Differential Neuropsychiatric Symptom Responses to Tacrine in Alzheimer's Disease
Abstract
Neuropsychiatric symptom responses to tacrine were investigated in an open-label study of Alzheimer's outpatients. Forty subjects were stratified into three groups (Mild, Moderate, and Severe) based on Mini-Mental State Examination scores. A significant reduction in total Neuropsychiatric Inventory score across all subjects was principally attributable to changes in the Moderate group. Apathy and disinhibition symptoms were significantly reduced overall. Whereas other symptoms showed differential responses in Mild and Severe subjects, all symptoms improved in Moderate subjects. These findings suggest that disease severity may significantly influence neuropsychiatric symptom responses to tacrine. Putative mechanisms underlying the observed pattern of responses are explored.
Neuropsychiatric disturbances such as delusions, personality alterations, dysphoria, anxiety, and agitation are common in Alzheimer's disease (AD) and may contribute significantly to clinical morbidity and feelings of caregiver distress.1–3 There is a marked cholinergic deficiency in AD, and much therapeutic effort in AD has focused on ameliorating cognitive symptoms with cholinergic-enhancing agents.4–6 Tacrine, a centrally active cholinesterase inhibitor and the first drug approved in the United States for the symptomatic treatment of cognitive symptoms in AD patients, has shown generally modest and limited efficacy in ameliorating symptoms of cognitive decline in AD.7–9 The relationship between central cholinergic deficits and neuropsychiatric symptoms in AD has received little systematic investigation.10–12 A previous open-label study in unselected AD patients12 reported a dose-dependent and statistically significant reduction in a global measure of neuropsychiatric symptoms (the Neuropsychiatric Inventory; NPI),13 during treatment with tacrine. AD patients with a moderate degree of cognitive deficit were observed to have the most robust neuropsychiatric symptom response. Building on the previous study, the primary goals of the current study were to investigate the differential response of individual neuropsychiatric symptoms in relation to disease severity. On the basis of these and other data, we discuss possible neurobiological substrates of clinical responses to cholinergic treatments in AD.
METHODS
Subjects
Fifty consecutive subjects who were enrolled in an outpatient university clinic and who met NINCDS-ADRDA criteria for possible or probable Alzheimer's disease14 participated in the study. Data from a subset of these subjects (n=28) have been reported previously.12 Ten subjects were excluded from the analysis for the following reasons: failure to complete the first assessment (2), incomplete baseline information because tacrine was started prior to baseline assessment (5), and change in dosing of a concomitantly administered psychotropic agent (3). Eight subjects (2 Mild, 3 Moderate, and 3 Severe) were on concomitant psychotropic medications during the study period; data from these subjects were included only if they had been on a stable dose regimen for at least 2 weeks prior to starting tacrine and during the course of tacrine therapy. Data are reported on 40 subjects who completed a baseline and at least one follow-up assessment while taking tacrine.
Treatment
A fixed dose-titration schedule of tacrine following recommended guidelines was employed.12 Forty subjects completed at least 6 weeks on a dose of 40 mg/day. Thirty-two subjects were maintained on a dose of 80 mg/day for at least 6 weeks; 21 of these completed at least 6 weeks at a dose of 120 mg/day. Sixteen subjects tolerated the maximum dose of 160 mg/day for at least six weeks. Of the 40 subjects on whom data are available, 21 reached a maximum dose of 120 mg/day or higher and only 8 failed to reach a dose of 80 mg/day. Liver enzyme elevation rarely caused treatment withdrawal, but it limited the rate of dose escalation in the majority of subjects who failed to achieve the maximum dosage during the study. Intolerable gastrointestinal side effects were the only other common reason for withdrawal or failure to reach the maximum dose. In subjects maintained at the same dose level for 12 weeks or more, data obtained after the initial 6 weeks of therapy are used for analysis.
