A Randomized, Double-Blind, Crossover Study of Methylphenidate and Lithium in Adults With Attention-Deficit/Hyperactivity Disorder
Abstract
The authors examined the efficacy of methylphenidate (MPH) and lithium to treat attention-deficit/ hyperactivity disorder (ADHD) in adults, using a randomized, double-blind, crossover design. Patients received 8 weeks of MPH treatment (up to 40 mg/day) and 8 weeks of lithium treatment (up to 1,200 mg/day), by random assignment. Independent evaluators blind to group assignment assessed response every 2 weeks and at the end of each phase. The primary outcome measure was the Conners' Adult ADHD Rating Scale sum score for the clusters of hyperactivity, impulsivity, and learning problems. Secondary outcome measures were scores of irritability, overt aggression, antisocial behavior, anxiety, and depression, and scores on tests of verbal learning and sustained attention. In this preliminary study, lithium and MPH produced similar improvements on the primary outcome measure and on measures of irritability, aggressive outbursts, antisocial behavior, anxiety, and depression.
Between 10% and 60% of children with attention-deficit/hyperactivity disorder (ADHD) may show persistence of this syndrome into adulthood.1 Despite this finding, the efficacy of psychoactive agents in adults with ADHD received far less attention than in children.2 Spencer et al.3 carried out a randomized, 7-week, placebo-controlled, crossover study of methylphenidate (MPH; up to 10 mg/kg per day) in 23 adults with ADHD and found a marked therapeutic response, as defined by a reduction of at least 30% on the Clinical Global Impression, in 78% of patients on MPH. Wilens et al.4 conducted a randomized, 6-week, placebo-controlled, parallel-design study of desipramine (up to 200 mg/day) in 48 adults with ADHD. Patients on desipramine showed a significant improvement in 12 of 14 symptoms of ADHD compared with the placebo group, with a response rate of 68% for the desipramine group and 0% for the placebo group. Spencer and co-workers5 carried out a 7-week, double-blind, placebo-controlled, crossover study of tomoxetine (a highly selective noradrenergic reuptake inhibitor) in 22 adults with ADHD. They found that tomoxetine was superior to placebo, with overall response rates of 53% and 10%, respectively. Wilens and colleagues6 carried out a controlled study of pemoline in 35 adults with ADHD. They found that pemoline was significantly superior to placebo, with response rates of 50% and 17%, respectively. Recently, Paterson et al.7 carried out a randomized, double-blind, placebo-controlled trial of dexamphetamine in a series of 68 adults with ADHD and reported a response rate of 58% for dexamphetamine versus 10% for placebo.
The efficacy of lithium to treat symptoms of ADHD and impulsivity has rarely been examined. Carlson et al.8 assessed the efficacy of MPH and/or lithium in a placebo-controlled study that included 7 children with ADHD and conduct disorder. They found a significant improvement in attention deficits and hyperactivity when lithium and MPH were combined. To our knowledge, the efficacy of lithium to treat adult ADHD has not been empirically examined in a controlled study.
We present a randomized, double-blind, crossover study of MPH and lithium in a series of adult individuals with ADHD. We expected that MPH would be superior to lithium to treat attentional deficits; that MPH and lithium would have similar efficacy to treat hyperactivity; and that lithium would be superior to MPH to treat impulsivity.
METHODS
Patients
A consecutive series of 32 patients who met DSM-IV criteria for ADHD9 were screened for participation in the study. Patients were attending the ADHD Clinic of the Department of Neuropsychiatry at FLENI because of their lifelong histories of inattention and/or hyperactivity. Patients with an IQ of less than 75, a history of substance abuse or alcoholism, or neurological disorders with central nervous system involvement, as well as pregnant or nursing women were excluded from the study. Patients on psychotropic medications underwent a 2-week washout period before entering the study. Our local Institutional Review Board approved the protocol, and all patients gave informed written consent after a full explanation of the study.
Psychiatric Examination
All patients included in the study were examined by a psychiatrist using Spanish-language versions of the following instruments:
Structural Interview for Adult ADHD:10 A questionnaire designed to assess life history, past psychiatric and medical history, developmental milestones, sexual development, medications, family history of ADHD, and social functioning. It also includes the Self-Rating Symptom Checklist and Physical Complaints Checklist for conducting a quick screening of psychiatric and physical symptoms. This interview was carried out with the patient and one or more close relatives (usually a sibling and/or a parent).
