A Review of Cholinergic Agents in the Treatment of Neurobehavioral Deficits Following Traumatic Brain Injury
Abstract
Although traumatic brain injury (TBI) frequently results in significant handicap, empirical investigations of pharmacological treatment of the neurobehavioral sequelae of TBI are rare. This review presents evidence that supports hypotheses of a cholinergic mechanism underlying some neurobehavioral sequelae of TBI, as well as a critical review of the preliminary evidence supporting the efficacy of cholinergic agents in TBI. Despite numerous methodological limitations, preliminary evidence exists for the efficacy of cholinergic agents in ameliorating attention and memory deficits following TBI. The authors highlight the need for large, randomized, double-blind, placebo-controlled trials that include a broad range of cognitive and behavioral outcome measures.
Traumatic brain injury (TBI) is an important health problem and has been widely reported to be one of the leading causes of death among young adults.1,2 TBI affects approximately 1.5 to 2 million people in the United States each year, with an estimated 70,000 to 90,000 cases resulting in significant functional impairment.3 In cases where TBI does not result in death, TBI survivors frequently sustain a variety of physical, neuropsychological, and emotional/behavioral impairments. Further, impairment in cognitive functioning has specifically been linked to poor long-term outcome in the areas of vocational functioning,4–6 independent living,7,8 and community integration.9 Young people, particularly young men between the ages of 15 and 24, are the most common victims of TBI and are thus faced with the possibility of a lifetime of disability and handicap.10
Treatment of the cognitive and behavioral impairments associated with TBI should result in decreased handicap, improved quality of life, and decreased societal impact. Attempts at ameliorating cognitive deficits following TBI have widely focused on neurocognitive rehabilitation. Although neurocognitive rehabilitation appears to be helpful from a clinical perspective, empirical investigations to date have failed to provide conclusive evidence of efficacy in improving outcome after TBI.3,11 Further, there are no presently established pharmacological strategies to address these outcomes in TBI. Given the lack of conclusive evidence regarding the efficacy of cognitive rehabilitation of TBI, and the lack of current pharmacological strategies to improve cognition, behavior, and quality of life in patients with TBI, investigation of new intervention strategies is clearly needed.
In this review, we present evidence for the potential benefit of cholinergic agents in treating cognitive, and possibly behavioral, impairment following TBI. We first review evidence underlying hypotheses regarding the possible efficacy of cholinergic agents in TBI and then review the literature investigating the use of cholinergic agents in TBI populations. Finally, we present recommendations regarding the types of outcome measures that should be included in clinical trials of cholinergic agents in TBI.
CHOLINERGIC AGENTS
A number of agents operating on the cholinergic system have been investigated as they relate to cognition and behavior in individuals with central nervous system disease, including Alzheimer's disease (AD) and TBI. Physostigmine, tacrine, and donepezil are acetylcholinesterase inhibitors that temporarily disrupt the hydrolysis of acetylcholine, thus temporarily increasing concentrations of acetylcholine in the brain. Both physostigmine and tacrine have problematic side effect profiles (cardiovascular and autonomic for physostigmine, gastrointestinal and hepatic for tacrine). Donepezil has recently been approved for the treatment of AD in the United States and Canada and has been relatively well tolerated, with low risk of liver toxicity. Lecithin and CDP-choline (or citicoline) are choline precursors that also work to enhance cholinergic activity in the brain.
THE CHOLINERGIC SYSTEM, AD, AND TBI
Support for the use of cholinergic agents in TBI comes from a number of sources: 1) growing understanding of the efficacy of cholinesterase inhibitors in AD; 2) growing knowledge of the effects of TBI on the cholinergic system in the brain; and 3) growing understanding of neuropathological and neurocognitive similarities between AD and TBI.
The Cholinergic System and AD
Acetylcholine has received significant attention in the literature on AD as a proposed mechanism for the cognitive impairments observed in this disorder. Individuals with AD demonstrate impairments in sustained and divided attention and memory for new material.12 These impairments are similar to those observed in control subjects who are administered cholinergic antagonists such as scopolamine, suggesting that these cognitive deficits are specifically related to cholinergic dysfunction. Indeed, studies have consistently demonstrated that the degree of hippocampal and cortical cholinergic dysfunction in the brains of AD patients is associated with the severity of dementia.13,14 Various cholinergic agents have been shown to improve the cognitive functioning of patients with AD.14 Two clinical trials have demonstrated that 5 mg or 10 mg of donepezil daily improves cognition in AD patients.15,16 A recent review of memory-enhancing drugs in AD17 found that measures of verbal rehearsal and verbal episodic memory most consistently responded to manipulation of the cholinergic system in patients with AD.