Behavioral and Cognitive Assessment
The NPI, a measure of neuropsychiatric symptoms shown to be valid and reliable in AD,13 was the principal outcome measure of this study. The following 10 behavioral symptoms in AD are assessed with the NPI: delusions, hallucinations, dysphoria/depression, agitation/aggression, anxiety, apathy/indifference, euphoria/elation, irritability/lability, disinhibition, and aberrant motor behaviors (e.g., pacing, stereotyped activities). The most prominent behavioral manifestations of a given domain that are present within a specified time frame (6 weeks in this study) are rated by informants in terms of frequency (1 to 4, indicating lower to higher frequency) and severity (1 to 3, reflecting mild to severe intensity). The product of the frequency and severity ratings constitutes the score (0 to 12) for each behavioral domain. The total NPI score is the sum of the 10 individual subsection scores. Cognitive function was assessed with the Mini-Mental State Examination (MMSE).15 Informed consent was obtained for all participating subjects and caregivers.
Data Analysis
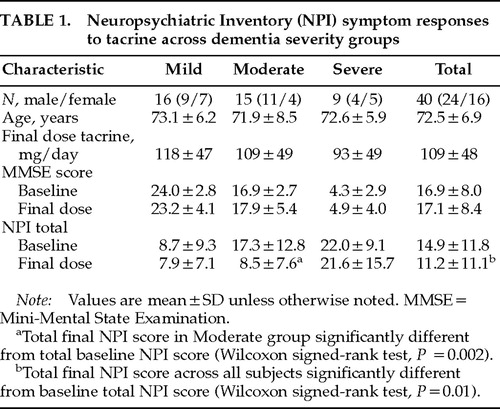
Stage-specific effects of dementia severity were examined by dividing subjects into three groups according to their baseline MMSE scores: Mild (MMSE score>20, n=16), Moderate (MMSE score between 11 and 20, n=15), and Severe (MMSE score≤10, n=9). Age differences across groups were examined with univariate analysis of variance (ANOVA). Group mean comparisons of the maximum (final) dose of tacrine, and between NPI baseline and change scores, were performed with the Kruskal-Wallis H statistic. Gender distributions across groups were assessed with the chi-square statistic. Paired group comparisons of mean MMSE and total NPI scores (comparing baseline scores and scores at the individual final dose of tacrine) and between baseline and final dose scores of individual NPI symptoms (in subjects with baseline or emergent symptoms) were assessed with the Wilcoxon signed-rank test, which results in the Z statistic. Pairwise associations between individual baseline NPI symptom subscores, and between individual symptom change scores, were examined by Spearman rank correlational analyses, correcting for ties. A P-value of 0.05 (two-tailed) was considered significant for all statistical analyses because of the exploratory nature of the study.
RESULTS
Effect of Dementia Severity on NPI Symptom Responses
Group differences in age (univariate ANOVA, F=0.12, df=2, P=0.89) and gender (χ2= 2.11, df=2, P=0.35) were not significant (Table 1). Across all subjects, the average maximum dose of tacrine attained was 109±48 mg/day. Group differences in the average final dose of tacrine were not significant (H=1.59, df=2, P=0.45). The mean change in MMSE score across all subjects was 0.25±3.5; moderately demented subjects showed the largest net increase in MMSE score (1.0±3.9), but neither change was significant (Z≤1.0, P>0.30). The mean baseline NPI score was 14.9±11.8 (range 0–40). The mean baseline NPI score for each of the three dementia severity groups was higher with increasing dementia severity, indicating an increased burden of neuropsychiatric symptoms with cognitive decline, and was significantly different across groups (H=9.71, df=2,3, P=0.008). Mean changes in NPI scores were also significantly different across groups (H=6.78, df=2,3, P=0.034). Whereas the net decrease in NPI total score was less than 1 in the Mild and the Severe groups (Z<±1.0, P>0.50), a marked (51%) reduction in total NPI score was observed in the Moderate group (mean difference –8.9, Z=–3.12, P=0.002). In the Moderate group, 8/15 subjects showed a decrease of 5 or more points in total NPI score, but none had an increase of greater than 1 point. In contrast, 4/16 Mild and 2/9 Severe subjects had reductions of 5 or more points in total NPI score; 3 subjects in each of these groups showed a net increase of 5 or more points in total NPI score during treatment. Across all subjects, a significant reduction in total NPI symptom score was observed following tacrine treatment (mean difference –3.8, Z=–2.45, P=0.01).
