Psychiatric, Motor, and Autonomic Effects of Bifrontal ECT in Depressed Parkinson’s Disease Patients
Abstract
Objective:
Depressive symptoms are a source of significant morbidity in Parkinson’s disease (PD). Electroconvulsive therapy (ECT) is a promising treatment for depression in PD (dPD); however, data remain limited, including data on optimal electrode placement. In this retrospective study, the investigators aimed to characterize the effects of bifrontal ECT for dPD on psychiatric and motor symptoms, as well as autonomic response.
Methods:
Clinical data were retrieved from a university-affiliated ECT service in Vancouver, British Columbia, for patients with dPD receiving bifrontal ECT between 2014 and 2018. Clinical Global Impression (depressive symptoms) and Unified Parkinson’s Disease Rating Scale (motor symptoms) scores and cardiovascular measurements during ECT, as well as doses of dopaminergic medications, were recorded.
Results:
Eight patients met criteria for inclusion. Six patients (75%) met response criteria for improvement of depressive symptoms, including 83% of patients who completed a full ECT course. Five patients went on to receive maintenance ECT, with only one patient relapsing by the 1-year follow-up (20%). For patients with motor scales reported, 60% showed a clinically significant improvement in motor symptoms. Among patients who completed ECT, a reduction in the median dopaminergic medication dose was also observed (−350 mg). Two patients discontinued ECT as a result of tolerability concerns. Participants demonstrated a relatively typical pattern of autonomic response to ECT, with low incidence of bradycardic events.
Conclusions:
The results provide preliminary evidence of the benefit of bifrontal ECT in dPD for both depressive and motor symptoms. The autonomic data suggest that most patients with dPD respond in a typical physiological manner to ECT stimulus; however, further investigation is needed.
Parkinson’s disease (PD) is a neurodegenerative movement disorder affecting approximately 1% of the population over age 60 (1) and is characterized by loss of dopaminergic neurons in the substantia nigra and α-synuclein cytoplasmic accumulation (2). Nonmotor symptoms (NMS), including autonomic, gastrointestinal, psychiatric, and neurocognitive, are now recognized as clinically important, with estimated prevalence in 98.6% of patients including psychiatric NMS in as many as 67% (3). Among psychiatric NMS, depression in PD (dPD) is common (4) and significantly impacts quality of life (5). Conventional treatment with pharmacotherapy is limited by modest efficacy and concerns around acceptability and polypharmacy (6, 7). Psychotherapy may be helpful, but barriers include limited access and comorbid neurocognitive decline (7).
Electroconvulsive therapy (ECT) is a noninvasive convulsive neurostimulation treatment indicated for depression, bipolar disorder, and schizophrenia (8). Main side effects include nausea, headache, and muscle aches, generally mild and self-limited; moderate severity side effects include postictal delirium/confusion and retrograde/anterograde memory loss (9).
Observational evidence suggests ECT is an effective and well-tolerated treatment for dPD as well as motor symptoms in patients with and without psychiatric comorbidity (10, 11). As summarized in a recent systematic review, the vast majority of studies report bitemporal electrode placement; however, some have used right unilateral placement (11–13). Bifrontal ECT, by comparison, has not previously been studied in dPD to our knowledge. However, evidence, including a meta-analysis, from patients in the general population with major depressive disorder suggests the potential for this placement to deliver efficacy comparable to bitemporal placement with fewer cognitive side effects (14, 15). Consequently, it deserves consideration for use in this population.
While the efficacy of bifrontal ECT in improving depressive symptoms has been established, the potential for a beneficial effect on motor symptoms in PD has not been clinically studied. Preclinical evidence suggests that the putative effect of ECT on PD motor symptoms may be linked to the release of dopamine, including increased dopamine in the corpus striatum of rats following electroconvulsive shock (16) and increased dopamine transporter uptake in the striatum in humans following ECT (17). Changes in dopamine transporter activity may allow patients with PD who are treated with ECT to require less dopaminergic medication.
In order to achieve these postulated effects, the electrode placement used would likely need to achieve striatal penetration of the electric field. Modeling work suggests that, while bitemporal electrode placement achieves this to the greatest extent among the three placements, bifrontal is also significantly superior to right unilateral in this respect; right unilateral placement has extremely limited deep penetration of the contralateral hemisphere (18). Taken together with the evidence that bifrontal placement is associated with fewer cognitive side effects than bitemporal, including delirium (9, 17), bifrontal ECT has the unique potential to optimize these two competing priorities in the treatment of depressed PD patients.