The Cholinergic System and TBI
There is clear evidence that disruption of the cholinergic system following traumatic injury to the brain results in cognitive deficits that affect outcome in individuals with TBI. Evidence suggests that an initial period of hypercholinergic activity in the brain following TBI18 is followed by a more chronic state of hypocholinergic activity. Evidence from both animal and human studies has demonstrated that TBI produces chronic changes in cholinergic function in the brain.19–21 Indeed, because of the structure of the skull cavity, TBI frequently results in injury to acetylcholine-rich hippocampal regions responsible for short-term memory formation.22 Postmortem examination of individuals who died as the result of a blunt head injury has revealed decreased cholinergic activity in bilateral temporal, cingulate, and parietal cortical areas.20
Further investigation has linked specific cognitive deficits to acetylcholinergic dysfunction. Impairments in focused and sustained attention, memory (e.g., acquiring new information for recall), and executive functioning are consistently reported by individuals with traumatic brain injury, their relatives, and their health care providers.6,22,23 As noted earlier, acetylcholine is abundant in the hippocampus, particularly in regions responsible for attentional filtering of incoming stimuli and short-term memory formation.22 Hippocampal and frontal cortical cholinergic systems are believed to play a key role in attention, learning, storage, and retrieval of new information,13,14 and cholinergic dysfunction is believed to play a key role in memory impairment following TBI.20 Animal studies have demonstrated that disruption in cholinergic neurotransmission results in deficits on tasks of motor and spatial memory in rats,21 while increasing cholinergic transmission attenuates cognitive deficits in rats with experimentally induced brain injury.24–26
Research with anticholinergic agents in humans (e.g., scopolamine) has demonstrated that disruption of the cholinergic system results in deficits in attention and memory that mimic those observed following TBI. In particular, scopolamine has been demonstrated to induce impairments in sustained and selective attention,27 recall of recent events, and performance on measures of verbal recall and recognition memory (i.e., word lists).13,28–30
Parallels Between TBI and AD
Investigation of pathophysiology and impairment profiles in AD reveals a number of similarities to those observed in TBI. Levin and Goldstein31 found that patients with a history of severe TBI had problems with actively organizing verbal information to be recalled; this finding is consistent with observations made in degenerative dementia.32 Further, postmortem examination of brain pathology has revealed pathophysiological similarities between AD and TBI.20 Specifically, there is evidence of cholinergic dysfunction in frontal and temporal cortical regions of individuals with TBI and AD.
Lawrence and Sahakian12 suggest that many of the cognitive impairments observed in patients with AD are primarily the result of damage to the basal forebrain cholinergic system, which projects to the prefrontal cortex and serves to “increase attentiveness to behaviorally relevant stimuli” (p. 47). In an experimental attempt to dissociate the neurochemical substrates of memory systems, Nissen et al.29 concluded that the cholinergic system plays a specific role in the storage of new information in long-term memory, rather than retrieval of this memory information. This finding parallels what is known about AD, specifically, that decreased cholinergic function in this disease results in relatively greater deficits in sustained attention and new learning of declarative information, with relative sparing of old recall and procedural memory. Since the type of memory impairment observed following TBI is similar to that observed in AD, it follows that memory deficits in TBI might be responsive to cholinergic treatment, as well. Indeed, the Rey Auditory Verbal Learning Test (RAVLT), a measure of verbal episodic memory on which performance has consistently been shown to improve in AD patients administered acetylcholinesterase inhibitors,17 has also shown improvement in patients with TBI who are administered donepezil.33 Findings such as these have led to preliminary efforts at examining the efficacy of cholinergic treatment strategies in TBI.
ACETYLCHOLINERGIC TREATMENT OF COGNITIVE IMPAIRMENT IN TBI
Methodology of the Literature Review
MEDLINE and PsycINFO listings for English-language journals were searched for the following terms: brain injury OR head injury AND cholinergic, choline, citicoline, donepezil, tacrine, physostigmine, and lecithin.
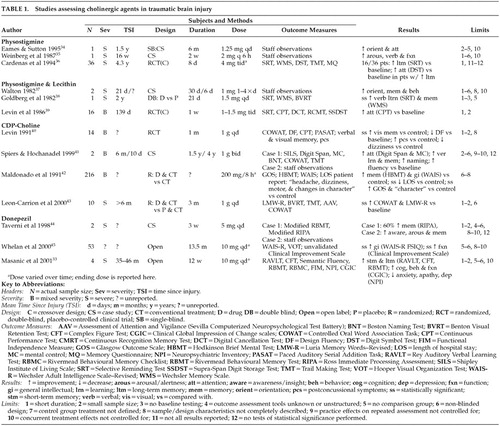
Our literature review revealed a total of 13 articles presenting original data related to the use of cholinergic agents to address cognitive impairments following TBI. These reports range from case studies to randomized, placebo-controlled designs. Table 1 presents a summary of these studies by drug type and highlights the limitations of each study.
Physostigmine
Two case studies have reported beneficial effects of physostigmine in patients with a history of severe TBI. Eames and Sutton34 described a 57-year-old male with a history of TBI who was administered physostigmine intravenously on 15 occasions over the course of 2 months. No objective assessment was conducted, but the authors reported that staff who were blind to the treatment noted reduced confusion, both immediately after the injections and progressively over the course of the treatment period. Further treatment by daily injections of physostigmine for 7 consecutive days was reported to result in improved cognition and language abilities. Finally, weekly intramuscular physostigmine injections over a 6-month period were noted to result in improvements in orientation, cognition, memory, and learning, as well as a decrease in confusion and paranoid ideation.