Effect of Tacrine on Individual NPI Symptoms
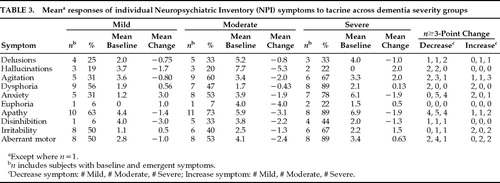
Across all subjects with baseline or emergent symptoms, a significant reduction in apathy scores (mean change –2.2, Z=–2.49, P<0.02) and a borderline significant reduction in disinhibition scores (mean change –1.9, Z=–1.98, P=0.05) occurred during treatment (Table 2). Excluding emergent symptoms, baseline symptoms of apathy (mean change –2.7, Z=–2.98, P=0.003), disinhibition (mean change –2.2, Z=–2.03, P=0.04), and aberrant motor behaviors (mean change –1.8, Z=–2.03, P=0.04) were significantly decreased during treatment. Hallucinations showed the largest mean decrease in symptoms present at baseline (mean change –4.6), but this difference was not significant (Z=–1.83, P=0.07).
A net reduction in the number of symptomatic subjects (i.e., number of subjects with emergent symptom minus number of subjects with symptom eliminated) during treatment was observed for apathy (–11), agitation (–6), delusions (–3), aberrant motor behaviors (–3), anxiety (–2), dysphoria (–2), and disinhibition (–1). A net increase in the number of symptomatic subjects during treatment occurred in euphoria (+1) and hallucinations (+2). An equal number of subjects with emergent symptoms of irritability had elimination of baseline irritable behaviors. More than three times as many subjects had decreases in apathy and disinhibition symptoms as had increases in these symptoms during treatment. Two or more times as many subjects had reduced symptoms of delusions and anxiety as had worsening of these symptoms. Hallucinations, agitation, dysphoria, and aberrant motor behaviors improved in more subjects than had increases in these symptoms. Irritability and euphoria were the only symptoms for which more subjects exhibited symptom exacerbations than symptom improvement during treatment.
Effect of Tacrine on Individual NPI Symptoms in Relation to Dementia Severity
Moderately demented subjects showed improvement in all symptoms during treatment (Table 3). In contrast, Mild subjects showed overall improvement in 6 symptoms (delusions, hallucinations, agitation, apathy, disinhibition, and aberrant motor behaviors), but exhibited net increases in the other 4 symptoms. In Severe subjects, 4 symptoms (delusions, anxiety, apathy, and disinhibition) improved and the other 6 worsened during treatment. Hallucinations were not reported in any severely demented subjects at baseline, but 2 such subjects developed mild hallucinatory symptoms during treatment. One of these subjects had concomitant elimination of delusions and a 5-point increase in MMSE score, whereas the other, who had the highest baseline NPI score (40 points) and showed the poorest response to treatment (NPI score increase of 15 points), had significant worsening of delusions and other symptoms. Hallucination scores were reduced by 3 or more points in 4/6 subjects (2 Mild and 2 Moderate subjects), and symptoms of disinhibition decreased in 3/9 subjects (1 each in the Mild, Moderate, and Severe groups) with these respective symptoms at baseline. Neither hallucinations nor disinhibition worsened or emerged to a significant degree. More subjects had significant decreases in symptoms of delusions, agitation, and aberrant motor behaviors than had significant symptom decreases. Dysphoria significantly improved in 2 and worsened in 2 Mild subjects, and no significant changes in euphoria were observed. Irritability was the only symptom for which more subjects worsened (2 Mild and 2 Severe) than improved (1 Moderate and 1 Severe).
For individual subjects, a change of 3 or more points in an individual symptom score was considered to be clinically important because this generally reflects a change between categories within a symptom score range (0 to 12) based on the following classification: Mild, 1 to 3; Moderate, 4 to 6; Severe, 8 to 12. Almost half (13/27) of the subjects with baseline apathy symptoms had a decrease of 3 or more points during treatment (4/10 Mild subjects, 5/11 Moderate subjects, and 4/8 Severe subjects). Only 4 subjects (2 Severe and 1 each in the Mild and Moderate groups) had an increase in apathy symptoms of 3 or more points. Anxiety symptoms significantly worsened in 3 subjects (1 Severe and 2 Mild) but showed a marked improvement in 9 subjects (5 Moderate and 4 Severe).
