Exploring Cognitive Impairment in Patients With Bilateral Capsular Genu Lesions
Abstract
Objective:
The authors investigated for presence of cognitive impairment after occurrence of bilateral lesions of the genu of the internal capsule (GIC). Clinical and neuropsychological features of unilateral GIC lesions have previously been studied, but the cognitive profile of bilateral lesions of the GIC has not been fully explored.
Methods:
An investigation was conducted of neurocognitive deficits and computerized tomography MRI findings among 4,200 stroke patients with bilateral GIC involvement who were admitted to the hospital between January 2010 and October 2018.
Results:
Eight patients with bilateral lesions of the capsular genu were identified and their data analyzed. Overall, behavioral and cognitive dysfunction were characterized by impairment of frontal, memory, and executive functions. Attention and abstraction were present among all eight patients (100%); apathy, abulia, and executive dysfunctions, among seven (87.5%); global mental dysfunction and planning deficits, among six (75.0%); short-term verbal memory deficits and language dysfunctions, among five (62.5%); long-term verbal memory deficits, among four (50.0%); and spatial memory deficits, reading, writing, counting dysfunctions, and anarthria, among two (25.0%). Four of the patients (50.0%) without a history of cognitive disorder showed severe mental deterioration compatible with the clinical picture of dementia. A clinical picture of dementia was still present in these patients 6 months after stroke.
Conclusions:
Bilateral lesions of the capsular genu appearing either simultaneously or at different times were significantly associated with executive dysfunctions.
The genu of the internal capsule (GIC) is the central region of the capsular structure placed in front of the thalamus (1). This structure contains both ascending and descending pathways, going to and coming from the cerebral cortex (Figure 1) (2). The GIC is supplied by the perforating branches directly off of the internal carotid artery (3). Vascular cognitive deficits are the result of a unilateral strategic lesion in this brain region, whereas bilateral lesions involving the thalamus or the caudate nuclei can cause severe and persistent cognitive deficits (4, 5). Poststroke cognitive deficits may occur after lesions in different locations (for example, after unilateral infarctions of the GIC) (6–8). The clinical syndrome is characterized by inattention, apathy, memory impairment, and language disorders and was described as thalamocortical disconnection syndrome (9, 10). Bilateral infarctions of the GIC are quite rare. Cognitive deficits after such lesions are neither well described nor understood. Therefore, we aimed to determine cognitive deficits such as memory, executive, and language disorders after bilateral capsular genu lesions, with the goal to uncover the functionality of this area.
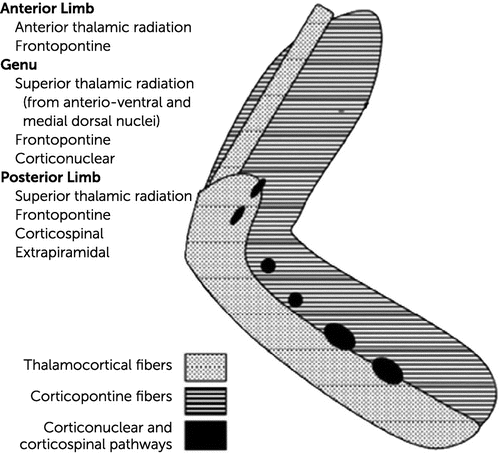
FIGURE 1. Schematic representation of cortical-subcortical frontal pathways crossing the genu of the capsula interna among eight patients with bilateral lesions of the capsular genu
Methods
Patient Selection and Imaging
During an 8-year period, data from 4,200 patients with first-ever stroke were collected and prospectively entered into our stroke registry (11). Among 2,350 patients with anterior circulation stroke, 58 (0.2%) patients had unilateral lesion of the GIC, and eight (0.03%) had bilateral lesions. Clinical, radiological, and detailed cognitive data are routinely acquired and archived in a database of the stroke registry. The following data were retrieved from the registry database: age, gender, previous stroke, risk factors, etiological subtypes, pathogenesis, and topography of lesions on CT and MRI. The cause of stroke was assessed according to criteria described previously (12) as large-artery disease, small-artery disease, cardioembolic, other, unknown, and intracerebral hemorrhage.
