Enlarged Cavum Septi Pellucidi in Patients With Schizophrenia
Abstract
Enlarged cavum septi pellucidi (CSP) is a neurodevelopmental anomaly that has been associated with schizophrenia. This study was designed to evaluate, in patients with schizophrenia, the relationship between the severity of this anomaly and measures of symptom and cognitive skills. Three groups were used: patients with large CSP (n=14), patients without large CSP (n=14), and healthy control subjects (n=14). In patients with large CSP, a significant, inverse relationship was found between size of CSP and measures of cognitive deficit. Thus, the greater the size of the anomaly, the greater the cognitive deficit. No relationship was found between severity of CSP and symptom measures.
Patients with schizophrenia have been documented to have significant abnormalities in midline brain regions such as the corpus callosum,1,2 septum pellucidum,3–5 and cerebellar vermis.6–8 With regard to the septum pellucidum, several studies (including several from our group) have documented that patients with schizophrenia have an increased rate of enlarged cavum septi pellucidi (CSP) compared with healthy control groups.3–5,9–14
However, only one study to date has evaluated the relationship between enlarged CSP and symptoms in patients with schizophrenia,5 and no study has evaluated the relationship between enlarged CSP and cognitive function. Enlarged CSP has been associated with cognitive dysfunction in heterogeneous pediatric groups showing neurologic abnormalities, mental retardation, and developmental delay.15–19 In addition, a study by Renier et al.20 showed that children with Apert's syndrome (acrocephalosyndactyly) had a variety of brain abnormalities such as ventricular enlargement, corpus callosum dysgenesis, and septum pellucidum abnormalities. However, only the septum abnormalities showed a relationship with cognitive dysfunction.
The current study was designed to evaluate the clinical correlates of enlarged CSP in patients with schizophrenia. On the basis of findings in other neurodevelopmental syndromes, we hypothesized that cognitive dysfunction would be related to the presence of and/or severity of enlarged CSP.
METHODS
Subjects
Patients and control subjects with and without enlarged CSP were identified from a pool of participants who were consecutive admissions to the University of Iowa Mental Health Clinical Research Center and/or participated in a brain imaging protocol. A previous study on the incidence of enlarged CSP and its relationship to brain morphology was conducted on patients and controls who were scanned through late 1995.9 The pool of subjects (patients and control subjects) used for the current study began with subjects who were admitted (or participated as controls) from October 1995 through March 1998. Each scan was rated for size of CSP as described below. Each patient was diagnosed as having schizophrenia based on either DSM-III-R or DSM-IV criteria and a structured interview, the Comprehensive Assessment of Symptoms and History (CASH).21
Fourteen patients were identified as having an enlarged CSP as defined below. These 14 patients were then matched on age and gender to 14 schizophrenia patients who did not have enlarged CSP and 14 healthy control subjects who did not have enlarged CSP.
The control group consisted of healthy volunteers who were recruited from the community through newspaper advertising. Each control subject was evaluated with an abbreviated version of the CASH and was excluded if there was any personal history of psychiatric illness or family history of schizophrenia. Patients or control subjects were excluded if they had a lifetime history of serious head trauma, neurological illness, serious medical or surgical illness, or recent, heavy psychoactive drug use or abuse. After complete description of the study to the subjects, written informed consent was obtained.
Symptom and Cognitive Measures
Clinical measures such as age at onset and duration of illness were obtained from the CASH. The Scale for the Assessment of Positive Symptoms (SAPS)22 and the Scale for the Assessment of Negative Symptoms (SANS)23 are embedded in the current section of the CASH, where the most severe level of symptoms during the previous month is recorded. Symptom items from the SANS and SAPS were grouped to represent three symptom dimensions: positive, negative, and disorganized.
Cognitive assessment was performed with a comprehensive battery designed to test a wide variety of functions. The entire battery is administered to patients when they are deemed clinically stable. This is done typically while patients are on their current medication regimen and prior to a 3-week medication washout. However, if the patient is admitted with no neuroleptic exposure, the testing is done prior to medication treatment. For the current study, general measures of overall cognitive function, verbal ability, and nonverbal ability were assessed. The measures used were the Wechsler Adult Intelligence Scale–Revised Full Scale IQ (FSIQ), Performance IQ (PIQ) and Verbal IQ (VIQ).24
MR Acquisition and Analysis
MR scans were obtained with a T1-weighted three-dimensional SPGR sequence on a 1.5-tesla GE Signa Scanner (TE=5, TR=24, flip angle=40, NEX=2, FOV=26, matrix=256×192) using the coronal plane, yielding 124 contiguous slices 1.5 mm thick.