Weinberg et al.35 described a 33-year-old male with a history of TBI who was administered intramuscular physostigmine, combined with methylphenidate, every 6 hours for a period of 2 weeks. Although no objective assessment was conducted apart from Glasgow Coma Scale (GCS) scores, the authors reported that the patient demonstrated improvements in alertness, ability to follow commands, participation in activities of daily living (ADLs), and verbalization for up to 2 hours following each injection. The patient's GCS score was noted to increase from 12 to 14 over the course of the 2-week treatment. In both the Eames and Sutton and the Weinberg et al. cases, discontinuation of physostigmine treatment resulted in deterioration of function to pre-treatment levels. Although neither group used objective assessment techniques or controlled for spontaneous recovery, placebo effects, or the effects of concurrent treatment, these case reports present anecdotal evidence of the possible benefit of cholinergic agents in ameliorating cognitive and behavioral impairments following TBI.
Cardenas et al.36 conducted a double-blind, placebo-controlled, crossover investigation of oral physostigmine in a group of 36 men with a history of TBI. Subjects were tested on a series of neuropsychological measures at baseline, immediately after each 8-day treatment phase, and at 1-month follow-up (see Table 1 for measures administered). Physostigmine treatment resulted in improvements in delayed recall, storage, and retrieval of verbal information on the Selective Reminding Test (SRT) in 44% of patients; “improvement” was defined as a 50% increase on the long-term storage or the long-term retrieval scores of the SRT. In addition, in the subgroup of patients who demonstrated improved memory in response to physostigmine, improvement in divided attentional skills on the Digit Symbol Test was also noted. Results of performance on other objective measures (i.e., Wechsler Memory Scale [WMS] and Trail Making Test [TMT]) were not reported by the authors. Performance on the SRT returned to pre-drug levels on follow-up.
Overall, these preliminary findings suggest a potential beneficial effect of physostigmine in ameliorating arousal and memory disturbance in some individuals with a history of TBI. However, the short half-life of the drug combined with the apparent failure to induce sustainable cognitive improvement presents a possible disadvantage for use in long-term treatment of individuals with TBI.
Physostigmine and Lecithin
The utility of physostigmine combined with lecithin, a choline precursor, has also been investigated in a variety of reports. Walton37 described two clinical cases in which intramuscular physostigmine injections were combined with lecithin to treat postconcussive symptoms following TBI. No objective outcome assessment was conducted with either patient. A 34-year old female patient with a history of severe TBI was noted to show clinical improvements 48 hours after commencement of treatment. Specifically, improvements in orientation, short-term memory, and decreased aggression were noted and were reported to progress over the next 14 days of treatment. This patient's functioning returned to pre-treatment levels once physostigmine and lecithin were stopped. A 23-year-old male patient who began treatment with physostigmine and lecithin four times daily, immediately following admission to the hospital with symptoms of confusion and confabulation, was noted to demonstrate improvements in behavior and orientation within 72 hours of initiation of treatment. No follow-up information was presented on this patient. Again, these case reports did not provide objective assessment results and did not control for spontaneous recovery, placebo effects, or the effects of concurrent treatment; however, they provide additional anecdotal evidence of the potential role of cholinergic agents in treating sequelae of TBI.
Goldberg et al.38 presented the results of a double-blind, placebo crossover investigation of oral physostigmine and lecithin in a 36-year-old man with a history of severe TBI. The patient was presented alternately with treatment or placebo three times daily over four 3-day periods, with neuropsychological assessment following each treatment/placebo administration (see Table 1 for measures administered). Statistically significant improvements in overall memory performance on the Wechsler Memory Scale and verbal memory storage and retrieval on the SRT were noted during the treatment phase, when compared with the placebo phase. No follow-up assessment was performed to assess the long-term effects of treatment.
Levin et al.39 conducted a double-blind, placebo-controlled, crossover investigation of physostigmine and lecithin in 16 male patients with a history of moderate to severe TBI. Subjects were assessed at baseline, after 1 week of treatment with lecithin and oral physostigmine or placebo, and after a 1-week washout period (see Table 1 for measures administered). Results indicated that patients who were treated with physostigmine first demonstrated improvement in sustained attention on the Continuous Performance Test (CPT), when compared with patients treated with placebo first; this improvement was maintained during the washout phase.
Taken together, these findings present further evidence for a possible beneficial effect of acetylcholinesterase inhibitors, combined with choline precursors, in ameliorating cognition and behavior in some individuals with a history of TBI. Again, the improvement appears to be transitory.
CDP-Choline
Cytidine diphosphoryl choline (CDP-choline or citicoline), a choline precursor, has been reported to ameliorate certain sequelae of TBI in several studies. Levin40 conducted a randomized, double-blind, placebo-controlled study of CDP-choline in 14 patients with a history of TBI. After emerging from posttraumatic amnesia, patients were administered either oral CDP-choline or placebo for 1 month. Neuropsychological assessment was conducted at baseline and following 1 month of treatment (see Table 1 for measures administered). Patients were also administered a structured interview related to postconcussional symptoms. Results indicated that, compared with placebo, patients treated with CDP-choline experienced greater improvement in visual recognition memory from baseline levels. Further, compared with placebo, patients in the CDP-choline group reported fewer postconcussional symptoms after treatment, including less headache, dizziness, and tinnitus. No follow-up assessment was conducted to ascertain whether the improvement in visual memory or postconcussional symptoms was sustained following termination of treatment.
