications as well as a feature of the illness. Similarly, anticholinergic medications or the anticholinergic properties of the antipsychotics would be expected to have a differential effect on the tests that measure memory. However, since the number of patients on an anticholinergic medication was small and the mean daily dose was not high, it is unlikely that the anticholinergics had a major effect on the overall measures of memory.
Possible differences between the socioeconomic classes of the two groups cannot be ruled out. However, similar levels of parental education in the two groups suggest that the groups were similar in terms of their socioeconomic classes. The similarity between the two groups on the WAIS Vocabulary and Information subtests is also assumed to reflect a similar premorbid level of intelligence between the groups.44
Overall, these results point to preserved set shifting and response inhibition abilities and a relative sparing of concept formation and mental flexibility in schizophrenia patients in the first 5 years of the illness. Study of individual executive functions separately by tests that are matched for the level of difficulty might support this finding and show that schizophrenia patients might be heterogeneous in terms of their cognitive abilities termed as executive functions.
ACKNOWLEDGMENTS
This study was supported by the Psychiatric Association of Turkey.
 |
 |
 |
1 Goldberg E, Seidman L: Higher cortical functions in normals and schizophrenia: a selective review, in Neuropsychology, Psychophysiology, and Information Processing: Handbook of Schizophrenia, Edited by Steinhauer SR, Gruzelier JH. Amsterdam, Elsevier Publishers, 1991, pp 553-597Google Scholar
2 Rund BR, Borg NE: Cognitive deficits and cognitive training in schizophrenic patients: a review. Acta Psychiatr Scand 1999; 100: 85-95Google Scholar
3 Mohamed S, Paulsen JS, O’Leary D, Arndt S: Generalized cognitive deficits in schizophrenia. Arch Gen Psychiatry 1999; 56:749–754Crossref, Medline, Google Scholar
4 Fey ET: The performance of young schizophrenics and young normals on the Wisconsin Card Sorting Test. J Consult Psychol 1951; 15:311–319Crossref, Medline, Google Scholar
5 Goldberg TE, Weinberger DR, Berman KF: Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry 1987; 44:1008–1014Crossref, Medline, Google Scholar
6 Weinberger DR, Berman KF, Illowsky BP: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, III: a new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry 1988; 45:609–615Crossref, Medline, Google Scholar
7 Morice R: Cognitive inflexibility and prefrontal dysfunction in schizophrenia and mania. Br J Psychiatry 1990; 157:50–54Crossref, Medline, Google Scholar
8 Harvey PD, Pedley M: Auditory and visual distractibility in schizophrenics: Clinical and medication status correlations. Schizophr Res 1989; 2:295–300Crossref, Medline, Google Scholar
9 Cornblatt B, Obuchowski M, Roberts S: Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol 1999; 11:487–508Crossref, Medline, Google Scholar
10 Saykin AJ, Gur RC, Gur RE, et al: Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
11 Gold JM, Randolph C, Carpenter CJ: Forms of memory failure in schizophrenia. J Abn Psychol 1992; 101:487–494Crossref, Medline, Google Scholar
12 Nelson HE, Pantelis C, Carruthers K: Cognitive functioning and symptomatology in chronic schizophrenia. Psychol Med 1990; 20:357–365Crossref, Medline, Google Scholar
13 Aylword E, Walker E, Bettes B: Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
14 Allen DN, Huegel SG, Seaton BE: Confirmatory factor analysis of the WAIS-R in patients with schizophrenia. Schizophr Res 1998; 34:87–94Crossref, Medline, Google Scholar
15 Hill SK, Ragland JD, Gur RC: Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of subtypes. J Clin Exp Neuropsychol 2002; 24:765–780Crossref, Medline, Google Scholar
16 Hoff AL, Kremen WS: Neuropsychology in schizophrenia: An update. Curr Opin Psychiatry 2003; 16:149–155Crossref, Google Scholar
17 Dieci M, Vita A, Silenzi C, et al: Non-selective impairment of Wisconsin Card Sorting Test performance in patients with schizophrenia. Schizophr Res 1997; 25:33–42Crossref, Medline, Google Scholar
