Postmortem Locus Coeruleus Neuron Count in Three American Veterans With Probable or Possible War-Related PTSD
Abstract
The authors investigated whether war-related posttraumatic stress disorder (WR-PTSD) is associated with a postmortem change in neuronal counts in the locus coeruleus (LC) since enhanced central nervous system (CNS) noradrenergic postsynaptic responsiveness has been previously shown to contribute to PTSD pathophysiology. Using postmortem neuromorphometry, the number of neurons in the right LC in seven deceased elderly male veterans was counted. Three veterans were classified as cases of probable or possible WR-PTSD. All three veterans with probable or possible WR-PTSD were found to have substantially lower LC neuronal counts compared to four comparison subjects (three nonpsychiatric veterans and one veteran with alcohol dependence and delirium tremens).To the authors’ knowledge, this case series is the first report of LC neuronal counts in patients with PTSD or any other DSM-IV-TR anxiety disorder. Previous postmortem brain tissue studies of Alzheimer’s Disease (AD) demonstrated an upregulation of NE biosynthetic capacity in surviving LC neurons. The finding reported is consistent with the similar upregulation of NE biosynthetic capacity of surviving LC neurons in veterans who developed WR-PTSD. Especially if replicated, this finding in WR-PTSD may provide further explanation of the dramatic effectiveness of propranolol and prazosin for the secondary prevention and treatment of PTSD, respectively. The LC neurons examined in this study are probably the origin of the first or second “leg” of what might be termed the PTSD candidate circuit. Larger neuromorphometric studies of the LC in veterans with WR-PTSD and in other development-stress-induced and fear-circuitry disorders are warranted, especially using VA registries.
For decades, postmortem human brain tissue studies have made slow but steady contributions to the psychiatric knowledge base. While early work focused on neurochemistry,1 recent postmortem techniques allow for neuron-counting studies of some of the smaller brain nuclei, such as those found in the brainstem reticular activating system (RAS).2–4 The locus coeruleus (LC), the noradrenergic arm of the RAS, comprises approximately 23,000 neurons on each side of the brainstem5 and provides over 70% of the noradrenergic innervation in the central nervous system (CNS) and probably a higher percent of the ascending noradrenergic innervation.6
There are no published morphometric studies of the LC in war-related posttraumatic stress disorder (WR-PTSD). We wanted to investigate whether chronic WR-PTSD is associated with lower LC neuronal counts. In this preliminary study, we compared the LC neuronal counts of three veterans whose clinical histories were strongly suggestive of a probable or possible WR-PTSD with four veterans of similar age whose clinical histories lacked any signs of WR-PTSD.
METHODS
Institutional Review Board Approval
All procedures were approved by the Institutional Review Board of the Central Arkansas Veterans Health Care System. Postmortem brain tissue was obtained during autopsy from psychiatric patients and medical comparison subjects who expired at the Central Arkansas Veterans Health Care System. Consent for autopsy with special provision for use in neuropsychiatric research was obtained postmortem from next of kin for all subjects.
Patient Selection
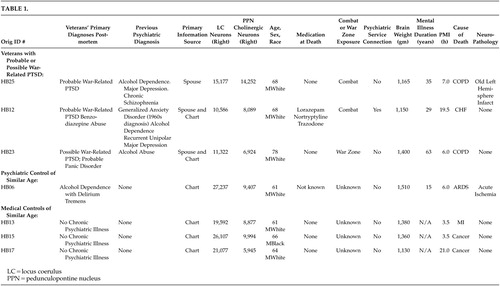
All seven patients presented in this paper were identified post hoc. The seven veterans discussed in this study were a part of nine psychiatric and medical comparison subjects included in a previous National Institute of Mental Health (NIMH) funded study of pedunculopontine nucleus (PPN) neuropathology in veterans with chronic schizophrenia.4,7 The data on all patients included in the present report were previously published in the above study. The patients were part of the comparison group in that study since none had any current psychosis, and they had PPN neuronal counts in the normal range for their age.4 In Table 1, we have retained the identification case numbers from the original (postmortem) study for comparison purposes.
Post hoc, we selected three veterans with a clinical history that was indicative of a diagnosis of a chronic probable or possible WR-PTSD. All three of these veterans had past histories of alcohol abuse or dependence. Therefore, we selected one psychiatric comparison veteran of similar age with a clinical history of alcohol dependence with delirium tremens but with no evident history of posttraumatic stress disorder (PTSD), panic disorder, or GAD. We also selected three medical comparison veterans of similar age with no evident history of alcohol abuse, anxiety disorder, or any other chronic mental illness in their medical charts.