Spiers and Hochanadel41 described two cases in which CDP-choline treatment appeared to facilitate cognitive improvement following TBI. A 39-year-old female was treated with CDP-choline for 1.5 years following a moderate TBI. Testing was performed at 6 months and 2 years after injury (see Table 1 for measures administered). The patient was noted to demonstrate improvement on tasks of verbal attentional span, mental control, word fluency, and confrontation naming. While no baseline testing of verbal or visual memory was conducted, the patient's 2-year follow-up performance fell in the superior range on the California Verbal Learning Test (CVLT), Complex Figure Test (CFT), and Auditory Consonant Trigrams (ACT). A 41-year-old male was also treated with CDP-choline for 4 years following a severe TBI. Although no neuropsychological assessment was conducted, the patient reported improvement in arousal and alertness following initiation of treatment at 10 days postinjury. These reports, although anecdotal and uncontrolled, provide preliminary evidence for the potential efficacy of choline precursors in improving cognition following TBI.
Maldonado et al.42 performed a single-blind randomized study of CDP-choline in 216 patients with moderate to severe TBI. Patients were randomly assigned to receive either conventional treatment (not described by the authors) or CDP-choline in addition to conventional treatment. Length of treatment was variable, depending on the progression of the patient's symptoms. Outcome was assessed with the Glasgow Outcome Scale (GOS), administered 3 months after injury. In addition, assessment of self-report of symptoms (e.g., headache, dizziness, motor dysfunction, and changes in character), Hodkinson Brief Mental Test (HBMT) of memory, and the Wechsler Adult Intelligence Scale (WAIS) was conducted when the patient left the intensive care unit and again at 3 months after injury. Results revealed that improvement in “changes in character” (not described by the authors) from baseline was statistically greater in the CDP-choline group; in addition, there was a trend toward improvement in motor symptoms (again not described by the authors) and WAIS performance in the CDP-choline group. Further, the percentage of patients falling in the “good recovery” range on the GOS after treatment was statistically greater in the CDP-choline group.
Leon-Carrion et al.43 also reported on the use of CDP-choline in 10 patients with a history of severe TBI. Patients were randomly assigned to receive either CDP-choline combined with memory rehabilitation or placebo combined with memory rehabilitation. Neuropsychological testing was conducted at baseline and at the end of the 3-month treatment period (see Table 1 for measures administered). Results indicated that patients in the CDP-choline group demonstrated statistically significant improvement in verbal memory (Luria Memory Words test) and verbal fluency (Controlled Oral Word Association Task [COWAT]), when compared with baseline; these improvements were not observed in the placebo group. The authors concluded that CDP-choline facilitates neuropsychological rehabilitation in patients with a history of TBI.
Donepezil
More recently, donepezil has become a drug of interest in TBI, because of its longer-acting properties and oral administration, when compared with physostigmine, and its recent approval for the treatment of AD in the United States and Canada. Taverni et al.44 described the use of donepezil in 2 patients with a history of severe TBI. A 21-year-old female with a history of TBI was treated with donepezil daily for 3 weeks; assessment of cognition was conducted at baseline and after 3 weeks, using an unvalidated test that incorporated modified versions of the Rivermead Behavioural Memory Test (RBMT) and the Ross Immediate Processing Assessment (RIPA). Results indicated 60% improvement from baseline. Further, subjective reports by staff and family indicated improvements in awareness, adaptive functioning, recall, and participation in group discussions. A 46-year-old male with a history of TBI was treated with donepezil daily for approximately 3 weeks. No objective measures were administered, but staff noted improvement in alertness and recall. The authors did not provide follow-up information regarding whether improvements were sustained following discontinuation of treatment.
In a recent open-label trial, Whelan et al.45 administered donepezil to 53 patients with a history of TBI for up to 2 years. Assessment was conducted at baseline and, on average, 12 months following initiation of treatment (see Table 1 for measures administered; no memory measures were used in this study). In addition, the first and second authors completed a “Clinician Improvement Scale,” which rated subjects according to improvement of function (e.g., mood, affect, energy, interest in daily activities, grooming, and social interaction) from baseline. Results indicated statistically significant improvement in WAIS-R Full Scale IQ scores, when compared with baseline, as well as statistically significant improvement in clinician ratings of function, when compared with baseline.
Our group recently completed an open-label trial of donepezil in 4 patients with TBI.33 We found that a 12-week trial of donepezil resulted in statistically significant improvements to 0.4, 1.04, and 0.83 standard deviations above baseline values on the RAVLT total recall, short-term recall, and long-term recall scores, respectively. Performance also improved to 1.56 and 1.38 standard deviations above baseline values on the CFT short-term and long-term recall scores, respectively. Although no improvement in functional abilities, as assessed by the Functional Independence Measures (FIM) and the Clinical Global Impression of Change (CGIC) scale, was associated with improved cognition, we hypothesized that a longer medication trial might be required in order to observe functional improvements in these patients. Our results also indicated improvements in emotional/behavioral functioning in patients treated with donepezil. Specifically, decline in anxiety, depression, and apathy scores on the Neuropsychiatric Inventory (NPI)46 was noted. This finding highlights the possibility of treating behavioral, in addition to cognitive, sequelae of TBI with acetylcholinesterase inhibitors.
