Clinical Correlates of Aggressive Behavior After Traumatic Brain Injury
Abstract
The authors assessed aggressive behavior in 89 patients with traumatic brain injury (TBI) and 26 patients with multiple trauma but without TBI using a quantitative scale (the Overt Aggression Scale) and examined its clinical correlates. Aggressive behavior was found in 33.7% of TBI patients and 11.5% of patients without TBI during the first 6 months after injury. Aggressive behavior was significantly associated with the presence of major depression, frontal lobe lesions, poor premorbid social functioning, and a history of alcohol and substance abuse. Interventions aimed at treatment of depression and substance abuse and enhancing social support may help reduce the severity of this disruptive behavior.
Traumatic brain injury (TBI) is a major health problem in industrialized societies. In the United States, the annual incidence of TBI can be conservatively estimated as 200 cases per 100,000 population.1 Since 1979, the case/mortality rate associated with traumatic brain injury has decreased from 24.6 per 100,000 population to 19.3 per 100,000 population per year.2 This decrease is probably due to improvements in vehicle safety as well as in trauma care. The incidence of new, long-lasting disabilities resulting from brain injury is approximately 33 per 100,000 population per year, or 83,000 new disabled patients each year.3
Associations between TBI and neuropsychiatric disorders have been recognized for many years.4 Aggressive behavior is one of the most socially and vocationally disruptive consequences of these neuropsychiatric disorders. Aggression endangers the safety of patients, families, and caregivers.5 It may prevent patients from receiving the care that they need and disrupt their rehabilitation process.6–8 Estimates of the frequency of aggressive behaviors during the acute period after TBI have ranged from 11% to 96%.6,9–11 This high variability is related to the use of different operational definitions of aggressive behavior as well as different assessment instruments. The Overt Aggression Scale (OAS) was devised to assess and quantify aggression.12 It has high reliability and validity and has been used to effectively rate aggression in patients with a wide range of disorders.12,13 Previous investigators have used the OAS to quantify the severity of aggression after TBI and to examine treatment strategies14 as well as to distinguish aggression from restlessness.11
The occurrence of aggressive behaviors has been associated with multiple etiological factors. It has been reported that aggression following TBI is associated with younger age and poor social functioning as well as with the type, extent, and location of brain lesions.7,15,16 In addition, previous studies have suggested an association between the occurrence of aggression and a history of alcohol or other substance abuse.17,18 In these previous studies, however, aggressive behavior was not adequately quantified.
In this study we examined the clinical correlates of aggressive behavior occurring during the early recovery period from TBI—that is, during the first 6 months after clearing of posttraumatic amnesia. We hypothesized that aggression would be significantly associated with younger age, poor premorbid social functioning, and the occurrence of depressive disorder. In addition, we predicted an association of aggressive behavior with frontal lobe lesions.
METHOD
Study Population
The study group consisted of 89 consecutive patients with closed head injury admitted to the University of Iowa Hospitals and Clinics (n = 58) and the Iowa Methodist Medical Center, in Des Moines, Iowa (n = 31). A diagnosis of TBI was substantiated by history of posttraumatic amnesia that lasted at least 30 minutes after the traumatic event. Patients with penetrating head injuries or associated spinal cord injury were excluded from the study.
Patients who had severe comprehension deficits (those who were unable to complete part II of the Token Test19), which precluded a thorough neuropsychiatric evaluation, were also excluded from the study. Our control group comprised 26 patients consecutively admitted to the University of Iowa Hospitals and Clinics with multiple traumas but without clinical or radiological evidence of nervous system involvement—that is, without primary or secondary brain damage or spinal cord injury. Sixty-seven of the 89 patients with TBI (75.3%) and 19 of the 26 patients in the control group (73.1%) were injured in a motor vehicle accident. All 115 patients gave written informed consent for participation in this study.