Interrelationships Among NPI Symptom Baseline and Change Scores
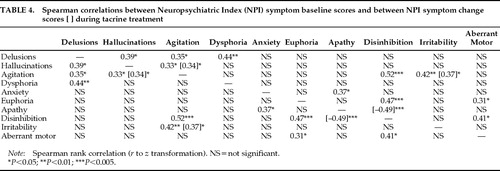
At baseline, scores for each NPI symptom exhibited at least one significant correlation with another NPI symptom score, reflecting symptoms that tended to occur together across individual subjects (Table 4). Two baseline symptom clusters were observed; agitation, hallucinations, and delusions were all significantly intercorrelated (Spearman's r≥0.33, Z≥2.05, P<0.05), as were disinhibition, euphoria, and aberrant motor behaviors (r≥0.31, Z≥1.95, P<0.05). Agitation and disinhibition were the most highly correlated baseline NPI symptoms (r=0.52, Z=3.23, P<0.005), linking the two symptom clusters. Three other pairs of NPI symptoms were correlated at baseline: agitation and irritability (r=0.42, Z=2.65, P<0.01), apathy and anxiety (r=0.37, Z=2.31, P<0.05), and dysphoria/depression and delusions (r=0.44, Z=2.77, P<0.01).
During treatment, three pairs of correlated symptom responses were observed, representing concurrent symptom changes across individual subjects. Two of these symptom response relationships paralleled baseline associations between symptom pairs; changes in agitation and hallucination scores were significantly related (r=0.34, Z=2.09, P<0.05), as were those for agitation and irritability (r=0.37, Z=2.29, P<0.05). Apathy and disinhibition scores, which were not significantly associated at baseline, showed the strongest (but inverse) response relationship (r=–0.49, Z=–3.07, P<0.005). That is, changes in apathy and disinhibition tended to occur together, but in opposite directions.
DISCUSSION
Findings of the Present Study
The present study significantly extends our previous report12 by examining differential responses of neuropsychiatric symptoms across different levels of dementia severity and symptom baseline and response interrelationships. Such data have not been reported and provide important insight into the nature and heterogeneity of clinical responses to cholinergic agents in AD. The present study also includes a 30% larger sample size, uses more conservative nonparametric statistical analyses, and takes account of emergent symptoms in change score comparisons (not just the effect of tacrine on baseline symptoms). Two main findings of this extended open-label study of tacrine in AD add further support to previous observations: 1) an overall significant reduction in neuropsychiatric symptoms reported on the NPI in a cross-section of AD patients representing different levels of dementia severity, and 2) a stage-specific (Moderate group) predilection for beneficial neuropsychiatric symptom responses. Previously observed significant or nearly significant reductions in anxiety and aberrant motor behaviors were not confirmed in the present study when emergent symptoms were included in the analysis. Significant reductions in apathetic and disinhibited behaviors were observed across all subjects with these symptoms, and, together with delusions, were the only symptoms to exhibit net decreases at all levels of dementia severity. All other symptoms showed differential stage-specific responses during tacrine treatment.
In addition to characterizing the stage-specific nature of individual neuropsychiatric symptom responses to tacrine in AD, this study extends the previous study by exploring relationships between baseline symptom scores and between treatment-based symptom change scores. Baseline agitation scores and agitation change scores during treatment were both correlated with these respective indices of two other symptoms, hallucinations and irritability. However, whereas agitation and hallucinations tended to decrease together in moderately demented subjects, symptoms of agitation and irritability tended to increase together in Mild and Severe subjects. The differential response relationships between agitation and other neuropsychiatric symptoms highlight the variable determinants of agitated behaviors across different stages of AD.2 The observed inverse relationship between changes in apathetic and disinhibited behaviors may appear somewhat paradoxical in that both symptoms showed net decreases across all subjects; within individual subjects, however, decreases in one of these symptoms were generally much greater than increases in the other. Despite the limitations imposed by an open-label design and relatively small sample size, several tentative conclusions emerge from this extended exploratory investigation.