Conners' Adult ADHD Rating Scale:10 A 93-item interviewer-rated scale that assesses symptoms of ADHD in adults. There are four possible answers for each question (not at all, just a little, pretty much, or very much), and item scores are subsumed into the following seven behavioral clusters: Conduct Disorder, Anxiety, Restlessness, Learning Problems, Antisocial Behavior, Hyperactivity, and Impulsivity.
Structured Clinical Interview for DSM-IV (SCID):11 A semistructured diagnostic interview for making the major Axis I DSM-IV diagnoses.
Hamilton Rating Scale for Depression (Ham-D):12 A 17-item interviewer-rated scale that measures psychological and autonomic symptoms of depression.
Hamilton Anxiety Rating Scale (Ham-A):13 An 11-item interviewer-rated scale that measures the severity of generalized or persistent anxiety.
Overt Aggression Scale (OAS):14 A measure of specific aspects of aggressive behavior based on observable criteria. Aggressive behaviors are divided into four categories: verbal aggression, and physical aggression against objects, self, and others.
Irritability Scale:15 A 14-item scale that is rated by a patient's relative. Scores range from 0 to 42; higher scores indicate more severe irritability.
Following the diagnostic scheme of Wilens et al.,4 a diagnosis of ADHD was made whenever patients 1) met DSM-IV criteria for ADHD both at age 7 years (this information was always checked with parents or siblings) and at the present evaluation; 2) described a chronic course of ADHD symptoms; and 3) endorsed moderate or severe impairment associated with the disorder.
Neuropsychological Examination
Blind to the psychiatric data, a comprehensive cognitive evaluation was carried out by a neuropsychologist using Spanish-language versions of the following:
Buschke Selective Reminding Test (SRT):16 Measures verbal learning and memory during a 10-trial list-learning task.
Wechsler Adult Intelligence Scale (WAIS):17 Measures general intellectual function.
Wechsler Memory Scale–Revised (WMS-R):18 Measures various aspects of memory function, such as verbal memory, visual memory, general memory, attention and concentration, and delayed recall.
Continuous Performance Test (CPT):19 This task measures attention deficits and impulsiveness. Patients are required to press a key only when a target letter (e.g., X) follows another two letters (e.g., E followed by A).
Study Design
This was a randomized, double-blind, crossover-design, 18-week study comparing lithium and MPH. Patients were withdrawn from any psychoactive medication for at least 2 weeks before the baseline evaluation. After baseline completion, patients were randomly assigned to lithium/MPH or MPH/lithium arms. Patients stayed 8 weeks on the first drug, followed by a 2-week washout period and 8 weeks on the second drug. Target dosages were 10 mg of MPH or 300 mg of lithium during weeks 1 and 2; 20 mg of MPH and 600 mg of lithium during weeks 3 and 4, 30 mg of MPH and 900 mg of lithium during weeks 5 and 6, and 40 mg of MPH and 1,200 mg of lithium during weeks 7 and 8. Because lithium levels may vary considerably among subjects on the same dose, a better strategy would have been to aim at specific lithium serum concentrations instead of using fixed doses. On the other hand, that procedure is logistically complicated and could have endangered the blindness of the study. Weekly supplies of MPH or lithium were dispensed in identical-appearing 10 mg and 300 mg capsules, respectively. Capsules were taken once a day (with breakfast) during the initial week or twice a day (with breakfast and lunch) during the remaining weeks of the trial.
All patients received biweekly evaluations with the following instruments: Conners' Adult ADHD Rating Scale, Ham-D, Ham-A, OAS, Buschke SRT, and CPT. Compliance was monitored by pill counts at each visit. Serum samples were drawn for lithium levels at each follow-up visit, whereas blood drawn for MPH levels was stored but not analyzed. All blood samples were obtained between 8:00 a.m. and 10:00 a.m. in a fasting state.