Radiological studies including computerized tomography (CT) or MRI were acquired immediately after admission to our center. Noninvasive imaging with CT and MRI was acquired per standard algorithm for acute stroke protocol, including noncontrast CT and axial T1- and T2-weighted spin-echo, diffusion-weighted image, and fluid attenuation inversion recovery sequences. MRI was performed by 1.5 and 3T scanners (Siemens Sonata, Siemens Medical Solutions, Erlangen, Germany). Two vascular neurologists with accreditation in neuroimaging and blinded to clinical variables retrospectively reviewed the noncontrast CT or MRI sequences acquired at admission. Axial MRI scans were reviewed in a consensus fashion to determine the presence or absence of GIC lesions on the basis of visual inspection. To avoid causal inferences, we excluded all types of prior infarctions, hemorrhages, and multiple additional lesions in different areas on CT scans and MRI. Carotid-vertebral Doppler and magnetic resonance angiography or CT angiography were also reviewed in a consensus fashion to determine the presence or absence of atherosclerotic lesions of intra- and extracranial vessels on the basis of visual inspection. Two-dimensional echocardiography and 24-hour electrocardiography (Holter) monitoring had been performed in all cases to determine whether a possible cardioembolism was present.
The study was reviewed and approved by the ethics committee of Ege University Medical Center, and written informed consent was obtained from all patients or relatives in place of patients.
Neuropsychological Assessment
All patients had undergone a comprehensive neuropsychological examination with a battery of tests 1 month after stroke because by then the pathophysiological effects of stroke were minimized. The results of each test were analyzed using normative cognitive data from a sample of 100 healthy volunteers recruited in our neuropsychology department (46% men; mean age=61 years [SD=12.1]; education level=9.3 years [SD=4.1]). Two clinical neuropsychologists with accreditation in neurocognitive science evaluated the results of the neurocognitive tests. Standardized neuropsychological tests were performed to assess cognitive functions, including global mental and executive functions, reasoning, working memory, verbal and visual memory, visuospatial skills, and language functions. These cognitive and behavioral characteristics were assessed with the following tests: Mini-Mental State Examination (MMSE) (13) to measure global mental function; Stroop test (14) to assess the ability to inhibit cognitive interference in reaction time on a task, attention, and executive function; Trail-Making Test, Parts A and B (15) to assess selective attention and task switching; Wisconsin Card-Sorting Test (16) to test set-shifting (i.e., the ability to display flexibility, planning, reasoning, multitasking, and goal-directed behavior); Rey Auditory Verbal Learning Test (17) to assess verbal learning, short-delay recall, long-delay recall, and long-delay cued recall; Wechsler Memory Scale–Revised (18) to determine episodic memory (information and orientation) and semantic (logical) memory; Controlled Oral Word Association test (19) to assess verbal fluency and executive functions; and Benton Visual Retention Test (20) to evaluate visuoperception and visuoconstructive abilities. Language function was tested by using the Token Test battery (21), and reading, writing, and counting were assessed with a brief psychogeriatric assessment schedule (22). A z-score (standard score) of each patient’s cognitive test point was calculated, a z-score range of less than three standard deviations was considered outside the limit of the normal distribution and interpreted as impairment of the related cognitive function. To determine prestroke cognitive status, we conducted an informant interview for dementia, mood disorders, and actual or former pharmacological therapy and validated the information obtained in the interview by reviewing the records of the primary care doctor or National Health Data System. None of the patients had a history of memory disorder or intellectual disorder consistent with the diagnosis of prestroke mild cognitive impairment or dementia.
Apathy and abulia are characterized by reduced goal-directed behavior, cognition, and emotion, and they differ in the severity of their behavioral and emotional presentations, with more apparent presentation of these symptoms in abulia (23). Because the exact definition of apathy and abulia does not appear in the DSM-5, we used clinical diagnostic criteria for poststroke apathy and abulia adapted from Marin et al. for the diagnosis (24, 25).