Neuropsychiatric symptom improvement is most apparent in patients with moderately severe cognitive impairment (MMSE range 11 to 20). The net effect of tacrine on composite NPI scores in moderately demented subjects was to reduce them to a level seen in mildly demented subjects. The pattern of observed responses is not consistent with “regression to the mean,” for this would predict the symptom reduction would be greatest in the most severely demented subjects—that is, in subjects with the lowest MMSE and highest NPI scores. Within the Severe group, subjects with lower MMSE scores generally had less improvement or worsening of NPI-rated symptoms than those with higher MMSE scores, consistent with the expectation that a cholinesterase-inhibitor agent will have less of an effect on more advanced AD cases because of severely diminished acetylcholine production. That patients with moderately severe dementia, and not those receiving the highest doses of tacrine, consistently improved suggests that the observed changes may be attributable to neurobiological differences across different levels of dementia severity and are not dose-dependent or placebo responses.
The observed association between psychotic symptoms (delusions and hallucinations) and agitation in AD is consistent with previous reports.16–18 However, only changes in hallucinations and agitation, principally reflecting concomitant symptom reductions in moderately demented subjects, were correlated during treatment. Delusional symptoms were significantly associated with depressive symptoms at baseline, showed consistent, if not dramatic, decreases at all dementia severity levels, and were eliminated in one-third (4/12) of subjects with this symptom at baseline. These findings are congruent with previous reports of an increased incidence of mood disturbances in AD patients with delusions19 and a reduction in delusional symptoms in AD patients treated with the cholinesterase inhibitor physostigmine.20,21 In contrast, hallucinations were markedly reduced in Moderate subjects but emerged in 2 Severe subjects. Together, these data suggest an overlapping cholinergic-sensitive pathophysiology linking delusions and hallucinations in AD, but the data highlight potential differences in these symptoms with respect to differential response patterns to cholinergic treatment.
Imbalances between cholinergic and monoaminergic transmission have been associated with both delusions and hallucinations, but these psychotic manifestations may differ in their anatomical substrates.22,23 Delusional symptoms, often paranoid in AD, have been most closely linked to dysfunction in limbic regions of the medial temporal lobe and caudate nucleus.23 Medial temporal areas typically bear the earliest and greatest pathological burden (including cholinergic deficits) in AD, and significantly reduced choline acetyltransferase activity has been observed in the caudate nuclei of both AD and dementia with Lewy bodies (DLB) patients compared with normal elderly subjects.24 Cholinergic deficits in neocortical (compared with medial temporal) areas are more severe in DLB than AD patients,24,25 and these deficits have been correlated with the presence of hallucinations.22 Acetylcholine has differential effects on thalamic and cortical neurons that combine to enhance cortical sensory signal detection and selection.26,27 To the extent that hallucinatory experiences may involve aberrant thalamocortical sensory information processing, cholinergic augmentation may ameliorate hallucinations by shifting the relative balance between spurious and actual sensory input. Patients with DLB may particularly benefit from cholinergic therapy because of the severity of neocortical cholinergic deficit and relative absence of AD neuropathology.28,29
Apathetic behaviors were the NPI-rated symptom most consistently responsive to tacrine treatment. Apathy—reflecting lack of spontaneity, reduced motivation, or behavioral disengagement—and disinhibited behaviors—generally representing behavioral excess with respect to social or self-generated activities—have been suggested to constitute the polar dimensions along which personality alterations in AD and other degenerative brain diseases are manifest.30,31 The significant inverse relationship observed between apathy and disinhibition change scores during treatment provides empirical support for this construct. Together, these findings suggest that tacrine may have a moderating effect on personality alterations in AD along the dimensions of apathy and disinhibition. Apathetic and disinhibited behavioral alterations are associated with dysfunction in separate frontal-subcortical circuits and are putatively linked to lesions of dopaminergic and serotonergic pathways, respectively.31–34 Cholinergic modulation of these monoamine neurotransmitter systems by tacrine35 may contribute to the amelioration of personality changes in Alzheimer's disease.