Severity of Brain Injury
We used two measures of severity of traumatic brain injury. The first was the 24-hour Glasgow Coma Scale (GCS) score. In this measure, scores from 13 to 15 indicate mild head injury, scores from 9 to 12 indicate moderate head injury, and scores from 4 to 8 indicate severe head injury.20 Patients who have a GCS score in the 13–15 range but who underwent intracranial surgical procedures or presented with focal lesions greater than 15 cc, however, were considered to have suffered a moderate head injury.21 The second measure we used was the duration of posttraumatic amnesia (PTA).22 The PTA period was estimated retrospectively through a structured interview that proved to have a high correlation with other prospective determinations of PTA duration. We also assessed whether medical complications occurred that might have contributed to secondary brain damage, such as hypoxia or arterial hypotension.
Traumatic brain injury was also classified according to the criteria proposed by the Traumatic Coma Data Bank (TCDB).23 This classification is based on the presence of a diffuse or mass (focal) lesion on initial CT scan.
Aggression Assessment
Aggressive behaviors were assessed with the OAS. The OAS defines four categories of aggressive behavior, each of which is given a weighted score. Verbal aggression is scored 1 to 4 points, physical aggression against objects, 2 to 5 points, and physical aggression against self or others, 3 to 6 points per incident. The aggression score (AS) is the sum of the weighted scores of the most severe behaviors in each category; the maximum AS is 21. We defined significant aggressive behavior as an AS more than 3 or a score of 3 with physically aggressive behavior. Aggression occurring in the context of delirium or during the period of posttraumatic amnesia was not considered in this study. Patients who had at least four episodes of significant aggressive behavior and had AS scores of 3 or greater constituted our aggressive group. The nonaggressive group included all patients who did not meet the aggressive behavior criteria during the 6-month period following PTA.
Assessment of premorbid aggressive behavior is hindered by potential retrospective bias, particularly among patients with TBI. In order to use a measure that would not be subject to such bias, we registered the frequency of aggressive behaviors that resulted in police intervention and legal actions (e.g., assault and domestic violence) among the TBI and control groups. Information on the latter was obtained from the patient, close relatives, and other informants who knew the patient well and from medical records.
Psychiatric Assessment
All patients were assessed by a fully trained psychiatrist using two semistructured interviews, a modified version of the Present State Examination24 designed to elicit symptoms of mood and anxiety disorders, and the Structured Clinical Interview for DSM-III-R (SCID).25 The severity of depressive and anxiety symptoms was assessed using the Hamilton Depression Rating Scale (HAM-D)26 and the Hamilton Anxiety Scale (HAM-A).27 The Mini-Mental State Examination (MMSE)28 was used as a global measure of cognitive functioning. Impairment in activities of daily living was assessed with the Functional Independence Measure (FIM).29 Social functioning was quantitatively assessed using the Social Functioning Exam (SFE) and the Social Ties Checklist (STC).30 SFE scores range from 0 (greatest satisfaction) to 1 (least satisfaction). STC scores range from 0 to 10, with higher scores indicating less social support. Initial SFE and STC scores assessed social functioning before the traumatic episode. The reliability and validity of each of these instruments have been demonstrated in brain-injured populations.31
Neuroimaging
CT and occasionally MRI scans were obtained as part of the standard clinical evaluation in the emergency and neurosurgery departments of institutions involved in the study. The nature, extent, and location of traumatic lesions were classified according to the Traumatic Coma Data Bank (TCDB) criteria and registered through the use of appropriate TCDB forms. A neurologist trained in assessment of structural neuroimaging scans who was blind to the RESULTS of the psychiatric examination read all scans.
Data Analysis
Background characteristics, results of neuropsychiatric assessment, and mean GCS score were analyzed by Student's t-test (two-tailed) using mean and standard deviations. Frequency distributions of background characteristics, results of neurological assessment, and major and minor depression were analyzed using chi-square tests, or Fisher's exact test (two-tailed), if sample sizes were prohibitively small.
Results
Comparison of TBI and Control Groups
The background characteristics of the study and control groups are summarized in Table 1. There were no significant differences between patients with traumatic brain injury and trauma patients without brain injury in age, gender, race, years of education, or Hollingshead social class. The degree of functional impairment as measured by FIM scores was not significantly different between the two groups. In addition, there were no significant differences between the TBI patients and the control group patients in the frequency of a history of mood or anxiety disorders, of alcohol or other substance abuse, or of aggressive behavior that warranted legal intervention prior to the traumatic episode. However, posttraumatic aggression was significantly more frequent among patients with brain injury than those in the control group (Fisher's exact test, P=0.03).