Results
Eight patients (0.3% of the registry; five men [63%] and three women [37%]) had bilateral lesions of the GIC. Their mean age was 68.3 years (SD=5.9; range, 59–74 years). Classical vascular risk factors included hypertension (78%), diabetes mellitus (50%), and hyperlipidemia (38%). Four of the eight patients (50%) had small-artery disease, and two (25%) had both bilateral severe stenosis at the origin of the internal carotid artery and cardioembolism.
On admission, patients were alert, but all had confusion and disorientation to time and made ambiguous citation about the facts of the relevant reality. On the following days, these patients (patients 1, 4, 5, 6, and 8) presented with abulia and hypokinesia with a tendency toward mutism. All patients presented with motor deficits without sensory and cerebellar disturbances, including facial-brachial-crural (50%), facial-brachial (37%), and facial motor weakness (13%). MRI showed that seven patients had hyperintense lesions of less than 1.5-cm diameter and one patient had a 1.3-cm-diameter hemorrhage with an old ischemic lesion in the right capsular genu (patient 8) (Figure 2).
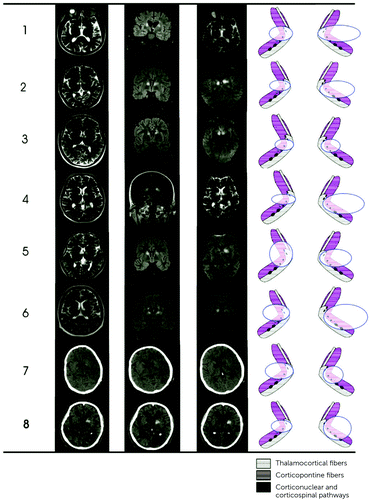
FIGURE 2. Cranial MRI in the acute phase of stoke among eight patients with bilateral lesions of the capsular genua
a Axial, coronal T2-weighted images (patients 1–6) show bilateral hyperintense lesions of the genu of internal capsule. Computerized tomography images of patient 7 show bilateral capsular infarcts. Patient 8 had an old ischemic lesion on the right genu of the internal capsule and hemorrhage in the left side.
Neuropsychological assessment showed impairment in mental flexibility and prominent disturbance in attention, shifting of attention, abstraction, memory, and executive function. Six patients’ MMSE scores were less than 24 points (Table 1). Four patients (patients 1, 4, 6, and 8) had impairment of speech fluency and difficulty with reading, writing, and calculation (Table 2). Six patients (patients 1, 2, 3, 5, 7, and 8) had slight dysarthria, and two had severe articulation deficit (patients 4 and 6). Short verbal and working memory were affected among almost all patients.
| Neuropsychological assessmentb | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Education level (years) | 10.0 | 12.0 | 5.0 | 12.0 | 8.0 | 5 | 11.0 | 15.0 | ||||||||||
| Global mental assessment | ||||||||||||||||||
| Mini-Mental State Examination | 27.0 | 1.6 | 16.0 | –6.88c | 23.0 | –2.50 | 24.0 | –1.88 | 20.0 | –4.38c | 18.0 | –5.63c | 17.0 | –6.25c | 24.0 | –1.88 | 23.0 | –2.50 |
| Stroop test | ||||||||||||||||||
| Name color print of noncolor words | 24.0 | 8.0 | 84.0 | 7.50c | 56.0 | 4.00c | 54.0 | 3.75c | 80.0 | 7.00c | 94.0 | 8.75c | 85.0 | 7.63c | 48.0 | 2.25 | 60.0 | 2.75 |
| Name color print of color words | 37.0 | 14.0 | 90.0 | 3.79c | 75.0 | 2.71 | 65.0 | 2.00 | 98.0 | 4.36c | 104.0 | 4.79c | 94.0 | 4.07c | 64.0 | 1.93 | 81.0 | 3.13c |
| Trail-Making Test | ||||||||||||||||||
| Part A | 38.0 | 12.0 | 78.0 | 3.33c | 66.0 | 3.17c | 52.0 | 1.17 | 72.0 | 2.83 | 78.0 | 3.33c | 82.0 | 3.67c | 60.0 | 1.83 | 76.0 | 3.17c |
| Part B | 91.0 | 55.0 | 270.0 | 3.25c | 210.0 | 2.16 | 220.0 | 2.35 | 260.0 | 3.07c | 280.0 | 3.44c | 290.0 | 3.62c | 220.0 | 2.35 | 290.0 | 3.62c |
| Token test | 32.4 | 2.1 | 26.0 | –3.05c | 28.0 | –2.10 | 31.0 | –0.67 | 22.0 | –4.95c | 26.0 | –3.05c | 21.0 | –5.43c | 30.0 | –1.14 | 24.0 | –4.00c |