Putative Mechanisms of Cholinergic Responsivity in AD
Individual variations in the topographic distribution, severity, and nature (muscarinic versus nicotinic) of cholinergic denervation in AD may contribute to specific neuropsychiatric manifestations and their relative responsivity to cholinergic augmentation therapy. A dynamic balance between functional (neurochemical) and structural neuropathologic alterations and the relative degree of alteration in other neurotransmitter systems may also influence differential symptom responsivity in relation to disease stage. Alterations in central monoamine metabolism, as well as changes in cholinergic function, have been implicated in the pathophysiology of noncognitive neuropsychiatric symptoms such as apathy, depression, aggression, psychomotor disturbances, and psychosis.32,33,36–38 Tacrine has also been shown to alter central dopaminergic and serotonergic metabolism.39 Alhainen and colleagues40 observed selective increases (toward normal levels) of CSF dopamine and serotonin metabolite levels in AD subjects who showed improved performance on attention and orientation tasks during tacrine treatment. The relative degree to which treatment-based reductions in neuropsychiatric symptoms are attributable to direct cholinergic augmentation, or are indirectly mediated by cholinergic modulation of monoamine transmitters, or reflect a direct influence on noncholinergic neurotransmitter systems is not known.35,41,42 Dementia severity in AD has been correlated to the degree of cholinergic, but not monoaminergic neurotransmitter deficit.43 Markers of cholinergic dysfunction thus may provide insight into the differential nature of neuropsychiatric symptom responsivity to cholinergic agents in relation to dementia severity.
Structural pathological changes in AD are typically most concentrated in medial temporal and in temporoparietal neocortical association areas, which generally parallel the topography of cholinergic deficits.44,45 However, pathological alterations are generally less abundant in frontal neocortical association areas in early to middle stages of AD, whereas the degree of cholinergic dysfunction is almost as severe as in medial temporal and more posterior neocortical areas. A relatively higher degree of cholinergic deficit than of structural pathological alterations in frontal lobe areas—as might be the case in moderately demented AD—may render functional deficits associated with these regions more susceptible to cholinergic therapy. Previous studies have observed that selected attentional and executive functions are the neuropsychological deficits in AD patients that are most responsive to tacrine therapy.46,47 In addition to these putative frontal lobe–mediated cognitive functions, neuropsychiatric symptoms including apathy, disinhibition, agitation, and psychosis have been associated with functional abnormalities in frontal lobe regions on radionuclide functional imaging studies.48,49 The observation that AD patients carrying an apolipoprotein E4 (APOE E4) allele are less responsive to cholinesterase-inhibitor therapy50 may be related to postmortem evidence that choline acetyltransferase, the synthetic enzyme for acetylcholine, is more severely depleted in frontal lobe regions of AD patients with the APOE E4 allele.51 Together, these observations impute a frontal lobe–associated functional topography of therapeutic response to cholinergic agents in AD.
ACKNOWLEDGMENTS
This study was supported by the Department of Veterans Affairs and National Institute on Aging Alzheimer Disease Center Grants AG10123 and AG10533. D.K. was also supported by the Augustus Rose Fellowship of the John Douglas French Foundation for Alzheimer Research and a Merck-AFAR Clinical Geriatric Pharmacology Fellowship. The authors (D.K. and J.L.C.) have served as consultants to or engaged in research sponsored by Parke-Davis, Eli Lilly, Esai-Pfizer, Sandoz, Bayer, Merck-Dupont, Sigma Tau, and Glaxo pharmaceutical companies and the National Institute on Aging. Tacrine (Cognex) is manufactured by Parke-Davis. A portion of this work was presented at the seventh annual meeting of the American Neuropsychiatric Association, Pittsburgh, PA, October 1995.