The autonomic response to ECT may also have implications for both efficacy and tolerability, but this has not been studied in patients with PD. The degree of sympathetic response may be related to efficacy, and both loss of sympathetic tone following seizure suppression and direct vagal nerve stimulation can lead to bradycardic events, affecting tolerability (19). Given the autonomic dysfunction commonly associated with PD, these patients may be vulnerable to aberrant autonomic responses to ECT that may affect efficacy or tolerability. Bifrontal electrode placement may again be advantageous given evidence for a lower risk of bradycardic and asystolic events compared with bitemporal placement (20, 21).
This retrospective study aimed to describe the clinical effects of bifrontal ECT on depressive and motor symptoms as well as autonomic response among patients with dPD. We hypothesized that bifrontal ECT would lead to clinically significant improvements in both depressive symptoms and motor symptoms of PD, and that ECT would decrease dopaminergic medication load. We also planned to investigate autonomic data gathered during ECT sessions as a hypothesis-generating exercise.
Methods
Study Design
Our study was approved by the University of British Columbia (UBC) Research Ethics Board. Patients receiving ECT at St. Paul’s Hospital in Vancouver, British Columbia, from January 1, 2014, to December 31, 2018, were screened, and included provided they had a neurologist-confirmed diagnosis of idiopathic Parkinson’s disease, had a psychiatrist-confirmed diagnosis of a depressive disorder, and received at least six ECT sessions during the study period. No specific exclusion criteria were applied. Patients were referred for consideration of ECT from neurologists at the UBC Pacific Parkinson’s Research Centre and subsequently referred for the procedure following assessment by a consulting psychiatrist. As our study was deemed minimal risk given its retrospective nature, informed consent was waived in conformance with Tri-Council ethics and institutional review board guidance. All data were anonymized and stored on a single secure, encrypted electronic device.
Our primary outcome was clinical improvement in depressive symptoms, measured by the Clinical Global Impression-Severity (CGI-S) and Improvement (CGI-I) scales (22) and administered retrospectively by the primary treating psychiatrist. Secondary outcomes included changes in motor symptoms, as measured by Part III of the Unified Parkinson’s Disease Rating Scale, Movement Disorders Society revision (MDS-UPDRS) (23), administered as part of routine clinical care by neurologists formally trained in the use of this scale; changes in doses of levodopa and other dopaminergic medications; and autonomic response to ECT, as measured by blood pressure and heart rate readings during treatment sessions.
Response of depressive symptoms to ECT was defined as both a minimum of a 1-point decrease on the CGI-S and a score of 1 or 2 (“very much improved” or “much improved”) on the CGI-I. For motor symptoms, clinically important difference (CID) criteria (24) were applied to changes in the MDS-UPDRS motor score for each patient (minimal change, 3–5 points; moderate, 6–10 points; and large, 11 or more points). For dopaminergic medications, a total levodopa equivalent dose was calculated at baseline and end of treatment for each patient, using the equivalency calculations developed by Tomlinson et al. (25) (see also Table S1 in the online supplement).
Procedure
ECT treatments were delivered at St. Paul’s Hospital in Vancouver. Written informed consent was obtained for all patients prior to the first treatment. Anticonvulsant and dopaminergic medications were held for 12 hours prior to the procedure. All patients received ECT using a Thymatron IV device. Bifrontal electrode placement was used for all patients throughout the treatment course, per site protocol using bifrontal as the standard initial electrode placement for index ECT. General anesthesia was provided with methohexital (1–1.5 mg/kg) for induction and succinylcholine (1 mg/kg) for neuromuscular blockade. Heart rate and blood pressure monitors were used during all treatments, and these data were systematically recorded.
Data Extraction
Baseline demographic and clinical data were collected. Hoehn and Yahr stage (26) was assessed based on the “on” state at baseline. Medications and doses, including levodopa equivalent dose (25), were recorded at baseline and end of treatment. ECT treatment data collected included electrode placement, electrical dose, seizure duration, and completion or noncompletion of ECT course. Clinical outcome data included baseline and posttreatment CGI-S and CGI-I scores, depressive relapse in the first year post-ECT, baseline and posttreatment MDS-UPDRS motor scores, and autonomic data (heart rate and blood pressure measurements) recorded during ECT treatment.