| Controlled Oral Word Association | 17.0 | 3.0 | 7.0 | –3.33c | 14.0 | –1.00 | 12.0 | –1.67 | 6.0 | –3.67c | 8.0 | –3.00c | 6.0 | –3.67 | 14.0 | –1.00 | 13.0 | –1.33 |
TABLE 1. Clinical findings of patients with bilateral lesions of the genu of the internal capsulea
| Neuropsychological assessmentb | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Wisconsin Card-Sorting Test | ||||||||||||||||||
| Categories achieved | 4.1 | 1.0 | 1.8 | –2.30 | 4.3 | 0.20 | 3.6 | –0.50 | 1.2 | –2.90 | 0.9 | –3.20c | 0.6 | –3.50c | 3.5 | –0.30 | 3.9 | –0.10 |
| Correct responses | 66.0 | 8.0 | 34.0 | –4.00c | 48.0 | –2.25 | 45.0 | –2.63 | 22.0 | –5.50c | 32.0 | –4.25c | 26.0 | –5.00c | 58.0 | –1.00 | 48.0 | –2.25 |
| Errors | 42.0 | 10.0 | 72.0 | 3.00c | 75.0 | 3.30c | 68.0 | 2.60 | 90.0 | 4.80c | 76.0 | 3.40c | 86.0 | 4.40c | 62.0 | 2.00 | 54.0 | 1.20 |
| Perseverative responses | 20.0 | 13.0 | 75.0 | 4.23c | 49.0 | 2.23 | 48 | 2.15 | 63.0 | 3.31c | 76.0 | 4.31c | 72.0 | 4.0c | 46.0 | 2.0 | 60.0 | 3.08c |
| Inability to maintain memory | 0.9 | 0.6 | 3.0 | 3.50c | 1.2 | 2.17 | 1.9 | 1.67 | 3.2 | –3.83 | 2.8 | 3.17c | 3.4 | 4.17c | 1.4 | 0.83 | 2.6 | 2.83 |
| Working memory (short-term memory), trial 1, list A | 6.3 | 1.2 | 2.0 | –3.58c | 4.5 | –1.50 | 4.0 | –1.92 | 2.2 | –3.42c | 2.4 | –3.25c | 1.9 | –3.67c | 5.2 | –0.92 | 4.2 | –1.75 |
| Short-term memory, digit span forward | 4.4 | 0.8 | 1.5 | –3.63c | 4.2 | –0.25 | 4.0 | –0.50 | 1.5 | –3.63c | 1.8 | –3.25c | 1.0 | –4.25c | 3.4 | –1.25 | 1.8 | –3.25c |
| Verbal memory, delayed recal | 10.2 | 2.1 | 3.0 | –3.43c | 7.0 | –1.52 | 8.0 | –1.05 | 3.0 | –3.43c | 4.0 | –2.95 | 3.0 | –3.43c | 6.0 | –2.0 | 5.0 | –2.48 |
| Recognition | 11.1 | 2.2 | 4.0 | –3.23c | 6.0 | –2.32 | 8.0 | –1.41 | 5.0 | –2.77 | 4.0 | –3.23c | 3.0 | –3.68c | 5.0 | –2.77 | 5.0 | –2.77 |
| Visual memory/visual memory span | 17.6 | 2.8 | 13.0 | –1.64 | 14.0 | –1.29 | 13.0 | –1.64 | 11.0 | –2.36 | 9.0 | –30.7c | 9.0 | –3.07c | 16.0 | –0.57 | 12.0 | –2.0 |
| Copy drawings | 12.8 | 1.8 | 7.0 | –3.22c | 12.0 | –0.44 | 11 | –1.0 | 7.0 | –3.22c | 6.0 | –3.78 | 6 | –3.78 | 11.0 | –1.0 | 10.0 | –1.56 |
| Counting, writing, and reading | 10.0 | 1.4 | 5.0 | –3.57c | 7.0 | –2.14 | 9 | –0.71 | 5.0 | –3.57c | 6.0 | –2.86 | 6.0 | –2.86 | 9.0 | –0.71 | 6.0 | –2.86 |
TABLE 2. Raw and z-scores of neuropsychological tests of patients with bilateral lesions of the genu of the internal capsulea
Four of the patients (patients 1, 4, 5, and 6) showed severe and persistent cognitive disorders 6 months after stroke. In the first month after stroke, the results of the cognitive assessment indicated severe deficits, especially in memory, executive, language, and frontal functions (Table 2). Frontal functions such as strategic planning, organized searching, ability to use environmental feedback, and behavior directed toward achieving a goal were significantly disturbed. Six months later, patients were considered by their family members to have loss of initiation and to be hypokinetic and apathetic with prominent intellectual deficits, and they were reported to be unable to perform their usual daily activities. Four patients were diagnosed as having dementia according to DSM-5 (26) and basic activities of daily living criteria; the other four patients (patients 2, 3, 7, and 8) displayed moderate memory deficits that did not disrupt activities of daily living.