Statistical Analysis
The primary efficacy measure of the study was the Conners' Adult ADHD Rating Scale sum score for the clusters of Hyperactivity, Impulsivity, and Learning Problems. Secondary efficacy measures were the Conners' Adult ADHD Rating Scale score clusters of Conduct Disorder, Restlessness, and Antisocial Behavior, and scores of depression (Ham-D), anxiety (Ham-A), overt aggression (OAS), irritability, attention (CPT), and verbal learning (Buschke SRT: total and delayed recall). Following the scheme of Spencer et al.,3 improvement in ADHD was defined as a reduction of greater than 30% in the Conners' Adult ADHD Rating Scale sum score of learning problems, hyperactivity, and impulsivity. Sample size estimation was based on Spencer and colleagues' finding that a sample of 23 patients was sufficient to detect a drug benefit at the P<0.001 level with power=0.80. Statistical analysis was carried out with repeated-measures analysis of variance (ANOVA) and post hoc t-tests, and included all patients randomized into the study with at least one postrandomized evaluation (intention to treat [ITT] analysis) with the last observation carried forward (LOCF). Factors for the repeated-measures ANOVA were sequence (MPH/ lithium or lithium/MPH), arm (first vs. second), and time (five evaluations). A significant sequence×arm interaction would indicate a significant overall drug effect, whereas a significant triple interaction would indicate an effect for a specific drug over time. Frequency distributions were calculated with a chi-square test and a Yates' correction for expected cell sizes less than 5. All P-values are two-tailed. Means are reported with standard deviations.
RESULTS
Demographic Findings
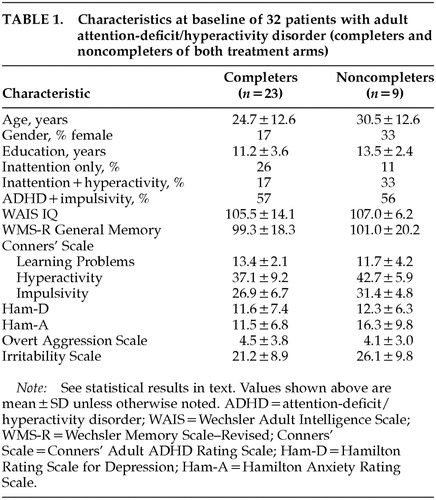
Four patients (2 on lithium and 2 on MPH) did not complete the first and second treatment arms, and 5 patients (3 on MPH and 2 on lithium) did not complete the second arm. Twenty-three patients completed both arms. They were 19 men and 4 women, with a mean age of 24.7±12.6 years (range 18–60), a mean education level of 11.2±3.6 years (range 7–19), a WAIS Full Scale IQ score of 105±14.1 (range 81–130), and a WMS-R Memory Quotient score of 99.3±18.3 (range 67–131). Twenty-two percent of the sample met DSM-IV criteria for inattention only; 15% had hyperactivity and impulsivity without inattention; and the remaining 63% had the full syndrome. Table 1 shows background information for both completers and noncompleters. There were no significant differences in age, education, full scale IQ, or Ham-D, Ham-A, or OAS scores between completers and dropouts. On the other hand, dropouts had significantly higher Conners' Adult ADHD Rating Scale scores in the clusters of Conduct Disorder (P<0.05), and Hyperactivity (P<0.05) compared with completers.
Eleven of the 32 patients (34%) had at least one lifetime comorbid psychiatric disorder; 8 had a major depressive syndrome, 2 had social phobia, and 1 had a history of panic attacks. Twenty of the 32 patients (63%) had a positive history of ADHD in first-degree relatives. Despite average intelligence, school problems were reported by 20 of the 32 patients (63%); 14 patients (44%) failed at least one grade, 2 (6%) were placed in special classes, and 4 (13%) were expelled from school. A history of serious working difficulties (e.g., frequent job changes or disciplinary measures) was positive for 80% of the sample. This information was always checked with parents and/or siblings.
Primary Efficacy Measures
Average daily doses of MPH (mg) for the 23 completers were the following: weeks 1–2, 10±0.0; weeks 3–4, 19.5±1.4; weeks 5–6, 38.9±5.2; and weeks 7–8, 38.9±5.2. Average daily doses of lithium carbonate (mg) and corresponding plasma lithium levels (mEq) were the following: weeks 1–2, 600±0.0 and 0.43±0.15, respectively; weeks 3–4, 886±62.5 and 0.50±0.18; weeks 5–6, 1,186±62.5 and 0.65±0.21; and weeks 7–8, 1,173±125 mg and 0.68±0.25. Final doses for both drugs did not match planned doses because some patients could not tolerate the targeted final dose.