All postacquisition processing was done by using a locally developed family of software called BRAINS. Details of the image analysis are published elsewhere.25–27 The MR data were converted to a three-dimensional data set. The brains were then resampled in 1-mm slices. The resampled slices were then visualized in multiple planes, simultaneously. This allowed for a thorough, millimeter-by-millimeter inspection of the entire brain in all three orientations (coronal, sagittal, and axial).
Rating of CSP
Brains were identified by a number only. Thus the rater (A.K.) was blind to diagnosis, sex, or age of the subject. The method for the present study entailed a quantitative approach, measuring the size of the cavum by its appearance in consecutive coronal 1-mm slices: a rating of 1 represents a cavum seen in only one coronal slice, a rating of 2 represents a cavum seen in two consecutive coronal slices, and so on. Because the images are 1 mm without gaps, the rating is then a reflection of the actual anterior-to-posterior length of the cavum, although partial voluming renders this an approximation only. For example, a CSP with a rating of 5 would be approximately 5 mm long.
Reliability for rating the CSP was established on a separate sample of 50 brains. Percentage agreement for two raters (A.K. and P.N.) was high, at 90%, and for those ratings on which the two raters disagreed, the difference was never by more than one point.
Determination of Enlargement of the CSP
A small CSP is common in a large proportion of healthy control subjects and is therefore considered to be a “normal variant.”4,28 Determination of how large a CSP must be in order to reflect pathology is not known exactly, although it is clear that an overly conservative estimate may erroneously include CSP that are in the upper range of the normal variant. The best estimate of size of normal-variant cavum is approximately 1–4 mm.28–31 To allow some room for partial volume effects and to eliminate “borderline” CSP, we defined enlargement as CSP length greater than 6 mm. All ratings were then collapsed into two groups: those that had CSP enlargement (rating of greater than 6) and those that did not (ratings of 0 to 6).
The group of patients with “large” CSP consisted of six subjects with a CSP of size 7, two with CSP size 8, two with CSP size 10, one with CSP size 18, and the remaining three subjects, who had complete nonfusion of the septal leaflets. The latter condition is termed a combined cavum septi pellucidi and cavum vergae. The CSP sizes for these three patients were documented as measuring, respectively, 45, 46, and 59 consecutive slices. By definition, the patient group without large CSP and the control group had CSP ratings of 0 to 6. See Figure 1 for examples of small and large CSP.
Statistical Analysis
To compare clinical data between the two patient groups, paired t-tests were used (two-tailed). To evaluate the relationship between size of CSP and various clinical measures, Spearman's correlations were calculated. Nonparametric tests were used because the size of CSP was not normally distributed and the use of nonparametric tests would be resistant to the influences of outliers.
RESULTS
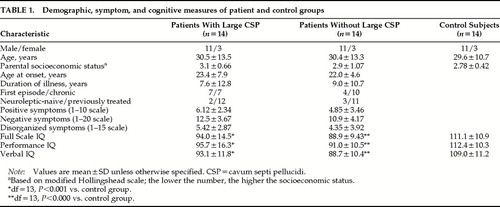
Patient and control sample demographics are shown in Table 1. The parental socioeconomic status for the patients with large CSP was lower than that of the patients without large CSP and control subjects. However, this was not statistically significant between any of the groups. The two patient groups were similar in age at onset and duration of illness. The patient group with large CSP comprised 7 first-episode patients (defined as first psychiatric hospitalization) and 7 with chronic illness. The patient group without large CSP had fewer first-episode patients (n=4) and more with chronic illness (n=10).
Symptom and cognitive data comparisons between the three groups are shown in Table 1. Scores on the three cognitive measures were not significantly different between the patients with large CSP and the patients without large CSP. Scores on all three cognitive measures were significantly higher for the control subjects compared with either patient group. The symptom data show that patients with large CSP had somewhat more severe pathology in all three domains (positive, negative, and disorganized symptoms); however, this did not reach statistical significance.