In an attempt to partly control for the impact of nonspecific neurodegenerative conditions on LC counts, we compared these with the number of PPN neurons in these same patients (see Table 1). Projections from the PPN modulate thalamic and locomotive functions. The PPN is comprised largely of cholinergic neurons and is the key component of the cholinergic arm of the RAS. Although physically contiguous with the LC, the PPN is not associated with noradrenergic systems. Therefore, general neurodegenerative conditions would be likely to affect the LC and the PPN equally. In contrast, conditions differentially affecting noradrenergic systems, such as chronic noradrenergic over-activation, should result in changes to the LC but not the PPN.
Caseness was determined postmortem by expert consensus based primarily on information obtained by a psychiatrist following the guidelines of the Diagnostic Evaluation After Death (DEAD),8 the only structured postmortem interview available at the time. The DEAD uses all available information, including chart review and information obtained from kin. When sufficient information is obtained, the DEAD may be a valid and reliable instrument for the two purposes it was used in this study: 1) to rule out the misdiagnosis of schizophrenia given many years earlier to patient HB25; and 2) to assess the severity of alcohol abuse by the veterans.2 The diagnosis of alcohol abuse and dependence relied on the criteria of Feighner et al.9
It is of note that none of these veterans carried a clinical diagnosis of Parkinson’s disease or Alzheimer’s disease (AD) in their charts, nor were these diseases evident at postmortem examination. There was also no clinical evidence of other conditions known to affect the LC, such as multiple system atrophy, progressive supranuclear palsy, or brain stem infarcts in any of these veterans.
Clinical Histories
Veteran HB25 (probable WR-PTSD) was a combat WW-II, Pacific Theater veteran who was later stationed in Hiroshima and served during the Korean War and was 68 years old at the time of death. Chart information suggested at least one episode of severe depression treated 24 years prior to his death. Collateral information from the veteran’s wife strongly suggested that the veteran had a history of exaggerated startle response, combat–related nightmares, avoidance of war reminders, avoidance of crowds, restricted range of affect, difficulty concentrating, chronic dysphoria, suicidal thoughts, little to no interest in any social activities, and chronic irritability resulting in difficulties on the job and arrests for fighting. Episodes of rage/violence were also frequently directed towards his family. He had a past history of alcohol dependence; however, it was in remission for 15 years at the time of death. There was no history of panic attacks. Two years before his death, he was admitted to a VA nursing home following a left hemispheric cerebrovascular accident suffered during lung resection due to adenocarcinoma of the lung. No metastases to the brain were noted at autopsy. The presence of a large, old infarct involving most of the left cerebrum in the territory of the middle cerebral artery was confirmed by postmortem examination. There were also scattered old microscopic infarcts in the right cerebrum. He was taking no medication at the time of his death.
Veteran HB12 (benzodiazepine abuse and probable WR-PTSD) was 68 years of age at the time of his death. He was a combat veteran and served for four years in WWII. This veteran had a diagnosis of benzodiazepine abuse at the time of his death and a history of alcohol dependence that had been in remission for 20 years. He had been hospitalized on several occasions for recurrent unipolar major depression and, as noted in his chart, for “possible panic attacks.” This veteran had a chart diagnosis of Generalized Anxiety Disorder (GAD) dating to the 1960’s, which was identified as combat-related on his VA medical record. His anxiety disorder diagnosis was made more than a decade prior to the recognition and DSM formulation of PTSD. It is likely this four-year WWII combat veteran’s combat-related anxiety and other symptoms are misdiagnosed manifestations of WR-PTSD.
Veteran HB23 (probable panic disorder and possible WR-PTSD) was 78 years old at the time of his death. History from this veteran’s wife indicated the frequent occurrence of typical spontaneous panic attacks in this veteran (which started in his teenage years preceding his military service). The panic attacks occurred at a frequency of roughly two per week or more and manifested by flushing, profuse sweating, shaking, dyspnea, sudden weakness, and feeling faint. There was a positive history of panic attacks in the veteran’s family. The veteran had a history of alcohol abuse that had been in remission for at least five years at the time of his death. He served as a cook in WWII; and while there was no chart documentation that he experienced severe trauma during the war, he manifested extreme avoidance of war reminders and intense distress when faced with war reminders. In addition, the veteran frequently exhibited insomnia and other symptoms of anxiety. Since this veteran met core PTSD symptoms from each of the three DSM-IV-TR symptom clusters, his postmortem consensus diagnoses were probable panic disorder and possible WR-PTSD.