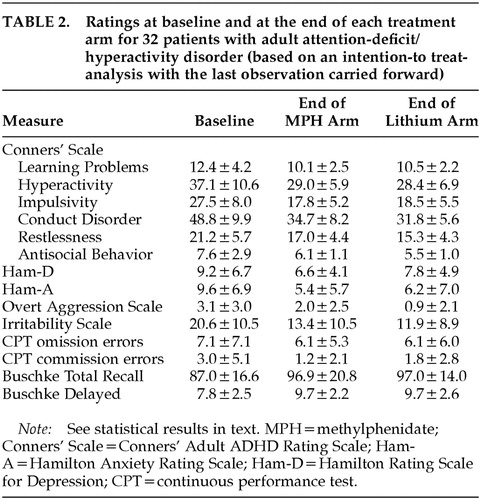
Mean ratings on all measures at baseline and at the end of each treatment arm are displayed in Table 2. Changes over time in Conners' Adult ADHD Rating Scale cluster scores are shown in Figure 1 for the Learning Problems cluster, Figure 2 for the Hyperactivity cluster, and Figure 3 for the Impulsivity cluster.
The rate of improvement, defined as 30% or greater reduction in the Conners' Adult ADHD Rating Scale sum score of Learning Problems, Hyperactivity, and Impulsivity compared with the first baseline evaluation, was 48% for MPH and 37% for lithium (95% confidence interval [CI], –12% to 34% for the observed difference between MPH and lithium). For completers, the rate of improvement was 47% for MPH and 43% for lithium (95% CI, –23% to 31% for the observed difference between MPH and lithium). Three of the 10 patients who were nonresponders to lithium during the first arm did respond to MPH during the second arm, but none of the 9 nonresponders to MPH during the first arm had a positive response to lithium during the second arm. There were no significant differences in lithium serum levels between lithium responders and nonresponders (mEq/l [mean±SD]=0.69±0.39 and 0.65±0.20, respectively).
A three-way repeated-measures ANOVA (Factor 1: sequence [MPH/lithium vs. lithium/MPH]; Repeated measures: arm [first vs. second] and time [5 evaluations]) for all 32 patients randomized into the study (ITT analysis with LOCF) was calculated for each of the three primary outcome measures. The Hyperactivity cluster of the Conners' Adult ADHD Rating Scale showed a significant arm effect (F=15.3, df=1,28, P<0.0001), with a significantly greater improvement during the first compared with the second arm; a significant time effect (F=13.1, df=4,112, P<0.0001), with a significant improvement over time for both arms; no significant effects for sequence (F=1.06, df=1,28, not significant) or sequence×arm interaction (F=0.32, df=1,28, not significant), with MPH and lithium showing similar efficacy; and no significant sequence×arm×time interaction (F=1,75, df=4,112, not significant), with MPH and lithium showing similar magnitudes of improvement over time. Similar results were found for the Impulsivity and Learning Disorders clusters of the Conners' Adult ADHD Rating Scale (Impulsivity: arm effect, F=6.83, df=1,28, P<0.05; time effect, F=22.4, df=4,112, P<0.0001; Learning Disorders: arm effect, F=9.72, df=1,28, P<0.01; time effect, F=13.1, df=4,112, P<0.01). The remaining main effects and interactions were not significant.
Hyperactivity and Impulsivity scores at the baseline evaluation for the second arm were significantly lower than scores for the initial baseline evaluation (Hyperactivity: first baseline score [mean±SD]=36.7±8.7, and second baseline score=31.6±7.7; t=3.23, df=28, P<0.001; Impulsivity: 27.4±6.8 and 22.7±6.7, respectively; t=3.71, df=28, P<0.001). There were no significant changes in scores of Learning Problems and Hyperactivity between the last evaluation of the first arm and the baseline evaluation for the second arm (Learning Problems: t=1.47, df=28, not significant; Hyperactivity: t=1.32, df=28, not significant), but scores of Impulsivity had a significant increment during this interval (t=2.57, df=28, P<0.05).
To examine whether AD symptoms reemerged during the washout period, we compared scores on the ADHD clusters for the baseline and the washout evaluations. Patients on MPH had significantly lower scores on the washout as compared to the baseline evaluation for the clusters of Learning Disorders (t=2.28, df=9, P<0.05), Hyperactivity (t=5.93, df=9, P<0.001), and Impulsivity (t=7.91, df=9, P<0.0001). Patients on lithium had significantly lower scores on the washout compared with the baseline evaluation for the cluster of Impulsivity (t=2.83, df=9, P<0.05), but no differences were found for the clusters of Learning Disorders (t=0.43, df=9, not significant) and Hyperactivity (t=1.87, df=9, not significant).