The results of the correlation analyses are shown in Table 2. For patients with large CSP, there was a significant negative correlation between size of CSP and multiple measures of cognitive functioning, including FSIQ, PIQ, and VIQ. Thus, the larger the CSP or the more severe the anomaly, the greater the cognitive impairment (see Figure 2). This relationship between size of CSP and cognition was not present in the group of patients without large CSP. There were no significant correlations between size of CSP and symptom measures in either patient group.
DISCUSSION
Several studies have already established that enlarged CSP is associated with schizophrenia and may be considered a marker for cerebral maldevelopment.3–5,10–13 The current study extends these findings by showing that not only is the presence of this anomaly associated with the illness, but there is a direct relationship between severity of this anomaly and cognitive function. That is, the larger the anomaly, the greater the cognitive dysfunction. Moreover, the relationship was not specific to any particular type of cognitive skill, but instead was related to global disturbances of cognitive functions: the inverse correlations between size of CSP and cognitive function were significant for full scale, verbal, and nonverbal IQ. This suggests not only that enlarged CSP is a marker for cerebral maldevelopment, but also that the larger the anomaly, the greater the functional deficit, likely reflecting a greater degree of neurodevelopmental aberration.
Enlarged CSP is not a finding specific to schizophrenia. Several other developmental syndromes, such as fetal alcohol syndrome,32 Sotos syndrome,33 and Apert's syndrome,20 have documented increased rates of enlarged CSP. Nor is the association between enlarged CSP and cognitive dysfunction specific to schizophrenia. As mentioned above, a study of Apert's syndrome by Renier et al.20 found that although brain abnormalities such as enlarged ventricles and corpus callosum dysgenesis were often present in these patients, only the septum pellucidum abnormalities were correlated with cognitive dysfunction (lower IQ). Studies by Bodensteiner and colleagues16–19 have repeatedly documented the association with large CSP and a broad range of developmental problems, including mental retardation, developmental delay, seizures, macro/microcephaly, and others. Their work clearly indicates that an enlarged CSP is an important marker for increased risk of disturbed brain development.
By what mechanism could a large CSP underlie global deficits in cognition? The septum pellucidum is part of the limbic system and plays an important role in the linkage between the hypothalamus, hippocampus, amygdala, habenula, and brainstem reticular formation.19,34 It is quite unlikely that a specific disturbance of a localized structure such as this would lead to such widespread functional deficit. More likely is that the developmental process by which the septum pellucidum is formed and matures may reflect the pathoetiology for widespread brain disturbance. Very early in development, the septum pellucidum is solid, then it cleaves to form a fluid-filled cavity along its length (the cavum septi pellucidi). In normal fetal development, these two leaflets fuse back together near the time of birth. Although the details of this process are not well studied, it appears that fusion of the two leaflets depends on a variety of other surrounding structures developing normally—namely, medial temporal structures such as the hippocampus and midline structures such as the corpus callosum, as well as the cerebral hemispheres.28 Other midline structures such as the thalamus may also play a role. Therefore, a disturbance of any or all of the possible surrounding structures may lead to an enlarged CSP. Thus, although the septum pellucidum is a limbic structure, the fusion of the septal leaflets requires normal development of a variety of other brain regions. If these other regions are also abnormally developed, more widespread functional deficit may be expected when the leaflets of the septum fail to fuse properly.
Of interest is the lack of correlation between symptom measures and size of CSP, especially in light of the relationship between CSP and cognitive function. Kwon et al.5 also found no relationship between measures of CSP and symptom ratings on the SANS and SAPS. This is also consistent with recent findings from our lab in another midline brain region, the cerebellar vermis.8 This study found that cerebellar vermis area (in particular, the anterior lobe) was decreased in male patients with schizophrenia compared with control subjects. Moreover, this area decrement was positively correlated with cognitive function but had no relationship to symptom measures. Taken together, these two studies5,8suggest that the pathophysiologies of symptoms and cognition may differ in their relationship to brain morphology.
It is important to emphasize that enlarged CSP is a relatively uncommon finding in schizophrenia. Although patients with schizophrenia have an increased incidence of this brain anomaly compared with healthy control subjects, it is present in only 10% to 30% of patients.3,5 Therefore, it is not possible to generalize these findings to all patients with the disorder. Nor is it possible to speculate that cognitive deficits of schizophrenia are mediated by neurodevelopmental anomalies such as enlarged CSP. Clearly, most patients with schizophrenia do not have enlarged CSP, but suffer from significant cognitive deficit nonetheless. Given that enlarged CSP and its association with cognitive deficits are found in many other developmental syndromes and are not specific to schizophrenia, the findings of this study simply highlight the functional significance of this brain anomaly in the context of yet another neurodevelopmental syndrome. Further investigation into the pathology of early brain development should continue to provide clues to the pathophysiology of this devastating illness.