Alcohol dependent control of similar age: Veteran HB06 (psychiatric comparison) was selected since he had evidence of alcohol dependence with delirium tremens at the time of his death. He had no history of PTSD, panic disorder, or other anxiety disorders. No combat exposure was noted in his chart. Autopsy revealed acute cerebral ischemia.
Medical comparison subjects of similar age: Veterans HB13, HB15, and HB17 (nonpsychiatric medical comparison subjects) were selected because they had no chart evidence of an anxiety disorder or alcohol dependence. There was no chart evidence of combat exposure. Brain autopsy was within normal limits in these three veterans.
The total number of comparison subjects in the original schizophrenia study was nine. Of those, seven are presented in Table 1. The remaining two veterans (HB03, HB22) were not included in this comparison since they were substantially younger (ages 37 and 46, respectively), see Tables 1–3 of Garcia Rill et al. for all available information on these two younger veterans.4
Laboratory Methods: At autopsy, the brain stem was separated from the remainder of the brain and bisected at the midline. Only the right half of the brain stem was used in the neuronal count studies described in this study. Immediately after dissection, the right half of the brain stem was placed in a solution of 4% paraformaldehyde with lmM MgCl2 for 4–6 hours, and then it was transferred to 20% sucrose with lmM MgCl2 for 3–4 days. Sagittal frozen sections (60 micrometers) were made to minimize cell-counting errors due to the anteroposterior orientation of the LC. LC neurons were defined as neuromelanin–pigment containing masses enclosed by a cell membrane. Many of the neurons contained a visible nucleus, but neurons without a visible nucleus also were counted. Cholinergic pedunculopontine nucleus (PPN) neurons were identified by processing sections for NADPH–diaphorase histochemistry as previously described.10 This method selectively labels cholinergic mesopontine neurons. Cell counts were performed using a Biographics Image Analysis system. The outlines of the sagittal sections were digitized (at 40×) and the locations of each cell entered (at 100×) and coded. Total numbers of neurons were extrapolated by multiplying the average cell number found in the digitized sections by the total number of sections in which that brain stem nucleus was present, including discarded sections. Correction for split–cell error11 was used. The individuals making the cell counts were unaware of diagnosis. In all cases at least two individuals carried out neuronal counts in each brain stem, and no individual entered all of the brain stems in a diagnostic group. Inter-observer reliability differed by less than five percent.2,4
RESULTS
The results are presented in Table 1, which summarizes the available relevant data for all seven deceased veterans.
DISCUSSION
Three veterans with probable or possible WR-PTSD were found to have substantially lower LC neuronal counts compared to four comparison subjects (three nonpsychiatric veterans and one veteran with alcohol dependence and delirium tremens). Krystal and Duman have recently pointed out that postmortem studies are sorely needed in PTSD research.12 To our knowledge, this is the first report of LC neuronal counts in veterans with a probable or possible WR-PTSD or with any other DSM-IV-TR anxiety disorder.
It is increasingly recognized that many “noncombat” troops (such as cooks) had been exposed to life-threatening and terrifying combat trauma in the form of artillery attacks, sniper fire, mines, and the carnage of war and, therefore, developed WR-PTSD. It should be emphasized that the veterans discussed in this study (who all died prior to 1990) were diagnosed in an era in which VA clinicians were unfortunately far less sensitive to the multiplicity of war-trauma and the high prevalence of WR-PTSD in noncombat troops. VA Clinicians, therefore, were likely to make a diagnosis other than PTSD, especially of paranoid schizophrenia, in veterans who had a noncombat military occupation specialty (MOS).
Although no valid inferential statistics are possible in such a small case series, the LC neuronal count in all three veterans with probable or possible WR-PTSD was substantially lower than in the comparison veterans. Larger postmortem neuromorphometric studies of the LC in WR-PTSD and other postdeployment, stress-induced and fear-circuitry disorders are warranted.