Secondary Efficacy Measures
The Conduct Disorder cluster of the Conners' Adult ADHD Rating Scale showed a significant arm effect (F=11.7, df=1,28, P<0.01), and a significant time effect (F=27.9, df=4,112, P<0.0001), and there were similar results for the Restlessness (arm, F=11.1, df=1,28, P<0.01; time, F=14.0, df=4,112, P<0.0001) and Antisocial Behavior clusters (arm, F=17.4, df=1,28, P<0.0001; time, F=12.1, df=4,112, P<0.0001).
Statistical analysis of the Irritability scale demonstrated significant arm (F=5.47, df=1,28, P<0.05) and time effects (F=12.0, df=4,112, P<0.0001), and similar results were found for the Ham-A (arm, F=5.88, df=1,28, P<0.05; time, F=11.7, df=4,112, P<0.0001). There was only a time effect for both the Ham-D (F=5.45, df=4,112, P<0.001) and the Overt Aggression Scale (F=5.73, df=4,112, P<0.001). No other significant main effects or interactions were found.
Neuropsychological Outcomes
A three-way ANOVA with repeated measures for the Buschke SRT showed a significant time effect for both the total recall (F=4.90, df=4,112, P<0.01) and the delayed recall sections (F=3.13, df=4,112, P<0.05): there was a significant improvement in verbal memory over time with both lithium and MPH. On the other hand, there were no significant main effects or interactions for either omission or commission errors on the CPT.
Dropouts and Side Effects
As noted above, 9 patients dropped out from the study, 4 of them because of side effects (3 on MPH and 1 on lithium). The 3 patients who dropped out while on MPH reported nausea and weight loss (1 patient), palpitations (1 patient), and skin rash (1 patient), whereas the patient who dropped out while on lithium reported motor slowness. Four patients dropped out because of lack of perceived benefit, and 1 patient had to move to another country. The rate of side effects was the following: headaches: lithium 21%, MPH 4%; diarrhea: lithium 11%, MPH 11%; nausea: lithium 4%; chest discomfort: lithium 3%; orthostatic hypotension: MPH 3%.
DISCUSSION
We examined the efficacy of MPH and lithium to treat a consecutive series of adults with ADHD by using a randomized, double-blind, crossover design. The main finding of this preliminary study was that MPH and lithium produced significant improvements of similar magnitudes on the ADHD clusters of Hyperactivity, Impulsivity, and Learning Disorders. Both drugs also produced significant improvements on other behavioral problems frequently associated with ADHD, such as irritability, aggressive outbursts, antisocial behavior, anxiety, and depression.
Before commenting further, we should point out several limitations of our study. First, the interpretation of crossover studies may be complicated by carryover effects. Main outcome measures in our study demonstrated a significant arm effect, which resulted from improvements during the first arm that were maintained during the second arm. On the other hand, there were no significant sequence by arm interactions for any of the primary or secondary outcome measures, suggesting that MPH and lithium had similar efficacy. However, the observed difference in efficacy between MPH and lithium had wide CI confidence intervals and a low power to detect true differences. The ultimate difference in the efficacy between these compounds will have to be examined in studies with a larger sample. The second limitation is that our study did not include a placebo arm, and thus the ultimate efficacy of lithium in adult ADHD is difficult to determine. However, the relative efficacy of MPH to treat adult ADHD as compared with placebo has been repeatedly demonstrated, and the present study showed no differences in primary or secondary outcome measures between MPH and lithium. Another limitation is that we did not measure serum levels of MPH. However, Spencer et al.3 could not find significant correlations between MPH serum levels and clinical response or side effects in adults with ADHD. Drug titration was rather slow and subjects reached maximum doses during the last two weeks of treatment. This strategy was selected to detect potential positive changes at low doses.