ACETYLCHOLINERGIC TREATMENT OF APATHY IN TBI
Personality changes, including irritability, impulsivity, emotional lability, aggression, and apathy are commonplace following TBI. Apathy is characterized by diminished initiative, diminished interest and concern, and diminished emotional responsiveness.47 Apathy following TBI is particularly problematic, as it can often have a negative impact on rehabilitation, return to work, and successful reintegration to the community following TBI.48 Estimates of the frequency of apathy following TBI range from 46%49 to 71%.48
There is growing evidence for a link between cholinergic dysfunction and the behavioral syndrome of apathy. Limbic and paralimbic structures are among the regions containing the highest acetylcholine levels in the brain.50 It is hypothesized that the nucleus basalis, which manufactures the choline acetyltransferase necessary for synthesis of acetylcholine and serves as a structural link between the limbic system and the cortex, may contribute to symptoms of apathy.51 Specifically, the nucleus basalis is believed to exert influence on the cortex in response to motivational and emotional information conveyed through limbic structures,52 and dysfunction of this structure may contribute to decreased ability to link emotional significance to other information about the world.
The relationship between apathy and the cholinergic system has been most extensively studied in Alzheimer's disease. Apathy is one of the most commonly reported behavioral symptoms of AD, estimated to occur in 72% of AD patients.51 There is growing evidence that cholinergic agents result in improvement in behavioral symptoms of AD, including apathy.53,54 Specifically, tacrine and metrifonate (another acetylcholinesterase inhibitor) have been used with AD patients to produce statistically significant declines in apathy.30,55 In a review of studies of cholinergic treatment of behavioral symptoms in AD, Cummings30 concluded that, along with visual hallucinations, apathy is the most consistent behavioral symptom to improve. Cummings hypothesized that the strong responsiveness of apathy to cholinergic agents may be related to improvement in attentional systems associated with cholinergic treatment; Cummings also suggests that the behavioral deficits common to other disorders associated with cholinergic dysfunction, such as TBI, may be treatable with cholinesterase inhibitors, as well.
Although cholinergic mechanisms of behavioral disorders in TBI have received relatively little attention, extrapolation from AD research suggests a rationale for treating the syndrome of apathy with cholinergic agents. Indeed, in an uncontrolled trial of 4 patients with a history of TBI, our group found that donepezil had a beneficial effect on apathy, as measured by the NPI.33 The present literature review revealed no additional studies addressing treatment of apathy with cholinergic agents following TBI. However, the prevalence of apathy following TBI suggests a need for further investigation of treatment mechanisms and strategies. Further investigation of the possible role of cholinergic agents in ameliorating other behavioral deficits common to both AD and TBI (e.g., irritability, disinhibition) is also needed.
OUTCOME MEASURES IN FUTURE STUDIES OF CHOLINERGIC AGENTS IN TBI
We propose that the following criteria be considered when selecting outcome measures for inclusion in future studies of cholinergic agents in TBI: 1) whether the measures address the spectrum of cognitive and/or behavioral realms commonly affected in TBI; 2) whether specific measures have demonstrated responsivity to acetylcholinergic manipulation in prior studies; and 3) whether the measures have demonstrated positive relationships with broader functional outcome in TBI.
The selection of appropriate outcome measures should also be guided by our understanding of the role of the cholinergic system in human cognition. It has been hypothesized that the cholinergic system serves to direct attentional processes to relevant stimuli, and that cholinergic disruption diminishes this ability.12 Other authors30 have suggested that the primary effect of cholinergic agents on cognition may be through their action on the attentional/executive system, which may have a modulating influence on other cognitive skills, including memory. One might postulate that the effect of cholinergic agents on cognition following TBI may be to improve the executive/control system, thereby resulting in improvements in performance on aspects of all cognitive processes (attention, memory, language, etc.) at lower levels.
These hypotheses lead us to suggest the need for a broader range of outcome measures in studies assessing the role of cholinergic agents in TBI. Few studies to date have included objective assessment of skills other than memory and attention. We hypothesize that cholinergic agents, through effects on basic attentional/executive systems, might be demonstrated to influence cognitive function in a variety of realms, if studied appropriately. In particular, we recommend that future studies include outcome measures that assess a broad range of cognitive abilities typically affected in TBI, including verbal and visual memory (e.g., RAVLT,56 CFT57), working memory (e.g., Brown-Peterson task58), sustained, selective and divided attention (e.g., CPT,59 Digit Symbol Test,60 TMT61), and executive functioning (e.g., Wisconsin Card Sorting Test,62 COWAT63). Further, more extensive investigation of cholinergic agents in the treatment of behavioral deficits following TBI is also indicated by the above review. We expect that cholinergic agents will improve symptoms of apathy in TBI, and recommend that future studies include instruments that address these realms (e.g., Apathy Evaluation Scale–Informant and Self-rated versions47). Finally, although speculative at this point, one might hypothesize that behavior in other realms (e.g., irritability, disinhibition) may also be improved through use of cholinergic agents. These realms should be addressed through use of a broader array of outcome measures designed to assess behavior following TBI, including the Katz Adjustment Scale,64 the Neurobehavioral Rating Scale,65 and the Portland Adaptability Inventory.66
Inclusion of measures that have demonstrated responsivity to acetylcholinergic manipulation in prior studies of normal controls, AD patients, and/or TBI patients might also prove useful in providing us with a greater understanding of the relationship between cognition and the cholinergic system. In particular, measures of verbal learning, including the RAVLT and the SRT, have been consistently demonstrated to respond to cholinergic agents in studies of both TBI33 and AD populations.17
Finally, we recommend that measures which have demonstrated relationships with broader TBI outcome be included in future studies. For example, performance on specific measures of verbal memory (i.e., RAVLT; CVLT; WMS Logical Memory subtest), visual memory (WMS), and attention/executive functioning (TMT, WCST) has been found to be correlated with return to work or school,4,6,67 general outcome,68 and community integration9 following TBI. Further, we recommend that measures which directly assess quality of life (e.g., Sickness Impact Profile69), functional performance (e.g., Patient Competency Rating Scale70), and community integration (e.g., Community Integration Questionnaire71) be included in future studies. Clearly, improvement in performance on discrete tasks of memory and attention is encouraging; however, our goal should be to demonstrate that such improvements translate into sustainable changes in functional abilities and quality of life for patients who are being treated.