Confounding factors frequently plague postmortem studies, especially in psychiatric populations. Replication in a larger sample using this or other cell-counting methods would strengthen the preliminary finding reported in this study. Age and the presence of neurodegenerative illnesses are known to decrease the number of neurons in the LC.14–17 Limitations that could be addressed include a better match for cause of death, medication, postmortem interval, and medical and psychiatric co-morbidities.
However, other studies of the LC in normal comparison subjects5 have shown counts similar to the LC counts of the comparison veterans in this study. Furthermore, the number of cells in the PPN (the cholinergic nucleus adjacent to the LC) were not consistently lower in the three WR-PTSD veterans.2,4 This suggests that there may not have been a general or regional degenerative condition that could account for the lower LC neuronal counts.
Additionally, the fact that the comparison veteran with alcohol dependence and delirium tremens (HB06) had similar LC neuronal counts to the nonpsychiatric (comparison) veterans suggests that alcohol dependence may not account for the lower LC neuronal count. Furthermore, a study by Halliday et al. found no differences in LC neuronal counts between alcoholics with Wernicke-Korsakoff syndrome and comparison subjects.13
Affective disorder is unlikely to be a confounding factor in our study since a recent study by Hoogendijk et al. reported no association between depression and loss of neurons in the LC in Alzheimer’s disease patients.3 Furthermore, Hoogendijk et al. point out the deficits in earlier studies of depression that have suggested such an association.3 Finally, LC neuronal counts in veterans with chronic psychotic illness have been found to not differ from healthy comparison subjects.2,4 These previous LC neuronal counts from veterans with chronic psychotic illness were higher than those in the three veterans with probable or possible WR-PTSD presented in this study.
In this study, only the right LC was available for neuromorphometry. However, recent bilateral studies of the LC in suicide victims and healthy comparison subjects5 have found LC neuronal counts in healthy comparison subjects that are very similar to those in this study (roughly double our unilateral counts), while those of suicide victims are also similar to the counts in our veterans with probable or possible WR-PTSD. Furthermore, the right LC may be preferred for studies of PTSD.18
Enhanced CNS noradrenergic postsynaptic responsiveness has been clearly shown to contribute to PTSD pathophysiology.19 On the surface, the observed decreased LC neuronal number reported in this study may seem paradoxical, given that CSF norepinephrine (NE) concentrations (a direct index of CNS noradrenergic activity20) and urine MHPG (an indirect index)21 are both elevated in WR-PTSD during life. Theoretically, it is possible that in our three WR-PTSD veterans, genetic or other pre-deployment factors are responsible for the smaller number of LC neurons. This may result in upregulation of post-synaptic NE receptors, even prior to warzone exposure resulting in low stress resilience, and may be a risk factor for PTSD (as is the case with smaller hippocampal size in combat-related PTSD).22
However, the most intriguing, and clinically most important possible explanation for our finding, draws on recent studies of antemortem CSF NE and postmortem LC neurochemistry in AD. Despite LC neuronal loss of a magnitude similar to that reported in this study in WR-PTSD, antemortem NE concentrations are normal or elevated in the CSF in AD.23
Postmortem brain tissue studies of AD24 have demonstrated an upregulation of the NE biosynthetic capacity in the surviving LC neurons. The finding reported here is consistent with a similar upregulation of NE biosynthetic capacity in surviving LC neurons following war-trauma-induced loss of neurons in the LC of veterans who develop WR-PTSD. If replicated, the neuromorphometric finding reported in this study may provide further explanation of the dramatic effectiveness of two generic postsynaptic NE receptor antagonists in PTSD: prazosin27 for the treatment of one of the key symptoms of WR-PTSD and propranolol25,26 for the secondary prevention (or as Friedman has cogently noted, a potential “morning after pill”).28for PTSD.
Finally, as Andreasen recently noted,29 DSM-IV has misconceptualized PTSD as a disorder induced by many forms of “abuse” and commonplace vicarious “traumas,” rather than as a disorder induced by direct threat to one’s own life30–32 (often in conjunction with severe physical injury).33 The latter is how PTSD is increasingly conceptualized by researchers in Europe and the rest of the world. One of the many unfortunate consequences of this DSM-IV misconceptualization is that PTSD is among the last brain disorders for which large postmortem tissue banks are being established.