Our present findings confirm the efficacy of MPH in the treatment of adult ADHD as demonstrated in other controlled studies. Efficacy (as defined by a reduction of at least 30% in the severity of ADHD symptoms) was 48% in our study, which is similar to the 57% efficacy reported by Wender et al.20 but lower than the 78% efficacy reported by Spencer et al.3 Our study also expands previous findings demonstrating significant improvement in behavioral domains such as irritability, aggression, anxiety, and depression—which, although not part of the ADHD cluster, are nonetheless frequently reported in adult ADHD. To our knowledge this is the first study to show the usefulness of lithium to treat adult ADHD. Although lithium had a somewhat lower overall efficacy than MPH (37% and 48% respectively), it produced similar improvements on scores of anxiety, depression, overt aggression, and anterograde verbal memory. Because our study did not include a placebo arm, whether the memory-related finding is a genuine effect of the medication or is due to a practice effect could not be ascertained. The prevalence and severity of side effects were similar for lithium and MPH.
A question that arises is whether the present findings may help to increase understanding of the mechanism of ADHD in adults. Several studies have reported high rates of antisocial personality disorder and substance use disorders and higher lifetime rates of oppositional disorder in adults with ADHD.21 Some of these disorders were reported to improve upon treatment with lithium,8 which may partially explain the improvement observed with lithium in the present study. Carlson21 recently reviewed a series of studies in children with mania, demonstrating a high overlap with ADHD because both disorders may feature impulsivity, inability to delay gratification, irritability, and overt aggressive episodes.22–24 In a study that included children with both manic and ADHD symptoms, Carlson and co-workers8 demonstrated that children's inattention and hyperactivity responded better to combined low-dose MPH and lithium than to either high-dose MPH or lithium alone. Malone et al.25 recently demonstrated the efficacy of lithium to treat aggressive children and adolescents with conduct disorder, a frequent comorbid condition of ADHD.
In summary, our preliminary study demonstrated that MPH and lithium have a similar efficacy to treat the cardinal symptoms of adult ADHD. Improvement was also found in other behavioral domains related to ADHD, such as irritability, aggression, anxiety, and depression. The question arises of which drug should be preferred to treat adult ADHD. Although the two drugs had a comparable overall efficacy and rate of side effects, MPH may be considered a better alternative because it does not require periodic analysis of blood levels.
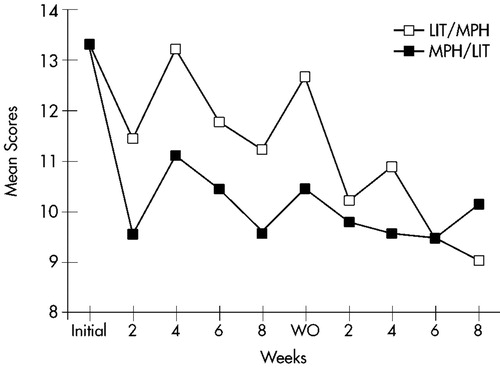
FIGURE 1. Learning Problems. Mean score changes over time for completers of both treatment arms (n=23) on the Learning Problems cluster of the Conners' Adult ADHD Rating Scale. LIT=lithium; MPH=methylphenidate; WO=washout.
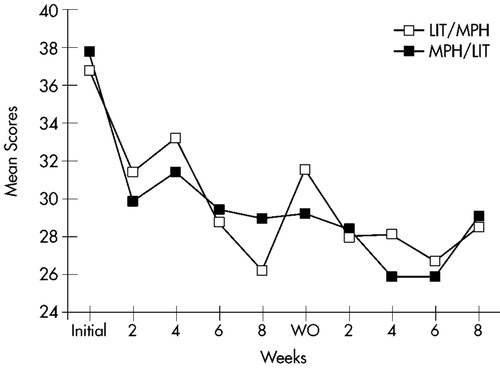
FIGURE 2. Hyperactivity. Mean score changes over time for completers of both treatment arms (n=23) on the Hyperactivity cluster of the Conners' Adult ADHD Rating Scale. LIT=lithium; MPH=methylphenidate; WO=washout.
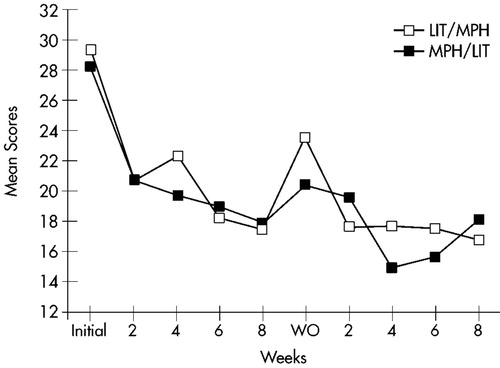
FIGURE 3. Impulsivity. Mean score changes over time for completers of both treatment arms (n=23) on the Impulsivity cluster of the Conners' Adult ADHD Rating Scale. LIT=lithium; MPH=methylphenidate; WO=washout.