CONCLUSIONS
Although many of the studies presented above are anecdotal case reports, and thus fail to control for the effects of spontaneous recovery, placebo effects, practice effects, and the effects of concurrent treatment, they do provide preliminary evidence to support further investigation of cholinergic agents in treating cognitive and behavioral impairments following TBI. Further, several small controlled trials have provided evidence for statistically and clinically significant efficacy of these agents in ameliorating specific cognitive deficits following TBI. The accumulating data do provide solid preliminary evidence that cholinergic agents are likely to be efficacious in ameliorating attention, memory, and perhaps executive impairment in some patients who have sustained a TBI. The apathy syndrome, and perhaps other aspects of behavior, may also improve. These more molecular improvements are likely to result in improved outcome and quality of life for individuals with TBI. However, there is a strong need for large-sample, randomized, double-blind, placebo-controlled studies of specific cholinergic agents in TBI populations. Such studies should include a broad range of outcome measures as suggested herein.
ACKNOWLEDGMENTS
The authors are grateful to Donald T. Stuss, Ph.D., for his conceptual contributions to this manuscript. Dr. van Reekum's work is supported by the Kunin-Lunenfeld Applied Research Unit of Baycrest Centre.
 |
1 Kraus JF, Black MA, Ilessol N, et al: The incidence of acute brain injury and serious impairment in defined populations. Am J Epidemiol 1984; 119:185-201Google Scholar
2 Klasbeek WD, McLaruin RL, Harris BSH, et al: The national head and spinal cord survey findings. J Neurosurg 1980; 53:519-531Google Scholar
3 NIH Consensus Development Panel on Rehabilitation of Persons with Traumatic Brain Injury: Rehabilitation of persons with traumatic brain injury. JAMA 1998; 282:974-983Crossref, Google Scholar
4 Brooks DN, McKinlay W, Symington C, et al: Return to work within seven years of severe head injury. Brain Inj 1987; 1:5-19Crossref, Medline, Google Scholar
5 Fraser R, Dikmen S, McLean A, et al: Employability of head injury survivors: first year post-injury. Rehabilitation Counseling Bulletin 1988; 31:276-288Google Scholar
6 Ip RY, Dornan J, Schentag C: Traumatic brain injury: factors predicting return to work or school. Brain Inj 1995; 9:517-532Crossref, Medline, Google Scholar
7 Dikmen S, Machamer J, Savoie T, et al: Life quality outcome in head injury, in Neuropsychological Assessment of Neuropsychiatric Disorders, 2nd edition. Edited by Grant I, Adams K. New York, Oxford University Press, 1996, pp 552-576Google Scholar
8 Little AJ, Templer DI, Persel, et al: Feasibility of the neuropsychological spectrum in prediction of outcome following head injury. J Clin Psychol 1996; 52:455-460Crossref, Medline, Google Scholar
9 Millis SR, Rosenthal M, Lourie IF: Predicting community integration after traumatic brain injury with neuropsychological measures. Int J Neurosci 1994; 79:165-167Crossref, Medline, Google Scholar
10 Colantonio A, Dawson DR, McLellan BA: Head injury in young adults: long-term outcome. Arch Phys Med Rehabil 1998; 79:550-558Crossref, Medline, Google Scholar
11 Salazar AM, Warden DL, Schwab K, et al: Cognitive rehabilitation for traumatic brain injury: a randomized trial. JAMA 2000; 283:3075-3081Crossref, Medline, Google Scholar
12 Lawrence AD, Sahakian BJ: Alzheimer disease, attention, and the cholinergic system. Alzheimer Dis Assoc Disord 1995; 9:43-49Crossref, Medline, Google Scholar
13 Bartus RT, Dean RL, Beer B, et al: The cholinergic hypothesis of geriatric memory dysfunction. Science 1982; 217:408-417Crossref, Medline, Google Scholar
14 Riekkinen M, Soininen H, Riekkinen P, et al: Tetrahydroaminoacridine improves the recency effect in Alzheimer's disease. Neuroscience 1997; 83:471-479Crossref, Google Scholar
15 Rogers SL, Friedhoff LT, the Donepezil Study Group: The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia 1996; 7:293-303Medline, Google Scholar
16 Rogers SL, Doody RS, Mohs RC, et al: Donepezil improves cognition and global function in Alzheimer disease. Arch Intern Med 1998; 158:1021-1031Crossref, Medline, Google Scholar
17 Simard M, van Reekum R: Memory assessment in studies of cognition-enhancing drugs for Alzheimer's disease. Drugs Aging 1998; 14:197-230Crossref, Google Scholar
18 McIntosh TK, Juhler M, Wieloch T: Novel pharmacological strategies in the treatment of experimental traumatic brain injury. J Neurotrauma 1998; 15:731-769Crossref, Medline, Google Scholar