Discussion
Consistent with the literature (27), our analysis of a large cohort of stroke patients shows bilateral capsular genu infarctions to be rare and associated with severe behavioral and cognitive deficits of frontal, attention, memory, and executive functions. Almost all patients with bilateral GIC had various types of bilateral facio-brachio-crural paresis and articulation deficits. Previous case reports with persons with unilateral capsular lesion have suggested moderate memory deficits that slightly affected everyday life activities and were compatible with the diagnosis of mild cognitive impairment (4–6, 8, 10). In addition, bilateral GIC infarctions induce severe and long-lasting cognitive deficits (28, 29), possibly as a result of destruction of mutually connected anatomical and functional pathways. However, it is difficult to draw a definitive conclusion regarding cognitive differences between these two groups because most of the case series do not directly compare the unilateral and bilateral capsular genu infarction groups.
An interesting finding was that executive dysfunction was a prominent deficit. For example, interference control (selective attention and cognitive inhibition), working memory, and cognitive flexibility (set shifting, mental flexibility, or mental set-shifting) were significantly disturbed. Medial-dorsal and ventral-anterior nuclei of the thalamus convey the projection tracts via internal capsule to the frontal lobe. These thalamo-cortical pathways form complex cortical-subcortical-frontal circuits that are regulated and relayed in the basal ganglia and the thalami before reaching the frontal cortex (30, 31). Therefore, it appears plausible that bilateral lesions damaging the pathway of this circuit can cause severe executive dysfunctions.
In addition, our patients had various memory deficits corroborating the existing evidence for a selective role of the GIC in memory systems. Different types of memory failure and their impact on patients’ lives after capsular genu lesions have been described in different reports. In our study, it seemed that approximately one third of patients had poor performance either on recognition tasks or in recall. However, in a detailed evaluation we have observed that only patient 5 had poor performance on recognition tasks with relatively well-preserved recall. Generally, it was interesting to observe that these patients had no insight into their inability to maintain memory and gave perseverative and false responses to questions. Taken together, these findings suggest a possible role for executive dysfunction interfering with memory. For instance, several studies have suggested a high correlation between executive functions and memory (32, 33).
The occurrence of memory deficits in patients with GIC lesions may be attributed to the involvement of two different limbic loops: the loop crossing the hippocampus fornix-anterior thalamic nucleus-cingulum hippocampus (34) and the loop involving the amygdala dorsomedial thalamic nucleus orbitofrontal cortex amygdala (35, 36). Previous studies have demonstrated that in the case of capsular genu lesions, the interruption of the thalamic projection fibers may cause a functional deactivation of the ipsilateral orbitofrontal cortex (7, 10).