Since the publication of an earlier version of this study in abstract form,34 there has been significant interest in establishing a large national tissue bank, specifically focused on WR-PTSD and on acute deployment-stress-induced disorders.28,35–38A replication of the findings of this study should be among the first to be conducted using the emerging PTSD tissue bank for several reasons. 1) The LC efferents may be the key segment and, arguably, the first or second “leg” of what may be termed the PTSD candidate circuit. 2) The LC is, perhaps, the best-studied nucleus in neuroscience and may be the most pharmacologically relevant in PTSD.39 3) The small size of the LC is a major asset for postmortem studies. 4) Neuron-counting techniques have a long track record, especially in research in Alzheimer’s disease, and are straightforward low-risk, high-reward postmortem techniques. Cell counting techniques are particularly suitable for postmortem studies of MZ twins discordant for WR-PTSD, especially the National Academy of Sciences-National Research Council WWII Veteran Twin Registry. Additionally, unlike gene-expression techniques, neuron-counting techniques probably have a low number of unknowns. The clinical and logistical pitfalls discovered and the lessons learned during the decade-long postmortem study presented here are outside the scope of this article. However, it is imperative that these lessons be incorporated in the development of the emerging postmortem tissue banks of WR-PTSD.
ACKNOWLEDGMENTS
An earlier version of this study was presented at the 1998 joint National Annual Meeting of the American Neuropsychiatric Association and Behavioral Neurology Society.
This study is based upon work supported in part by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, VA Pacific Islands Health Care System, Spark M. Matsunaga Medical Center.
This study was also supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award, and the VA National Center for Posttraumatic Stress Disorder; the National Institutes of Health grant RO1MH-45729 and the Stanley Foundation
The authors thank Craig N. Karson, M.D. (Sanofi-Aventis Parmaceuticals) for initiating this study and supervising the postmortem clinical reassessment and Roger Amick, M.D. for conducting the clinical chart review. The authors also thank E. Fuller Torrey, M.D. for encouraging this study on WR-PTSD research and John H. Krystal, M.D.
 |
1 Bracha HS, Kleinman JE: Postmortem studies in psychiatry. Psychiatr Clin N Am 1984; 7:473–485Crossref, Medline, Google Scholar
2 Karson CN, Garcia-Rill E, Biedermann J: The brain stem reticular formation in schizophrenia. Psychiatry Res Neuroimaging 1991; 40:31–48Crossref, Medline, Google Scholar
3 Hoogendijk WJ, Sommer IE, Pool CW, et al: Lack of association between depression and loss of neurons in the locus coeruleus in Alzheimer disease. Arch Gen Psychiatry 1999; 56:45–51Crossref, Medline, Google Scholar
4 Garcia-Rill E, Biedermann J, Chambers T, et al: Mesopontine neurons in schizophrenia. Neurosci 1995; 66:321–335Crossref, Medline, Google Scholar
5 Arango V, Underwood MD, Mann JJ: Fewer pigmented locus coeruleus neurons in suicide victims: preliminary results. Biol Psychiatry 1996; 39:112–120Crossref, Medline, Google Scholar
6 Moore R, Bloom F: Central catecholamine neuron epinephrine systems. Annu Rev Neurosci 1979; 2:113–168Crossref, Medline, Google Scholar
7 Garcia-Rill E: The pedunculopontine nucleus. Prog Neurobiol 1991; 36:363–389Crossref, Medline, Google Scholar
8 Salzman S, Endicott J, Clayton P, et al: Diagostic Evaluation After Death (DEAD) 1983Google Scholar
9 Feighner JP, Robins E, Guze SB, et al: Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 1972; 26:57–63Crossref, Medline, Google Scholar
10 Garcia-Rill E, Biedermann J, Chambers T, et al: Mesopontine neurons in schizophrenia. Neurosci 1995; 66:321–335Crossref, Medline, Google Scholar
11 Konigsmark B: Methods for counting neurons in Contemporary Methods in Neuroimaging New york, Springer-Verlag 1970Google Scholar
12 Krystal JH, Duman R: what’s missing in PTSD? Post-mortem studies. Psychiatry (in press)Google Scholar
13 Halliday G, Ellis J, Harper C: The locus coeruleus and memory: a study of chronic alcoholics with and without the memory impairment of Korsakoff’s psychosis. Brain Res. 1992; 598:33-37Google Scholar
