Pituitary Volume in Medication-Naïve Adults With Obsessive Compulsive Disorder
Abstract
Pituitary volume is considered to reflect hypothalamic-pituitary-adrenal axis dysregulation, and this has been studied in various psychiatric disorders. This study demonstrates that pituitary volume as assessed through the region of interest manual tracing method in 50 medication-naïve adult patients with obsessive-compulsive disorder was not significantly different compared with 40 healthy control subjects (687.80±126.60 versus 694.73±131.59, F=0.55, p=0.46). The authors also compared the patients with obsessive-compulsive disorder without any comorbid axis I conditions (N=35) with healthy control subjects and found no difference in the pituitary volumes (681.62±130.85 versus 694.72±131.59, F=0.90, p=0.35). This emphasizes the need to examine hypothalamo-pituitary axis structures after taking into consideration various potential confounders such as medications and depression.
Hypothalamic-pituitary-adrenal axis (HPA) dysregulation has been described as a pathophysiological mechanism in various psychiatric disorders including depression, posttraumatic stress disorder, eating disorders, psychosis, and obsessive-compulsive disorder (OCD).1 OCD is characterized by recurrent intrusive thoughts or images that cause a significant amount of distress and anxiety. Obsessions increase during stressful events, and hence OCD is considered to be stress responsive.2 In that context, HPA axis abnormalities have been proposed to be important pathophysiological mechanisms leading to the impaired physiological stress response regulation in OCD.3 There have been some recent studies examining the volume of pituitary gland, a key component of the HPA axis, in OCD, and they demonstrate smaller pituitary volumes in comparison with healthy control subjects.4–6 The HPA axis and pituitary volume may be affected by many factors including concurrent use of antidepressants and comorbid depression. Thus, there is a need to ascertain the specificity of the pituitary volume changes to the pathophysiology of OCD without the potential confounding effect of treatment. To evaluate this, we examined the pituitary volume in a large sample of medication-naïve adult subjects in comparison with health control subjects using structural MRI. We also examined this aim separately in a subset of comorbidity-free patients with OCD compared with healthy control subjects.
Methods
The study subjects consisted of 50 medication-naïve patients with OCD and 40 matched health control subjects. The patients were recruited from the specialty OCD clinic at the National Institute of Mental Health And Neurosciences, Bangalore, India. The patients recruited never were exposed to psychotropic medications. Written informed consent was obtained from all subjects before assessment. The Institute Ethics Committee at the National Institute of Mental Health And Neurosciences reviewed and approved the research project from which the results of the present study are derived. The diagnosis of OCD was made according to the DSM-IV criteria.7 The Mini International Neuropsychiatric Interview was used to ascertain the diagnosis and to evaluate the presence of comorbid psychiatric conditions.8 The subjects were also evaluated with the Yale-Brown Obsessive Compulsive Scale (YBOCS) that includes a symptom checklist and severity rating scale.9 The Montgomery-Åsberg Depression Rating Scale (MADRS)10 was used to measure depressive symptoms. The clinical assessments were conducted by trained mental health professionals in the specialty OCD clinic, and the diagnosis was further confirmed by a consultant of the clinic (Y.C.J.R./G.V.S.). However, formal interrater reliability exercises were not conducted for the clinical data collection. Healthy control subjects who volunteered for participation in the study were recruited from consenting individuals from the community who were age, sex, and handedness matched. All subjects were right handed. Healthy control subjects were not evaluated using the severity rating scales (YBOCS and MADRS) because they screened negative for the psychiatric disorders on the Mini International Neuropsychiatric Interview. Subjects did not have a history of medical illness or substance dependence. There was no family history of psychiatric illness, including alcohol dependence syndrome, in their first-degree relatives.