Bilateral GIC lesions among four of our patients led to dementia as a result of a persistent and severe decrease in cognitive functions. Among these patients, the involvement of strategic anatomical pathways that maintain behavior and memory functions caused global mental deficit. Moreover, bilateral lesions may have disrupted the process of improving cognitive functions over time with plasticity and compensation mechanisms. Thus, among patients with acute dementia after stroke, the coexistence of bilateral capsular genu lesions should be considered (37–40).
Special attention should also be paid to a possible synergistic effect between strategically located lesions, such as infarcts in basal ganglia (41), thalamus, angular gyrus (42), cingulate cortex (43), frontal subcortical region (44), and bilateral capsular lesions.
Our study has some limitations. First, we did not assess the relation between dementia and brain atrophy (45). Second, two of our patients with bilateral carotid stenosis presented with apathy and attentional short-term verbal and executive dysfunctions. Previous studies have shown that bilateral carotid stenosis may lead to phonemic, categorical, and visual-cognitive dysfunctions (46). Moreover, severe carotid stenosis may influence cognitive deterioration over a long period in asymptomatic patients (47). In our cases, we did not observe other strategic small lesions (<15 mm) and microhemorrhages that might be independent risk factors for cognitive disorders (48, 49). However, because we did not perform a cognitive analysis comparing patients with unilateral and bilateral lesions, our results should be interpreted cautiously and as descriptive rather than comparative.
In summary, bilateral GIC lesions are rare, but they can cause a pervasive poststroke behavioral and cognitive dysfunction related to an interruption of connections between anterior and dorsomedial parts of the thalamus, prefrontal cortex, and anterior cingulate cortex (50, 51).
1 : White fiber dissection of brain; the internal capsule: a cadaveric study. Turk Neurosurg 2010; 20:314–322Medline, Google Scholar
2 : Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System; An Approach to Cerebral Imaging. New York, Thieme, 1988Google Scholar
3 : Arteries and Veins of the Human Brain. Springfield, Ill., Charles C Thomas, 1969Google Scholar
4 : Acute caudate vascular lesions. Stroke 1999; 30:100–108Crossref, Medline, Google Scholar
5 : Bilateral thalamic infarction: clinical, etiological and MRI correlates. Acta Neurol Scand 2001; 103:35–42Crossref, Medline, Google Scholar
6 : A follow-up study of cognitive impairment due to inferior capsular genu infarction. J Neurol 1999; 246:764–769Crossref, Medline, Google Scholar
7 : Abulia from unilateral capsular genu infarction: report of two cases. J Neurol Sci 1996; 143:181–184Crossref, Medline, Google Scholar
8 : Verbal memory deterioration after unilateral infarct of the internal capsule in an adolescent. Cortex 1990; 26:597–609Crossref, Medline, Google Scholar
9 : Abulia and cognitive impairment in two patients with capsular genu infarct. Acta Neurol Scand 2001; 104:185–190Crossref, Medline, Google Scholar
10 : Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology 1992; 42:1966–1979Crossref, Medline, Google Scholar
11 : The Ege Stroke Registry: a hospital-based study in the Aegean region, Izmir, Turkey. Analysis of 2,000 stroke patients. Cerebrovasc Dis 1998; 8:278–288Crossref, Medline, Google Scholar
12 : Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999; 53:126–131Crossref, Medline, Google Scholar
13 : “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
14 : Studies of interference in serial verbal reactions. J Exp Psychol Gen 1992; 121:15–23Crossref, Google Scholar
15 : Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19:203–214Crossref, Medline, Google Scholar
16 : Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 2001; 21:7733–7741Crossref, Medline, Google Scholar
17 L’examen clinique en psychologie. Paris, Presses Universitaires de France, 1958.Google Scholar
18 : Wechsler Memory Scale—Revised (WMS-R) Manual. San Antonio, Tex., Psychological Corporation, 1987Google Scholar