18 Seltzer J, Conrad C, Cassens G: Neuropsychological profiles in schizophrenia: paranoid versus undifferentiated distinctions. Schizophr Res 1997; 25:131–138Crossref, Medline, Google Scholar
19 Pantelis C, Yücel M, Wood SJ: Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. Austr New Zeal J Psychiatry 2003; 37:399–406Crossref, Medline, Google Scholar
20 American Psychiatric Association: Diagnostic and Statistical Manual for Mental Disorders, 4th ed. Washington, D.C., American Psychiatric Press, 1994Google Scholar
21 First MB, Spitzer RL, Gibbom M: Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C., American Psychiatric Press, Inc., 1997Google Scholar
22 Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, Iowa, University of Iowa, 1984Google Scholar
23 Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, Iowa, University of Iowa, 1984Google Scholar
24 Benton AL: The Revised Visual Retention Test: Clinical and Experimental Applications, 4th ed. New York, The Psychological Corporation, 1974Google Scholar
25 Weintraub S, Mesulam M: Mental state assessment of young and elderly adults in behavioral neurology, in Principles of Behavioral Neurology, Edited by Mesulam M. Philadelphia, FA Davis Company, 1985Google Scholar
26 Benton AL, Hamsher K, Varney NR: Contributions to Neuropsychological Assessment. New York, Oxford University Press, 1983Google Scholar
27 Rey A: L’examen clinique en psychologie [The clinical exam in psychology]. Paris, Presses Universitaires de France, 1964Google Scholar
28 Golden CS: Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, Stoelting Co, 1978Google Scholar
29 Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery. Tucson, Neuropsychology Press, 1985Google Scholar
30 Wechsler D: The Measurement and Appraisal of Adult Intelligence, 4th ed. Baltimore, Williams & Wilkins, 1958Google Scholar
31 Wechsler D: A standardized memory scale for clinical use. J Psychol 1945; 19:87–95Crossref, Google Scholar
32 Heaton RK: Wisconsin Card Sorting Test Manual. Odesa, Psychological Assessment Resources, 1981Google Scholar
33 Baldessarini RJ, Frankenburg FR: Clozapine: a novel antipsychotic agent. N Engl J Med 1991; 324:746–754Crossref, Medline, Google Scholar
34 Meltzer HY: The role of serotonin in schizophrenia and the place of serotonin-dopamine antagonist antipsychotics. J Clin Psychopharm 1995; 15(suppl 1):2-3Google Scholar
35 Corrigan JD, Hindelkey NS: Relationship between parts A and B of the Trailmaking Test. J Clin Psychol 1987; 43:402–408Crossref, Medline, Google Scholar
36 Vadhan NP, Serper MR, Harvey PD: Convergent validity and neuropsychological correlates of the Schedule for the Assessment of Negative Symptoms Attention Subscale. J Nerv Ment Dis 2001; 189:637–641Crossref, Medline, Google Scholar
37 Hutton SB, Puri BK, Duncan LJ: Executive function in first-episode schizophrenia. Psychol Med 1998; 28:463–473Crossref, Medline, Google Scholar
38 Goldstein G, Beers SR, Shemansky WJ: Neuropsychological differences between schizophrenic patients with heterogenous Wisconsin Card Sorting Test performance. Schizophr Res 1996; 21:13–18Crossref, Medline, Google Scholar
39 Loring DW (ed): INS Dictionary of Neuropsychology. New York, Oxford University Press, 1999 p 64Google Scholar
40 Elliott R, McCenna PJ, Robbins TW, Sahakian BJ: Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med 1995; 25:619–630Crossref, Medline, Google Scholar
41 Chapman LJ, Chapman JP: The measurement of differential deficit. J Psychiatr Res 1978; 14:303–311Crossref, Medline, Google Scholar
42 Russell EW: A reference scale for constructing neuropsychological test batteries. J Clin Exp Neuropsychol 1987; 9:376–392Crossref, Medline, Google Scholar
43 Cirillo MA, Seidman LJ: Verbal declarative memory dysfunction in schizophrenia: From clinical assessment to genetics and brain mechanisms. Neuropsychol Rev 2003; 13:43–77Crossref, Medline, Google Scholar
44 DeQuardo JR, Goldman RS, Tandon R: Comparison of indices of premorbid function in schizophrenia. Schizophr Res 1995; 15:283–290Crossref, Medline, Google Scholar