19 Dewar D, Graham DI: Depletion of choline acetyltransferase activity but preservation of M1 and M2 muscarinic receptor binding sites in temporal cortex following head injury: a preliminary human postmortem. J Neurotrauma 1996; 13:181-187Crossref, Medline, Google Scholar
20 Murdoch I, Perry EK, Court JA, et al: Cortical cholinergic dysfunction after human head injury. J Neurotrauma 1998; 15:295-305Crossref, Medline, Google Scholar
21 Shao L, Ciallella JR, Yan HQ, et al: Differential effects of traumatic brain injury of vesicular acetylcholine transporter and MS muscarinic receptor mRNA and protein in rat. J Neurotrauma 1999; 16:555-566Crossref, Medline, Google Scholar
22 Arcieniegas D, Adler L, Topkoff J, et al: Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating and a hypothesis for further investigation. Brain Inj 1999; 13:1-13Crossref, Medline, Google Scholar
23 Kaitaro T, Koskinen S, Kaipio M-L: Neuropsychological problems in everyday life: a 5-year follow-up study of young severely closed-head-injured patients. Brain Inj 1995; 9:713-727Crossref, Medline, Google Scholar
24 Pike BR, Hamm RJ: Postinjury administration of BIBN 99, a selective muscarinic M2 receptor antagonist, improves cognitive performance following traumatic brain injury in rats. Brain Res 1995; 686:37-43Crossref, Medline, Google Scholar
25 O'Dell DM, Hamm RJ: Chronic postinjury administration of MDL 26,479 (suritozole), a negative modulator of GABAA receptor, and cognitive impairment in rats following traumatic brain injury. J Neurosurg 1995; 83:878-883Crossref, Medline, Google Scholar
26 Dixon CE, Ma X, Marion DW: Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma 1997; 14:161-169Crossref, Medline, Google Scholar
27 Rusted JM, Warburton DM: Cognitive models and cholinergic drugs. Neuropsychology 1989; 21:31-36Google Scholar
28 Drachman DA: Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology 1977; 27:783-790Crossref, Medline, Google Scholar
29 Nissen MJ, Knopman DS, Schacter DL: Neurochemical dissociation of memory systems. Neurology 1987; 37:789-794Crossref, Medline, Google Scholar
30 Cummings JL: Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry 2000; 157:4-15Crossref, Medline, Google Scholar
31 Levin HS, Goldstein FC: Organization of verbal memory after severe closed-head injury. J Clin Exp Neuropsychol 1986; 8:643-656Crossref, Medline, Google Scholar
32 Weingartner H, Grafman J, Boutelle W, et al: Forms of memory failure. Science 1983; 221:380-382Crossref, Medline, Google Scholar
33 Masanic CA, Bayley MT, van Reekum, R, et al: Open-label study of donepezil in traumatic brain injury. Arch Phys Med Rehabil 2001; 82:896-901Crossref, Medline, Google Scholar
34 Eames P, Sutton A: Protracted post-traumatic confusional state treated with physostigmine. Brain Inj 1995; 9:729-734Crossref, Medline, Google Scholar
35 Weinberg RM, Auerbach SH, Moore S: Pharmacologic treatment of cognitive deficits: a case study. Brain Inj 1987; 1:57-59Crossref, Medline, Google Scholar
36 Cardenas DD, McLean A, Farrell-Roberts L, et al: Oral physostigmine and impaired memory in adults with brain injury. Brain Inj 1994; 8:579-587Crossref, Medline, Google Scholar
37 Walton RG: Lecithin and physostigmine for posttraumatic memory and cognitive deficits. Psychosomatics 1982; 23:435-436Crossref, Medline, Google Scholar
38 Goldberg E, Gerstman LJ, Mattis S, et al: Effects of cholinergic treatment on post-traumatic anterograde amnesia. Arch Neurol 1982; 39:581Crossref, Medline, Google Scholar
39 Levin HS, Peters BH, Kalisky Z, et al: Effects of oral physostigmine and lecithin on memory and attention in closed head injured patients. Central Nervous System Trauma 1986; 3:333-342Crossref, Medline, Google Scholar
40 Levin HS: Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci 1991; 103:S39-S42Google Scholar
41 Spiers PA, Hochanadel G: Citicoline for traumatic brain injury: report of two cases, including my own. J Int Neuropsychol Soc 1999; 5:260-264Crossref, Medline, Google Scholar
42 Maldonado VC, Perez JBC, Escario JA: Effects of CDP-choline on the recovery of patients with head injury. J Neurol Sci 1991;103:S15-S18Google Scholar
43 Leon-Carrion J, Dominguez-Roldan JM, Murill-Cabezas F, et al: The role of citicholine in neuropsychological training after traumatic brain injury. Neurorehabilitation 2000; 14:33-40Medline, Google Scholar