Imaging Method
MRI scans were done with a 1.5-T scanner (Magnetom Vision; Siemens, Erhlangen, Germany). A T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo sequence was performed (TR=9.7 ms, TE=4 ms, nutation angle=12°, and slice thickness is 1 mm with no interslice gap), yielding 160 sagittal slices. All measurements were automatically calculated using the 3D Slicer software11 in coded images, with the rater blind to the subject identity. The pituitary was outlined and measured by the rater (J.C.N.) using the computer mouse controlled pointer. Figure 1 shows one representative section depicting the tracing. The rater was blind to the subjects’ clinical details at the time of the brain measurements on coded MRI sections. High intrarater and interrater reliability (two raters: J.C.N. and D.J.) was established (intraclass correlation coefficient>0.80) by independent blinded measurements of 10 randomly chosen scans. The pituitary boundaries for outlining during the manual tracing were chosen from a previous study.4
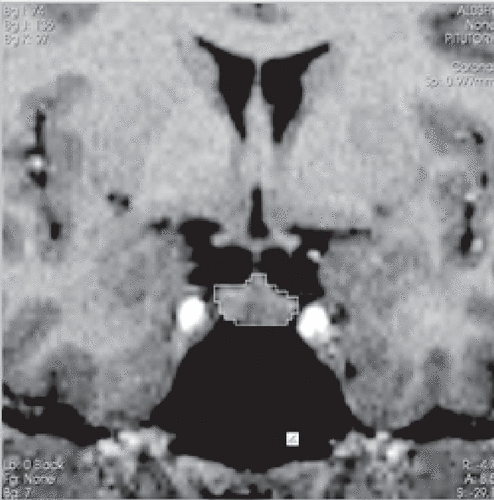
FIGURE 1. One Representative Section Depicting the Manual Tracing of the Pituitary in a Coronal Section
Statistical Analysis
The data were normatively distributed as assessed using the Shapiro-Wilk test. Independent t test and chi-square test were used for continuous and discrete variables for baseline comparisons. The difference in pituitary volume between patients with OCD and healthy control subjects were examined using analysis of covariance, with age, sex, and intracranial volume as covariates. We performed Pearson’s correlation for the OCD group to examine the relationship between pituitary volumes and illness severity scores: p≤0.05 was considered statistically significant.
Results
The demographic and clinical details are summarized in Table 1. All the patients with OCD and healthy control subjects were matched for age and sex. The mean intracranial volume (in mL) was comparable between the groups (1453.81±158.02 versus1420.70±145.11, p=0.29). The mean age of onset of OCD was 20.90±7.83 years, and the median illness duration was 60 months. There was no significant difference in the pituitary volume between the two groups (687.80±126.60 versus 694.73±131.59, F=0.55, p=0.46). In addition, there was no difference in the volume on a separate analysis of male patients and female patients compared with the healthy control subjects of the respective sex. There were no significant correlations between YBOCS total scores and pituitary volume among OCD subjects (r=0.08, p=0.59). We found no significant correlations between the pituitary volume and duration of illness (r=0.05, p=0.73), age of onset (r=0.11, p=0.17), and MADRS score (mean=12.78±9.27; r=0.07, p=0.65). Among the subjects with OCD, 13 (26%) had comorbid lifetime depression and two (4%) had comorbid social anxiety disorder. We also compared the patients with OCD without any comorbid axis I conditions (N=35) with healthy control subjects and found no difference in the pituitary volumes (681.62±130.85 versus 694.72±131.59, F=0.90, p=0.35). A separate analysis comparing OCD patients without depression and healthy control subjects (p=0.42), and OCD patients with depression and healthy control subjects (p=0.18) did not yield significant results.