Analysis
Outcomes were examined as an overall group and within the subgroups of patients who completed a full course of ECT (completers) and those who discontinued ECT before undergoing a full course (noncompleters). Descriptive statistics (median, range) were calculated and reported for continuous demographic and clinical data points. Measures of autonomic response to ECT (blood pressure and heart rate) were reported using mean values and standard deviation, owing to the much larger number of data points and heterogeneity in size of dataset per patient.
Results
Eight patients (male, N=6; median age, 72 years, range, 63–78 years) with concurrent diagnoses of depression and Parkinson’s disease were included in the study. All were assessed as markedly ill to extremely ill with respect to depressive symptoms at baseline. There was a wide variation in baseline severity of PD motor symptoms and dopaminergic medication load (Table 1).
| Characteristic | Patient A | Patient B | Patient C | Patient D | Patient E | Patient F | Patient G | Patient H |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Male | Male | Male | Male |
| Age (years) | 63 | 78 | 69 | 74 | 74 | 69 | 70 | 77 |
| Psychiatric diagnosis | MDD | MDD | MDD | MDD | MDD | MDD | MDD | MDD |
| Duration of depressive illness (years) | 7 | 6 | NA | 4 | NA | 11 | 20 | 5 |
| Stage of Parkinson's disease (Hoehn and Yahr) | 2 | 4 | 2 | 3 | 2 | 3 | 2 | 2 |
| CGI-Sb (baseline) | 5 | 6 | 7 | 6 | 5 | 6 | 6 | 5 |
| CGI-S (end of treatment) | 5 | 6 | 6 | 4 | 3 | 4 | 4 | 4 |
| CGI-Ic | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 2 |
| MDS-UPDRSc motor (baseline) | 14 | 59 | 39 | 37 | 23 | 67 | NA | NA |
| MDS-UPDRS motor (end of treatment) | 19 | 56 | 27 | 27 | 30 | NA | 36 | NA |
| Time between MDS-UPDRS assessments, months | 14 | 14 | 8 | 13 | 13 | NA | NA | NA |
| Number of psychotropic medications | 3 | 2 | 0 | 2 | NA | 3 | 3 | 2 |
| Number of benzodiazepines | 1 | 1 | 0 | 0 | NA | 1 | 1 | 1 |
| LED, mg (baseline) | 2,361 | 1,450 | 1,300 | 1,300 | NA | 1,200 | 1,928.5 | 400 |
| LED, mg (end of treatment) | 2,561 | 1,928.5 | 700 | 950 | NA | 1,450 | 1,995 | 600 |
| Completed course of ECT | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| Criteria for depressive response met | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
TABLE 1. Demographic and clinical characteristics of depressed patients with Parkinson’s disease (N=8)a
Six patients (75%) completed a full ECT course and two discontinued. Mean electrical doses delivered over the course of ECT were moderate (246.8 mC [SD=115.5]), and seizure duration was generally adequate (mean 41.6 seconds [SD=18.8]). Serious adverse effects reported were laryngospasm related to anesthetic (N=1) and postictal delirium (N=1), each of which led to a discontinuation of treatment. This episode of delirium was the only such occurrence recorded in 213 total ECT treatments, for an incidence of 0.47%; it occurred in a patient who had incidentally experienced a concussion 2 weeks prior to the treatment where the delirium occurred.
Six out of eight patients demonstrated depressive symptom response to ECT treatment (75%), including five out of six ECT completers (83%). Five patients went on to receive maintenance ECT; one patient relapsed 4 months after discontinuation of maintenance treatment.
For motor symptoms, among patients with complete MDS-UPDRS motor data at baseline and end of treatment, three out of five patients met criteria for a clinically significant reduction in severity on the basis of the CID criteria following treatment with ECT (one with minimal reduction, one with moderate, and one with large), whereas two experienced an increase (one with minimal increase and one with moderate increase).