 |
 |
 |
 |
1. Cummings JL, Victoroff JI: Noncognitive neuropsychiatric syndromes in Alzheimer's disease. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 1990; 3:140–158Google Scholar
2. Mega MS, Cummings JL, Fiorello T, et al: The spectrum of behavioral changes in Alzheimer's disease. Neurology 1996; 46:130–135Crossref, Medline, Google Scholar
3. Mangone CA, Sanguinetti RM, Baumann PD, et al: Influence of feelings of burden on the caregiver's perception of the patient's functional status. Dementia 1993; 4:287–293Medline, Google Scholar
4. Perry EK, Tomlinson BE, Blessed G, et al: Correlation of cholinergic abnormalities with senile plaques and mental test scores in dementia. British Medical Journal 1978; 2:1457–1459Crossref, Medline, Google Scholar
5. Bartus RT, Dean RL III, Beer B, et al: The cholinergic hypothesis of geriatric memory dysfunction. Science 1982; 217:408–417Crossref, Medline, Google Scholar
6. Schneider LS: Clinical pharmacology of aminoacridines in Alzheimer's disease. Neurology 1994; 43(suppl 4):S64–S79Google Scholar
7. Eagger SA, Levy R, Sahakian BJ: Tacrine in Alzheimer's disease. Lancet 1991; 337:989–992Crossref, Medline, Google Scholar
8. Knapp MJ, Knopman DS, Solomon PR, et al: A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. JAMA 1994; 271:985–991Crossref, Medline, Google Scholar
9. Farlow M, Gracon S, Hershey L, et al: A controlled trial of tacrine in Alzheimer's disease: the Tacrine Study Group. JAMA 1992; 268:2523–2528Crossref, Medline, Google Scholar
10. Sunderland T, Tariot PN, Cohen RM, et al: Anticholinergic sensitivity in patients with dementia of the Alzheimer's type and age-matched controls. Arch Gen Psychiatry 1987; 44:418–426Crossref, Medline, Google Scholar
11. Cummings JL, Kaufer DI: Neuropsychiatric aspects of Alzheimer's disease: the cholinergic hypothesis revisited. Neurology 1996; 47:876–883Crossref, Medline, Google Scholar
12. Kaufer DI, Cummings, JL, Christine D: Effect of tacrine on behavioral symptoms in Alzheimer's disease: an open-label study. J Geriatr Psychiatry Neurol 1996; 9:1–6Crossref, Medline, Google Scholar
13. Cummings JL, Mega M, Gray K, et al: The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
14. McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
15. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
16. Wragg RE, Jeste DV: Overview of depression and psychosis in Alzheimer's disease. Am J Psychiatry 1989; 146:577–587Crossref, Medline, Google Scholar
17. Flynn FG, Cummings JL, Gornbein J: Delusions in dementia syndromes: investigation of behavioral and neuropsychological correlates. J Neuropsychiatry Clin Neurosci 1991; 3:364–370Link, Google Scholar
18. Aarsland D, Cummings JL, Yenner G, et al: Relationship of aggressive behavior to other neuropsychiatric symptoms in patients with Alzheimer's disease. Am J Psychiatry 1996; 153:243–247Crossref, Medline, Google Scholar
19. Cummings JL, Ross W, Absher J, et al: Depressive symptoms in Alzheimer disease: assessment and determinants. Alzheimer Dis Assoc Disord 1995; 2:87–93Crossref, Google Scholar
20. Cummings JL, Gorman DG, Shapira J: Physostigmine ameliorates the delusions of Alzheimer's disease. Biol Psychiatry 1993; 33:536–541Crossref, Medline, Google Scholar
21. Gorman DG, Read SR, Cummings JL: Cholinergic therapy of behavioral disturbances in Alzheimer's disease. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 1993; 6:229–234Google Scholar
22. Perry EK, Marshall E, Kerwin JM, et al: Evidence of a monoaminergic:cholinergic imbalance related to visual hallucinations in Lewy body dementia. J Neurochem 1990; 55:1454–1456Crossref, Medline, Google Scholar
23. Cummings JL: Psychosis in neurologic disease: neurobiology and pathogenesis. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 1992; 5:144–150Google Scholar
24. Langlais PJ, Thal LJ, Hansen L, et al: Neurotransmitters in basal ganglia and cortex of Alzheimer's disease with and without Lewy bodies. Neurology 1993; 43:1927–1934Crossref, Medline, Google Scholar
25. Perry EK, Irving D, Kerwin JM, et al: Cholinergic transmitter and neurotrophic activities in Lewy body dementia: similarity to Parkinson's and distinction from Alzheimer disease. Alzheimer Dis Assoc Disord 1993; 7:69–79Crossref, Medline, Google Scholar
26. McCormick DA: Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 1992; 39:337–388Crossref, Medline, Google Scholar