| Variable | Patients (N=50) | Healthy Control Subjects (N=40) | t/χ2 | p |
|---|---|---|---|---|
| Age (years) | 26.42±6.59 | 25.45±5.47 | 0.75 | 0.46 |
| Sex (male:female) | 31:19 | 27:13 | 0.30 | 0.59 |
| Comorbid conditions | ||||
| Depression [N( %)] | 13 (26) | 0 | — | — |
| Social anxiety [N (%)] | 2 (4%) | 0 | — | — |
| Age of onset of OCD (years) | 20.90±7.83 | — | — | — |
| YBOCS | ||||
| Obsession score | 13.10±4.08 | — | — | — |
| Compulsion score | 12.74±4.89 | — | — | — |
| Total score | 25.76±8.22 | — | — | — |
| MADRS score | 12.78±9.27 | — | — | — |
TABLE 1. Clinical and Demographic Characteristics of Patients and Control Subjectsa
Discussion
We did not find a significant difference in the pituitary volume between patients with OCD and healthy control subjects. We also did not observe any correlation between pituitary volume and the OCD severity. Some previous studies reported smaller pituitary volumes in pediatric4 and adult subjects5,6 with OCD. Evidence suggesting HPA axis dysfunction has been reported among patients with OCD in the literature, with studies demonstrating elevated levels of cortisol,12 corticotrophin-releasing hormone,13 and oxytocin,14 in addition to demonstrating smaller pituitary gland volume among patients with OCD compared with healthy control subjects.4–6
Although previous studies have shown decreased pituitary volume in patients with OCD, the volume of this structure may be linked to a variety of factors. MacMaster et al.4 and Atmaca et al.6 demonstrated smaller volume of pituitary in pediatric and adult patients, respectively. However, replication of these findings is essential, considering some of the important confounders in most of these studies. First, the specificity of pituitary volume changes to diagnosis and comorbid conditions appear to be an important consideration. For instance, 26% of our subjects with OCD had comorbid lifetime depressive and anxiety disorders even though the current mean MADRS score was not high. However, the relatively smaller number of participants with OCD with comorbid depression in our study might not have been sufficiently powered to detect a difference in the region of interest analysis. In addition, comorbid personality disorders can also affect HPA axis structure and function,15 and this has not been assessed formally in this study or in previous studies on pituitary volume in OCD. Second, the mean age of onset of illness could also affect the pituitary because HPA dysregulation during peripubertal age may have greater consequences. The mean age at onset of OCD is late adolescence in our sample compared with peripubertal onset in some other studies on the topic. Third, the role of psychotropic medications on the pituitary needs to be considered. Antidepressants have a potentially significant effect on the functioning of HPA axis.16 Although Jung et al5 demonstrated a smaller pituitary volume in 12 medication-naïve individuals, this needs further examination in view of a small sample size. Our study reports on a large number of medication-naïve individuals with OCD, and this is in contrast to the previous studies. Finally, the factors apart from the main diagnoses such as life events play an important role on HPA regulation,1 and hence it is important to take these factors into consideration while examining the brain changes.
Thus, although the HPA axis may play a role in the pathophysiology of OCD, one needs to carefully consider the above factors to ascertain the illness marker role of the HPA axis structures. In a cross-sectional study of this kind, it would be possible only to detect possible associations, and the stress-responsive nature of OCD might not be related to the development of the condition; rather, it could be a consequence of it. It would be interesting and informative to study the pituitary volume in a large sample medication-naïve patients with OCD without any comorbidity compared with healthy control subjects, because HPA abnormalities are more commonly seen with depression.17 Additionally, longitudinal studies would provide evidence for the pathoplastic effect of illness course and duration on pituitary structure.
1 : The role of life events and HPA axis in anxiety disorders: a review. Curr Pharm Des 2012; 18:5663–5674Crossref, Medline, Google Scholar
2 : How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21:55–89Medline, Google Scholar
3 : Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J Psychiatr Res 2007; 41:928–933Crossref, Medline, Google Scholar
4 : Pituitary volume in pediatric obsessive-compulsive disorder. Biol Psychiatry 2006; 59:252–257Crossref, Medline, Google Scholar
5 : Volumetric differences in the pituitary between drug-naïve and medicated male patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:605–609Crossref, Medline, Google Scholar
6 : Smaller pituitary volume in adult patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci 2009; 63:516–520Crossref, Medline, Google Scholar
7 : Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
8 : The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(Suppl 20):22–33, quiz 34–57Medline, Google Scholar
9 : The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
10 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
11 : 3D Slicer, in IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 2004, pp 632–635Google Scholar
12 : Melatonin and cortisol secretion in patients with primary obsessive-compulsive disorder. Psychiatry Res 1992; 44:217–225Crossref, Medline, Google Scholar
13 : Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:9–20Crossref, Medline, Google Scholar
14 : Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder. Comparison with Tourette’s syndrome and healthy controls. Arch Gen Psychiatry 1994; 51:782–792Crossref, Medline, Google Scholar
15 : An MRI study of pituitary volume and parasuicidal behavior in teenagers with first-presentation borderline personality disorder. Psychiatry Res 2008; 162:273–277Crossref, Medline, Google Scholar
16 : The effects of antidepressants on the hypothalamic-pituitary-adrenal axis. Drug News Perspect 2006; 19:603–608Crossref, Medline, Google Scholar
17 : Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol 2012; 22:1–16Google Scholar