Changes in median levodopa equivalent doses from baseline to posttreatment were calculated. Among all patients overall and in the subgroup of ECT noncompleters, a small increase in median levodopa equivalent dose was observed during the study period (+150 mg), which was more pronounced among noncompleters (+272.5 mg). Among patients who completed a full course of ECT, there was a substantial decrease (−350 mg). Among patients who had complete MDS-UPDRS motor data (N=5), the two patients with moderate-to-large decreases in their score from baseline to posttreatment also had a concomitant decrease in levodopa equivalent dose. These two patients both completed a course of ECT; notably, they were also the only patients in our study sample who were not taking a benzodiazepine at the start of treatment.
Figure 1 illustrates the progression of mean heart rate and blood pressure during the titration (initial) ECT session. This demonstrates a relatively typical pattern of sympathetic surge followed by a gradual decline in heart rate and blood pressure. Bradycardia (heart rate <60) was observed in 7.5% of recordings immediately poststimulus, with no incidents of asystole reported. No anticholinergic agent rescue use occurred. Across all study data, the minimum poststimulus systolic and diastolic blood pressure and heart rate recordings were 80 mmHg and 46 mmHg and 42 bpm, respectively. Analysis of the immediate poststimulus autonomic response during each of the first 10 treatments demonstrated a gradual diminishment of peak heart rate and blood pressure over time, as patients received further treatments (see Figure S1 in the online supplement).
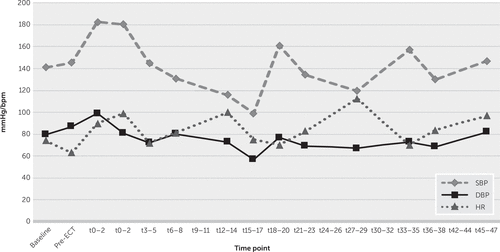
FIGURE 1. Autonomic response during the first ECT (titration) session among depressed patients with Parkinson’s diseasea
a Data points represent mean values for blood pressure and heart rate for all patients at each time point. Time points on the X axis correspond to the number of minutes following ECT stimulus delivery. Baseline denotes the measurement at arrival to ECT suite. Pre-ECT denotes the measurement following anesthetic induction prior to ECT stimulus delivery. DBP=diastolic blood pressure, HR=heart rate, SBP=systolic blood pressure.
Discussion
Significance
Our retrospective study provides the first preliminary clinical evidence, to our knowledge, in support of the feasibility and effectiveness of bifrontal ECT in treating dPD. This placement has evidence for similar efficacy with fewer cognitive side effects versus bitemporal in the general population (14). Indeed, a much lower incidence of delirium was observed in our study (one event in more than 200 treatments) than other reports (11, 27). Postponement of treatments after concussion is emphasized, because the affected patient experienced a concussion 2 weeks prior to treatment. Factors potentially explaining the low incidence of post-ECT delirium include the use of a less invasive placement than conventional, and the fact that our patients were systematically asked to hold the night and morning dose of levodopa before ECT. Our sample included patients with baseline disease from stage 2 to 4, as rated on the Hoehn and Yahr scale. The only patient with stage 4 disease discontinued ECT before completion because of adverse effects, highlighting the potential limitations of this treatment in patients with advanced motor disease.
Depressive Symptoms
We observed a 75% response rate of depressive symptoms to ECT, including 83% of patients who completed an ECT course. This rate is comparable to prior observational data on ECT for dPD and to the general adult population (11). This highlights bifrontal placement as potentially useful in dPD. Additionally, the low relapse rate among patients who responded and subsequently received maintenance ECT (20%) suggests a role for long-term treatment as a strategy for relapse prevention.
Motor Symptoms
Among patients with complete data on motor symptoms via the MDS-UPDRS motor subscale, three out of five patients demonstrated clinically significant improvement following ECT, including two with at least moderate improvement. The remaining two patients showed minimal to moderate worsening, to a degree consistent with estimates of typical motor symptom progression in the absence of treatment changes (28).
We also found a decrease in median levodopa equivalent dose among patients who completed a full course of ECT. Interestingly, of the three out of five patients who demonstrated MDS-UPDRS motor improvement, the two who completed a full course of ECT had larger score improvements and concomitant decreases in dopaminergic medication load. This suggests that ECT may lower the necessary dose of dopaminergic agents for some patients. Although medications were possibly decreased due to side effects, the apparent dose-response (levodopa/levodopa equivalent dose decrease only seen in ECT completers) supports the plausibility of a causal link. Of note, the two patients with decreases in both MDS-UPDRS motor and levodopa equivalent dose were the only patients in the sample not taking benzodiazepines, which are potential inhibitors of ECT effectiveness.