44 Taverni JP, Seliger G, Lichtman SW: Donepezil mediated memory improvement in traumatic brain injury during post acute rehabilitation. Brain Inj 1998; 12:77-80Crossref, Medline, Google Scholar
45 Whelan F, Walker MS, Schultz SK: Donepezil in the treatment of cognitive dysfunction associated with traumatic brain injury. Ann Clin Psychiatry 2000; 12:131-135Crossref, Medline, Google Scholar
46 Cummings JL, Mega M, Gray K, et al: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308-2314Crossref, Medline, Google Scholar
47 Marin RS, Biedrzycki RC, Firinciogullari S: Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143-162Crossref, Medline, Google Scholar
48 Kant R, Duffy JD, Pivovarnik A: Prevalence of apathy following head injury. Brain Inj 1998; 12:87-92Crossref, Medline, Google Scholar
49 Andersson S, Krogstad JM, Finset A: Apathy and depressed mood in acquired brain damage: relationship to lesion localization and psychophysiological reactivity. Psychol Med 1999; 29:447-456Crossref, Medline, Google Scholar
50 Cummings JL: Changes in neuropsychiatric symptoms as outcome measures in clinical trials with cholinergic therapies for Alzheimer disease. Alzheimer Dis Assoc Disord 1997; 11:S1-S9Google Scholar
51 Cummings JL, Back C: The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer's disease. Am J Geriatr Psychiatry 1998; 6:S64-S78Google Scholar
52 Mesulam M-M, Mufson EJ, Levey AI, et al: Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 1983; 214:170-197Crossref, Medline, Google Scholar
53 Kaufer D, Cummings JL, Christine D: Differential neuropsychiatric symptom responses to tacrine in Alzheimer's disease: relationship to dementia severity. J Neuropsychiatry Clin Neurosci 1998; 10:55-63Link, Google Scholar
54 Levy ML, Cummings JL, Kahn-Rose R: Neuropsychiatric symptoms of cholinergic therapy for Alzheimer's disease. Gerontology 1999; 45:S15-S22Google Scholar
55 Mega MS, Masterman DM, O'Connor SM, et al: The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol 1999; 56:832-842Crossref, Google Scholar
56 Rey A: L'examen clinique en psychologie. Paris: Presses Universitaires de France, 1964Google Scholar
57 Osterrieth PA: Le test de copie d'une figure complexe. Archives de Psychologie 1944; 30:206-356Google Scholar
58 Peterson LR, Peterson MJ: Short-term retention of individual verbal items. J Exp Psychol 1959; 58:193-198Crossref, Medline, Google Scholar
59 Conners CK, Multi-Health Systems Staff: Conners' Continuous Performance Test. Toronto, MHS, 1995Google Scholar
60 Wechsler D: WAIS-R manual. New York, The Psychological Corporation, 1981Google Scholar
61 Reitan RM, Wolfson D: The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Tucson, AZ, Neuropsychology Press, 1993Google Scholar
62 Grant DA, Berg EA: A behavioral analysis of the degree of reinforcement and ease of shifting to new responses in a Weigl-type card sorting problem. J Exp Psychol 1948; 38:404-411Crossref, Medline, Google Scholar
63 Benton AL, Hamsher KdeS: Multilingual Aphasia Examination. Iowa City, IA, AJA Associates, 1989Google Scholar
64 Katz MM, Lyerly SB: Methods for measuring adjustment and social behavior in the community, I: rationale, description, discriminative validity and scale development. Psychol Rep 1963; 13:503-535Crossref, Google Scholar
65 Levin HS, High WM, Goethe KE, et al: The Neurobehavioral Rating Scale assessment of the behavioral sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry 1987; 50:183-193Crossref, Medline, Google Scholar
66 Lezak M: Neuropsychological Assessment, 3rd edition. New York, Oxford University Press, 1995Google Scholar
67 Kibby MY, Schmitter-Edgecombe M, Long C: Ecological validity of neuropsychological tests: focus on the California Verbal Learning Test and the Wisconsin Card Sorting Test. Archives of Clinical Neuropsychology 1998; 13:523-534Medline, Google Scholar
68 Levin HS, Grossman RG, Rose JE, et al: Long-term neuropsychological outcome of closed head injury. J Neurosurg 1979; 50:412-422Crossref, Medline, Google Scholar
69 Bergner M, Bobbitt RA, Carter WB, et al: The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19:787-805Crossref, Medline, Google Scholar
70 Ben-Yishay Y, Silver S, Piasetsky E, et al: Relationship between employability and vocational outcome after intensive holistic cognitive rehabilitation. J Head Trauma Rehabil 1987; 2:35-48Crossref, Google Scholar
71 Willer B, Rosenthal M, Kreutzer JS, et al: Assessment of community integration following rehabilitation for traumatic brain injury. J Head Trauma Rehabil 1983; 8:75-87Crossref, Google Scholar