14 Vijayshankar N, Brody H: A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. J Neuropathol Exp Neurol 1979; 38:490–497Crossref, Medline, Google Scholar
15 Chan-Palay V, Asan E: Alterations in catecholaminergic neurons of the locus coeruleus in severe dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol 1989; 287:373–392Crossref, Medline, Google Scholar
16 Mann D, Yates P: Pathological basis for neurotransmitter changes in Parkinson’s disease. Neuropathol Appl Neurobiol 1983; 9:3–19Crossref, Medline, Google Scholar
17 Tomlinson B, Irving D, Blessed G: Cell loss in the locus coeruleus in senile dementia of the Alzheimer type. J Neurol Sci. 1981; 49:419-428Google Scholar
18 Porges SW: Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–233Crossref, Medline, Google Scholar
19 Southwick SM, Krystal JH, Morgan CA, et al: Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry 1993; 50:266–274Crossref, Medline, Google Scholar
20 Geracioti TD, Baker DG, Ekhator NN, et al: CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 2001; 158:1227–1230Crossref, Medline, Google Scholar
21 Mellman TA, Kumar A, Kulick-Bell R, et al: Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry 1995; 38:174–179Crossref, Medline, Google Scholar
22 Gilbertson MW, Shenton ME, Ciszewski A, et al: Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002; 5:1242–1247Crossref, Medline, Google Scholar
23 Elrod R, Peskind ER, DiGiacomo L, et al: Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry 1997; 154:25–30Crossref, Medline, Google Scholar
24 Szot P, Leverenz JB, Peskind ER, et al: Tyrosine hydroxylase and norepinephrine transporter mRNA expression in the locus coeruleus in Alzheimer’s disease. Brain Res Mol Brain Res 2000; 84:135–140Crossref, Medline, Google Scholar
25 Pitman RK, Sanders KM, Zusman RM, et al: Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002; 51:189–192Crossref, Medline, Google Scholar
26 Vaiva G, Ducrocq F, Jezequel K, et al: Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry 2003; 54:947–949Crossref, Medline, Google Scholar
27 Raskind MA, Peskind ER, Kanter ED, et al: Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003; 160:371–373Crossref, Medline, Google Scholar
28 Friedman MJ, Harris WW: Toward a national PTSD brain bank. Psychiatr 2004; 67:384–390Crossref, Medline, Google Scholar
29 Andreasen NC: Acute and Delayed Posttraumatic Stress Disorders: A History and Some Issues. Am J Psychiatry 2004; 161: 1321-1323Google Scholar
30 Vaiva G, Brunet A, Lebigot F, et al: Fright (effroi) and other peritraumatic responses after a serious motor vehicle accident: prospective influence on acute PTSD development. Can J Psychiatry 2003; 48:395–401Crossref, Medline, Google Scholar
31 Vaiva G, Ducrocq F, Cottencin O, et al: Immediate fright reaction: an essential criterion in the development of posttraumatic stress disorder (PTSD). Can J Psychiatry 2000; 45:939Medline, Google Scholar
32 Bracha HS, Jehel L, Vaiva G, et al: STRS-A3: Check-list de symptomes de la reaction de peur initiale; 2005 (unpublished research)Google Scholar
33 Jehel L, Paterniti S, Brunet A, et al: Prediction of the occurrence and intensity of post-traumatic stress disorder in victims 32 months after bomb attack. Eur Psychiatry 2003; 18:172–176Crossref, Medline, Google Scholar
34 Bracha HS, Freeman TW, Garcia-Rill E, et al: Postmortem locus coeruleus neuron counting in anxiety disorders. J Neuropsychiatry Clin Neurosci 1997; 9(4), 644 (abstract)Google Scholar
35 Osuch E, Ursano R, Li H, et al: Brain environment interactions: stress, posttraumatic stress disorder, and the need for a postmortem brain collection. Psychiatr 2004; 67:353–383Crossref, Medline, Google Scholar
36 Krystal JH, Duman R: What's missing in posttraumatic stress disorder research? Studies of human postmortem tissue. Psychiatr 2004; 67:398–403Crossref, Medline, Google Scholar
37 Deep-Soboslay A, Martin CE, Kleinman JE: The development of a posttraumatic stress disorder brain collection. Psychiatr 2004; 67:416–418Crossref, Medline, Google Scholar
38 Raskind MA: The challenge of a PTSD brain bank. Psychiatr 2004; 67:404–406Crossref, Medline, Google Scholar
39 Pitman RK, Delahanty DL: Conceptually driven pharmacologic approaches to acute trauma. CNS Spectr 2005; 10:99–106Crossref, Medline, Google Scholar