Possible mechanisms for the effect of ECT on PD motor symptoms may include enhancement of striatal dopamine release (20–22) or of the permeability of the blood-brain barrier to levodopa (29), the latter potentially related to our finding that ECT decreased dopaminergic medication requirement for some patients. This effect would be clinically useful, given the adverse effects of higher doses dopaminergic agents, including psychosis and impulse control disorders (30). The patients who demonstrated both motor improvement and decrease in levodopa equivalent dose may represent a subset of PD patients with particularly ECT-responsive motor pathology. Further study is necessary to determine whether ECT responsiveness relates to disease subtype or motor symptom pattern. Another potential contributor to the improvement in motor symptoms in our sample is amelioration of the psychomotor retardation commonly seen in depression, which may confound changes in motor symptoms, especially bradykinesia. Differentiating this effect from motor symptom improvement per se will require larger samples and likely component analysis of changes in symptom clusters on the UPDRS-MDS (i.e., bradykinesia versus rigidity) in response to ECT.
Autonomic Response
Aberrant autonomic responses to ECT may affect efficacy or tolerability. Given the autonomic dysfunction commonly associated with PD, these patients may be vulnerable, highlighting the importance of investigating autonomic response in our study, which was, to our knowledge, the first such attempt in patients with PD. Our data showed relatively typical autonomic responses following ECT stimulus, with gradually less pronounced sympathetic peaks over time (see Figure S1 in the online supplement). Additionally, no severe bradycardic (heart rate <40) or asystolic events were detected. Our data suggest that patients with PD can generally tolerate ECT from an autonomic perspective. The absence of severe bradycardia or asystole is consistent with the safety profile of bifrontal ECT compared with bitemporal (20, 21).
Limitations
Chief among our study’s limitations are the relatively small sample size, lack of control group, and retrospective, unblinded approach to analysis. Additionally, quantification of the motor effects of ECT was constrained by the incomplete reporting of rating scales in our sample. In addition, we cannot judge the generalizability of our findings to other samples of patients with PD, as this is the first study of its kind examining bifrontal ECT in PD and was conducted at a single site. For these reasons, while we have observed an encouraging rate of response for both depressive and motor symptoms, we cannot make definitive conclusions about the effectiveness of bifrontal ECT for dPD or whether it offers any advantage to other placements based on our data. However, given the limited evidence available on this topic, our data provide support for important and testable hypotheses related to the clinical effectiveness and tolerability of ECT in depressed patients with PD.
Future Directions
Despite growing evidence for ECT’s effectiveness in dPD, concerns about adverse effects and stigma (31) may be limiting uptake. Determination of optimal electrode placement and identification of patients at higher risk for adverse events are important further areas of investigation, which may improve acceptability. More study of autonomic data is necessary to better understand the relationship between autonomic response and both tolerability and efficacy. Ultimately, randomized controlled studies are needed to establish the true efficacy and tolerability of this procedure in dPD, including the comparative performance of different electrode placements, and to investigate associations with autonomic response and neurocognitive effects.