27. Perry EK, Perry RH: Acetylcholine and hallucinations: disease-related compared to drug-induced alterations in human consciousness. Brain Cogn 1995; 28:240–258Crossref, Medline, Google Scholar
28. Liberini P, Valerio A, Memo M, et al: Lewy-body dementia and responsiveness to cholinesterase-inhibitors: a paradigm for heterogeneity in Alzheimer's disease? Trends Pharmacol Sci 1996; 17:155–160Google Scholar
29. McKeith IG, Galasko D, Kosaka K, et al: Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB): report on the consortium on DLB international workshop. Neurology 1996; 47:1113–1124Crossref, Medline, Google Scholar
30. Cummings JL: Personality alterations in Alzheimer's disease: assessment and characterization. Bull Clin Neurosci 1989; 54:80–87Google Scholar
31. Kaufer DI, Cummings JL: Personality alterations in degenerative brain disorders, in Neuropsychiatry of Personality Disorders, edited by Ratey J. Cambridge, MA, Blackwell Scientific, 1995, pp 172–209Google Scholar
32. Palmer AM, Stratmann GC, Procter AW, et al: Possible neurotransmitter basis of behavioral changes in Alzheimer's disease. Ann Neurol 1988; 23:616–620Crossref, Medline, Google Scholar
33. Marin RS: Differential diagnosis and classification of apathy. Am J Psychiatry 1990; 147:22–30Crossref, Medline, Google Scholar
34. Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
35. Adem A: Putative mechanisms of action of tacrine in Alzheimer's disease. Acta Neurol Scand Suppl 1992; 139:69–74Crossref, Medline, Google Scholar
36. Zubenko GS, Moossey, Martinez J, et al: Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol 1991; 48:619–624Crossref, Medline, Google Scholar
37. Palmer AM, DeKosky ST: Monoamine neurons in aging and Alzheimer's disease. J Neural Transm [Gen Sect] 1993; 91:135–159Crossref, Medline, Google Scholar
38. Raskind MA, Peskind ER: Neurologic bases of noncognitive behavioral problems in Alzheimer's disease. Alzheimer Dis Assoc Disord 1994; 8(suppl 3):54–60Google Scholar
39. Soininen H, Unni L, Shillcutt S: Effect of acute and chronic cholinesterase inhibition on biogenic amines in rat brain. Neurochem Res 1990; 15:1185–1190Crossref, Medline, Google Scholar
40. Alhainen K, Helkala E-L, Reinikainen K, et al: The relationship of cerebrospinal fluid monoamine metabolites with clinical response to tetrahydroaminoacridine in patients with Alzheimer's disease. J Neural Transm [P-D Sect] 1993; 5:185–192Crossref, Google Scholar
41. Freeman SE, Dawson RM: Tacrine: a pharmacological review. Prog Neurobiol 1991; 36:257–277Crossref, Medline, Google Scholar
42. Soares JC, Gershon S: THA: historical aspects, review of pharmacological properties and therapeutic effects. Dementia 1995; 6:225–234Medline, Google Scholar
43. Bierer LM, Haroutunian V, Gabriel, S, et al: Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of cholinergic deficits. J Neurochem 1995; 64:749–760Crossref, Medline, Google Scholar
44. Arnold SE, Hyman BT, Flory J, et al: The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex 1991; 1:103–116Crossref, Medline, Google Scholar
45. Procter AW, Lowe, SL, Palmer AM, et al: Topographical distribution of neurochemical changes in Alzheimer's disease. Brain Res 1988; 14:939–944Google Scholar
46. Alhainen K, Helkala E-L, Riekkinen P: Psychometric discrimination of tetrahydroaminoacridine responders in Alzheimer's patients. Dementia 1993; 4:54–58Medline, Google Scholar
47. Sahakian BJ, Owen AM, Morant NJ, et al: Further analysis of the cognitive effects of tetrahydroaminoacridine (THA) in Alzheimer's disease: assessment of attentional and mnemonic function using CANTAB. Psychopharmacology 1993; 110:395–401Crossref, Medline, Google Scholar
48. Craig AH, Cummings JL, Fairbanks L, et al: Cerebral blood flow correlates of apathy in Alzheimer's disease. Arch Neurol 1996; 53:1116–1120Crossref, Medline, Google Scholar
49. Sultzer DL, Mahler ME, Mandelkern MA, et al: The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 1995; 7:476–484Link, Google Scholar
50. Poirier J, Delisle MC, Quirion R, et al: Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer's disease. Proc Nat Acad Sci USA 1995; 92:12260–12264Crossref, Medline, Google Scholar
51. Soininen H, Kosunen O, Helisalmi S, et al: A severe loss of choline acetyltransferase in the frontal cortex of Alzheimer's patients carrying apolipoprotein epsilon 4 allele. Neurosci Lett 1995; 187:79–82Crossref, Medline, Google Scholar