ACKNOWLEDGMENTS
This research was supported in part by NIMH Grants MH31593, MH40856, and MHCRC 43271; the Nellie Ball Trust Fund, Iowa State Bank and Trust Company, Trustee; and Research Scientist Award MH00625.
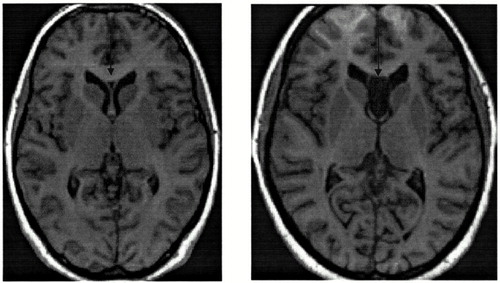
FIGURE 1. Axial view on the left is of a small cavum septi pellucidi (CSP)Axial view on the right is of a combined CSP and cavum vergae: total nonfusion of the septal leaflets.
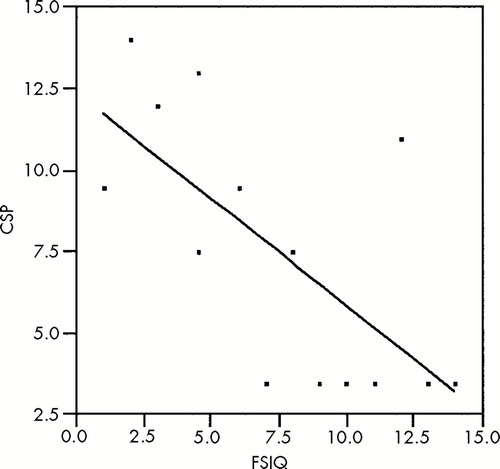
FIGURE 2. Cavum septi pellucidi (CSP) size versus Full Scale IQValues are ranked for nonparametric analysis. CSP range 7–59, FSIQ range 77–120; Spearman's r=–0.682, P=0.007.
 |
 |
1 Woodruff P, McManus I, David A: Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry 1995; 58:457–461Crossref, Medline, Google Scholar
2 Tibbo P, Nopoulos P, Arndt S, et al: Corpus callosum shape and size in male patients with schizophrenia. Biol Psychiatry 1998; 44:405–412Crossref, Medline, Google Scholar
3 Nopoulos P, Swayze V, Flaum M, et al: Cavum septi pellucidi in normals and patients with schizophrenia as detected by MRI. Biol Psychiatry 1997; 41:1102–1108Google Scholar
4 Nopoulos PC, Giedd JN, Andreasen NC, et al: Frequency and severity of enlarged cavum septi pellucidi in childhood-onset schizophrenia. Am J Psychiatry 1998; 155:1074–1079Google Scholar
5 Kwon JS, Shenton ME, Hirayasu Y, et al: MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am J Psychiatry 1998; 155:509–515Crossref, Medline, Google Scholar
6 Rossi A, Stratta P, Mancini F, et al: Cerebellar vermal size in schizophrenia: a male effect. Biol Psychiatry 1993; 33:354–357Crossref, Medline, Google Scholar
7 Tran KD, Smutzer GS, Doty RL, et al: Reduced Purkinje cell size in cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry 1998; 155:1288–1290Google Scholar
8 Nopoulos P, Ceilley J, Gailis E, et al: An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the “cognitive dysmetria” concept. Biol Psychiatry 1999; 46:703–711Crossref, Medline, Google Scholar
9 Nopoulos P, Swayze V, Andreasen N: Pattern of brain morphology in patients with schizophrenia and large cavum septi pellucidi. J Neuropsychiatry Clin Neurosci 1996; 8:147–152Link, Google Scholar
10 Degreef G, Bogerts B, Falkai P, et al: Increased prevalence of the cavum septum pellucidum in magnetic resonance scans and post-mortem brains of schizophrenic patients. Psychiatry Res Neuroimaging 1991; 45:1–13Crossref, Google Scholar
11 Degreef G, Lantos G, Bogerts B, et al: Abnormalities of the septum pellucidum on MR scans in first-episode schizophrenic patients. Am J Neuroradiol 1992; 13:835–840Medline, Google Scholar
12 DeLisi LE, Hoff AL, Kushner M, et al: Increased prevalence of cavum septum pellucidum in schizophrenia. Psychiatry Res Neuroimaging 1993; 50:193–199Crossref, Medline, Google Scholar
13 Jurjus GJ, Nasrallah HA, Olson SC, et al: Cavum septum pellucidum in schizophrenia, affective disorder and healthy controls: a magnetic resonance imaging study. Psychol Med 1993; 23:319–322Crossref, Medline, Google Scholar
14 Scott TF, Price TP, George MS, et al: Cerebral malformations and schizophrenia. J Neuropsychiatry Clin Neurosci 1993; 5:287–293Link, Google Scholar