1 : The prevalence of Parkinson’s disease: a systematic review and meta-analysis . Mov Disord 2014 ; 29 : 1583 – 1590 Crossref, Medline, Google Scholar
2 : Parkinson’s disease: the quintessential neuropsychiatric disorder . Mov Disord 2011 ; 26 : 1022 – 1031 Crossref, Medline, Google Scholar
3 : The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease . Mov Disord 2009 ; 24 : 1641 – 1649 Crossref, Medline, Google Scholar
4 : A systematic review of prevalence studies of depression in Parkinson’s disease . Mov Disord 2008 ; 23 : 183 – 189, quiz 313 Crossref, Medline, Google Scholar
5 : Quality of life and depression in Parkinson’s disease . J Neurol Sci 2006 ; 248 : 151 – 157 Crossref, Medline, Google Scholar
6 : Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials . BMC Neurol 2010 ; 10 :49Crossref, Medline, Google Scholar
7 : The role of cognitive-behavioural therapy for patients with depression in Parkinson’s disease . Parkinsons Dis 2011 ; 2011 :737523Medline, Google Scholar
8 Jaffe R (ed): The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. Washington, DC , American Psychiatric Publishing , 2008 Google Scholar
9 : Predictors of electroconvulsive therapy postictal delirium . Psychosomatics 2014 ; 55 : 272 – 279 Crossref, Medline, Google Scholar
10 : Non-invasive brain stimulation for Parkinson’s disease: a systematic review and meta-analysis of the literature . J Neurol Neurosurg Psychiatry 2005 ; 76 : 1614 – 1623 Crossref, Medline, Google Scholar
11 : Electroconvulsive therapy for depression in Parkinson’s disease: systematic review of evidence and recommendations . Neurodegener Dis Manag 2016 ; 6 : 161 – 176 Crossref, Medline, Google Scholar
12 : Unilateral ultra-brief pulse electroconvulsive therapy for depression in Parkinson’s disease . Acta Neurol Scand 2017 ; 135 : 407 – 411 Crossref, Medline, Google Scholar
13 : Electroconvulsive therapy in Parkinson’s disease . Arq Neuropsiquiatr 2015 ; 73 : 856 – 860 Crossref, Medline, Google Scholar
14 : Systematic review and meta-analysis of bifrontal electroconvulsive therapy versus bilateral and unilateral electroconvulsive therapy in depression . World J Biol Psychiatry 2012 ; 13 : 248 – 258 Crossref, Medline, Google Scholar
15 : Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy . N Engl J Med 1993 ; 328 : 839 – 846 Crossref, Medline, Google Scholar
16 : Electroconvulsive therapy (ECT) in Parkinson’s disease: ECS and dopamine enhancement . J ECT 2014 ; 30 : 122 – 124 Crossref, Medline, Google Scholar
17 : Iodine-123 fluoropropyl-carbomethoxy-3-β-(4-iodophenyltropane) single-photon emission computed tomography findings before and after electroconvulsive therapy in major depressive disorder with Parkinsonism . J ECT 2011 ; 27 : 331 – 333 Crossref, Medline, Google Scholar
18 : Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study . J Neural Eng 2011 ; 8 :
19 : Electroconvulsive therapy—induced hemodynamic changes unmask unsuspected coronary artery disease . J Clin Anesth 1990 ; 2 : 37 – 41 Crossref, Medline, Google Scholar
20 : The effect of electrode placement and pulsewidth on asystole and bradycardia during the electroconvulsive therapy stimulus . Int J Neuropsychopharmacol 2011 ; 14 : 585 – 594 Crossref, Medline, Google Scholar
21 : Absence of asystole during bifrontal stimulation in electroconvulsive therapy . J ECT 2010 ; 26 : 100 – 103 Crossref, Medline, Google Scholar
22 : ECDEU Assessment Manual for Psychopharmacology . Rockville, Md , , 1976 , 218 – 222 Google Scholar
23 : Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results . Mov Disord 2008 ; 23 : 2129 – 2170 Crossref, Medline, Google Scholar
24 : The clinically important difference on the unified Parkinson’s disease rating scale . Arch Neurol 2010 ; 67 : 64 – 70 Crossref, Medline, Google Scholar
25 : Systematic review of levodopa dose equivalency reporting in Parkinson’s disease . Mov Disord 2010 ; 25 : 2649 – 2653 Crossref, Medline, Google Scholar
26 : Parkinsonism: onset, progression, and mortality. 1967 . Neurology 2001 ; 57 ( Suppl 3 ): S11 – S26 Medline, Google Scholar
27 : ECT-induced delirium in depressed patients with Parkinson’s disease . J Neuropsychiatry Clin Neurosci 1991 ; 3 : 405 – 411 Link, Google Scholar
28 : Progression of motor symptoms in Parkinson’s disease . Neurosci Bull 2012 ; 28 : 39 – 48 Crossref, Medline, Google Scholar
29 : The permeability of the blood-brain barrier during electrically induced seizures in man . Eur J Clin Invest 1977 ; 7 : 87 – 93 Crossref, Medline, Google Scholar
30 : Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson’s disease . Neurotherapeutics 2014 ; 11 : 78 – 91 Crossref, Medline, Google Scholar
31 : The decision making process of older adults who elect to receive ECT . J Psychosoc Nurs Ment Health Serv 2008 ; 46 : 45 – 52 Crossref, Medline, Google Scholar