 |
 |
1 Biederman J: Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry 1998; 59:4-16Crossref, Medline, Google Scholar
2 Wender PH: Pharmacotherapy of attention deficit/hyperactivity disorder in adults. J Clin Psychiatry 1998; 59(suppl 7):76-79Google Scholar
3 Spencer TJ, Wilens TE, Biederman J: A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1995; 52:434-443Crossref, Medline, Google Scholar
4 Wilens T, Biederman J, Prince J, et al: A double-blind, placebo-controlled trial of desipramine for adults with ADHD. Am J Psychiatry 1996; 153:1147-1153Crossref, Medline, Google Scholar
5 Spencer T, Biederman J, Wilens T, et al: Tolerability and efficacy of tomoxetine for adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998; 155:693-695Crossref, Medline, Google Scholar
6 Wilens T, Biederman J, Spencer T, et al: Controlled trial of high doses of pemoline for adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 1999; 19:257-264Crossref, Medline, Google Scholar
7 Paterson R, Douglas CH, Hallmayer J, et al: A randomised, double-blind, placebo-controlled trial of dexamphetamine in adults with attention deficit hyperactivity disorder. Aust NZ J Psychiatry 1999; 33:494-502Crossref, Medline, Google Scholar
8 Carlson GA, Rapport MD, Kelly KL, et al: The effects of methylphenidate and lithium on attention and activity level. J Am Acad Child Adolesc Psychiatry 1992; 31:262-270Crossref, Medline, Google Scholar
9 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
10 Barkley RA: Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford, 1990Google Scholar
11 Spitzer RL, Williams JBW, Gibbon M, et al: The Structured Clinical Interview for DSM-III-R (SCID I): history, rationale, and description. Arch Gen Psychiatry 1992; 49:624-629Crossref, Medline, Google Scholar
12 Hamilton MA: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
13 Hamilton MA: The assessment of anxiety states by rating. British Journal of Medical Psychology 1959; 32:50-55Crossref, Medline, Google Scholar
14 Yudofsky SC, Silver JM, Hales RE: Treatment of aggressive disorders, in Textbook of Psychopharmacology, edited by Shatzberg AF, Nemeroff CB. Washington, DC, American Psychiatric Press, 1995Google Scholar
15 Starkstein SE, Migliorelli R, Tesón A, et al: Prevalence and correlates of apathy and irritability in Alzheimer's disease. Eur J Neurol 1995; 2:1-7Crossref, Google Scholar
16 Buschke H, Fuld PA: Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974; 24:1019-1025Crossref, Medline, Google Scholar
17 Wechsler D: Wechsler Adult Intelligence Scale (WAIS). New York, The Psychological Corporation, 1955Google Scholar
18 Wechsler D: Wechsler Memory Scale-Revised (WMS-R). New York, The Psychological Corporation, Harcourt Brace Jovanovich, 1987Google Scholar
19 Rosvold ME, Mirsky AF, Sarason I, et al: A continuous performance test of brain damage. J Consult Psychol 1956; 20:343-350Crossref, Medline, Google Scholar
20 Wender PH, Reimher FW, Wood D, et al: A controlled study of methylphenidate in the treatment of attention deficit disorder, residual type in adults. Am J Psychiatry 1985; 142:547-552Crossref, Medline, Google Scholar
21 Carlson GA: Mania and ADHD: comorbidity or confusion. J Affect Disord 1998; 51:177-187Crossref, Medline, Google Scholar
22 Faraone SV, Biederman J, Wozniak J, et al: Is comorbidity a marker for juvenile onset mania? J Am Acad Child Adolesc Psychiatry 1997; 36:1046-1055Crossref, Medline, Google Scholar
23 Biederman J, Russell R, Soriano J, et al: Clinical features of children with both ADHD and mania: does ascertainment source make a difference? J Affect Disord 1998; 51:101-112Crossref, Medline, Google Scholar
24 Heath J, Warner K: Lithium for prepubertal depressed children with family history predictors of future bipolarity: a double-blind, placebo-controlled study. J Affect Disord 1998; 51:165-175Crossref, Medline, Google Scholar
25 Malone RP, Delaney MA, Luebbert JF, et al: A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Neurol 2000; 57:649-654Google Scholar