15 Miller ME, Kido D, Horner F: Cavum vergae: association with neurologic abnormality and diagnosis by magnetic resonance imaging. Arch Neurol 1986; 43:821–823Crossref, Medline, Google Scholar
16 Schaefer G, Bodensteiner J, Thompson J: Subtle anomalies of the septum pellucidum and neurodevelopmental deficits. Dev Med Child Neurol 1994; 36:554–559Crossref, Medline, Google Scholar
17 Breeding L, Cowan L, Bodensteiner J, et al: The cavum septi pellucidi: a magnetic resonance imaging study of prevalence and clinical associations in a pediatric population. J Neuroimaging 1991; 1:115–118Crossref, Google Scholar
18 Bodensteiner J, Schaefer G: Wide cavum septum pellucidum: a marker of disturbed brain development. Pediatr Neurol 1990; 6:391–394Crossref, Medline, Google Scholar
19 Bodensteiner J, Schaefer G, Craft J: Cavum septi pellucidi and cavum vergae in normal and developmentally delayed populations. J Child Neurol 1998; 13:120–121Crossref, Medline, Google Scholar
20 Renier D, Arnaud E, Cinalli G, et al: Prognosis for mental function in Apert's syndrome. J Neurosurg 1996; 85:66–72Crossref, Medline, Google Scholar
21 Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing psychopathology and diagnosis. Arch Gen Psychiatry 1992; 49:615—623Crossref, Medline, Google Scholar
22 Andreasen NC: The Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA, The University of Iowa, 1984Google Scholar
23 Andreasen NC: The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA, The University of Iowa, 1983Google Scholar
24 Wechsler D: Wechsler Adult Intelligence Scale–Revised Manual, New York, The Psychological Corporation, 1981 Google Scholar
25 Andreasen NC, Cohen G, Harris G, et al: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125–133Link, Google Scholar
26 Andreasen NC, Cizadlo T, Harris G, et al: Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 1993; 5:121–130Link, Google Scholar
27 Harris G, Andreasen NC, Cizadlo T, et al: Improving tissue segmentation in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr 1999; 23:144–154Crossref, Medline, Google Scholar
28 Sarwar M: The septum pellucidum: normal and abnormal. Am J Neuroradiol 1989; 10:989–1005Google Scholar
29 Barr M, Kiernan J: The Human Nervous System: An Anatomical Viewpoint. Philadelphia, JB Lippincott, 1988Google Scholar
30 Shaw CM, Alvord EC: Cava septi pellucidi et vergae: their normal and pathological states. Brain 1969; 92:213–224Crossref, Medline, Google Scholar
31 Roberts M, Hanaway J, Morest D: Atlas of the Human Brain in Section, 2nd edition. Philadelphia, Lea and Febiger, 1987Google Scholar
32 Johnson V, Swayze V, Sato Y, et al: Fetal alcohol syndrome: Craniofacial and central nervous system manifestations. Am J Med Genet 1996; 61:329–339Crossref, Medline, Google Scholar
33 Schaefer GB, Bodensteiner JB, Buehler BA, et al: The neuroimaging features of Sotos syndrome. Am J Med Genet 1997; 68:462–465Crossref, Medline, Google Scholar
34 Bruyn G: Agenesis septi pellucidi, cavum septi pellucidi, cavum vergae and cavum veli interpositi, in Handbook of Clinical Neurology, edited by Vinken PF, Bruyn GW. Amsterdam, Elsevier, 1977, pp 299–366Google Scholar