19 : Neuropsychological Interpretation of Objective Psychological Tests. New York, Springer Science and Business Media, 2000Google Scholar
20 : Benton Visual Retention Test. San Antonio, Tex., Psychological Corporation, 1992Google Scholar
21 : The token test: a sensitive test to detect receptive disturbances in aphasics. Brain 1962; 85:665–678Crossref, Medline, Google Scholar
22 : A brief psychogeriatric assessment schedule: validation against psychiatric diagnosis and discharge from hospital. Br J Psychiatry 1975; 127:489–493Crossref, Medline, Google Scholar
23 : Disorders of diminished motivation. J Head Trauma Rehabil 2005; 20:377–388Crossref, Medline, Google Scholar
24 : Apathy following stroke. Can J Psychiatry 2010; 55:350–354Crossref, Medline, Google Scholar
25 : Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3:243–254Link, Google Scholar
26 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association Publishing, 2013Google Scholar
27 : Capsular infarcts: the underlying vascular lesions. Arch Neurol 1979; 36:65–73Crossref, Medline, Google Scholar
28 : Neuroprotective Therapy for Stroke and Ischemic Disease. New York, Springer, 2017Crossref, Google Scholar
29 : Current Diagnosis and Treatment: Geriatrics, 2nd ed. New York, McGraw Hill, 2014Google Scholar
30 : Memory loss from a subcortical white matter infarct. J Neurol Neurosurg Psychiatry 1988; 51:866–869Crossref, Medline, Google Scholar
31 : Memory without context: amnesia with confabulations after infarction of the right capsular genu. J Neurol Neurosurg Psychiatry 1996; 61:186–193Crossref, Medline, Google Scholar
32 : Impact of executive dysfunctions on episodic memory abilities in patients with ruptured aneurysm of the anterior communicating artery. Brain Cogn 2003; 53:354–358Crossref, Medline, Google Scholar
33 : Learning disorders after ruptured aneurysms of the anterior communicating artery. Rev Neurol (Paris) 1998; 154:508–522Medline, Google Scholar
34 : Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 1990; 85:119–146Crossref, Medline, Google Scholar
35 : The contribution of the anterior thalamic nuclei to anterograde amnesia. Neuropsychologia 1993; 31:1001–1019Crossref, Medline, Google Scholar
36 : Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
37 : Capsular genu syndrome. Neurology 1990; 40:1499–1502Crossref, Medline, Google Scholar
38 : Diencephalic amnesia. Brain 1990; 113:1–25Crossref, Medline, Google Scholar
39 : Acute capsular infarction. Location of the lesions and the clinical features. Neuroradiology 1985; 27:248–253Crossref, Medline, Google Scholar
40 : Mnestic performance profile of a bilateral diencephalic infarct patient with preserved intelligence and severe amnesic disturbances. J Clin Exp Neuropsychol 1993; 15:627–652Crossref, Medline, Google Scholar
41 : Post-stroke subjective cognitive impairment is associated with acute lacunar infarcts in the basal ganglia. Eur J Neurol 2013; 20:547–551Crossref, Medline, Google Scholar
42 : Angular gyrus syndrome simulating Alzheimer’s disease. Arch Neurol 1982; 39:616–620Crossref, Medline, Google Scholar
43 : Stroke location is an independent predictor of cognitive outcome. Stroke 2016; 47:66–73Crossref, Medline, Google Scholar
44 : Frontal subcortical ischemia is crucial for post stroke cognitive impairment. J Neurol Sci 2011; 309:92–95Crossref, Medline, Google Scholar
45 : Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke 2010; 41:e41–e46Crossref, Medline, Google Scholar
46 : Cerebral hemodynamics and cognitive performance in bilateral asymptomatic carotid stenosis. Neurology 2012; 79:1788–1795Crossref, Medline, Google Scholar
47 : Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology 2013; 80:2145–2150Crossref, Medline, Google Scholar
48 : Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 2016; 73:934–943Crossref, Medline, Google Scholar
49 : Cerebral cortical microinfarcts on magnetic resonance imaging and their association with cognition in cerebral amyloid angiopathy. Stroke 2018; 49:2330–2336Crossref, Medline, Google Scholar
50 : MRI Atlas of Human White Matter. New York, Elsevier, 2005Google Scholar
51 : Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 2008; 1142:266–309Crossref, Medline, Google Scholar