Clinical Correlates of Aggressive Behavior
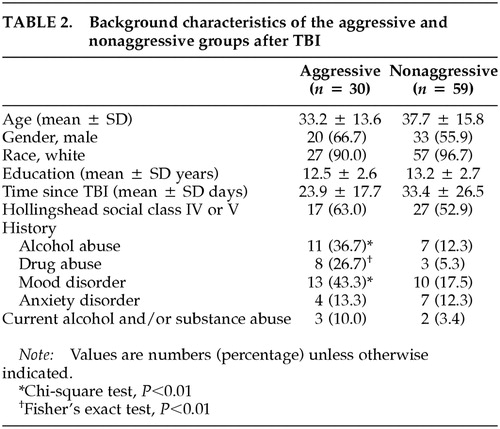
Of the 89 patients who suffered a traumatic brain injury, 30 of them (33.7%) met the aforementioned criteria for the presence of significant aggressive behavior during the first 6 months after the traumatic episode. The remaining 59 patients (66.3%) constitute the nonaggressive group.
The background characteristics of the two groups are summarized in Table 2. There were no significant differences between the aggressive and the nonaggressive groups in age, gender, race, years of education, socioeconomic status, or history of anxiety disorder. In addition, the frequencies of hypoxia and hypotension, the two most significant complications contributing to secondary brain damage, were not significantly different between the aggressive and the nonaggressive groups (hypoxia, 9.6% versus 7.5%; hypotension, 4.8% versus 5.0%). Patients in the aggressive group had a significantly higher frequency of a history of a mood disorder (χ2 = 6.72, df = 1, P = 0.01), alcohol abuse (χ2 = 7.12, df = 1, P = 0.008), and substance abuse (Fisher's exact test, P = 0.007) than those in the nonaggressive group. However, there was no significant difference between the two groups in the frequency of alcohol or substance abuse during the month preceding the onset of aggression. In addition, five of 30 TBI patients with aggression had a history of legal intervention for aggressive behavior, compared with one of 59 nonaggressive TBI patients (Fisher's exact test, P = 0.02; data not shown).
Results of the neuropsychiatric evaluations are summarized in Table 3. A diagnosis of major depression was significantly more frequent in the aggressive group than in the nonaggressive group (χ2 = 6.54, df = 1, P = 0.01). Of the 30 patients in the aggressive group, aggressive behavior and major depressive disorder occurred simultaneously in 17 (56.7%) of them. There was no significant difference in the frequency of minor depression between patients in the aggressive and nonaggressive groups. Compared with the nonaggressive group, patients in the aggressive group had significantly higher HAMD scores (t=–3.51, df =87, P=0.0007) and HAMA scores (t=–3.37, df=87, P=0.001) and had significantly poorer social functioning (t=–3.27, df=87, P=0.002). There were no significant differences between the aggressive and nonaggressive groups in MMSE, FIM, and STC scores.
Neurological findings are summarized in Table 4. Severity of brain injury as measured by either GCS scores or the duration of PTA was not significantly different between aggressive and nonaggressive patients. Fourteen patients had focal frontal lobe lesions, six patients had focal frontal lobe lesions and radiological evidence of diffuse injury, 18 patients had focal lesions in other areas of the brain, nine patients had non-frontal-lobe focal brain lesions and radiological evidence of diffuse injury, and 39 patients had diffuse brain injury. There was no between-group difference in the frequency of right or left hemisphere lesions. However, patients in the nonaggressive group had a greater frequency of diffuse injury than aggressive patients (χ2 = 3.95, df = 1, P = 0.047), and the frequency of frontal lobe lesions was significantly higher among patients in the aggressive group (χ2 = 8.05, df = 1, P =0.005). In addition, patients with focal frontal lobe lesions showed significantly higher mean AS scores than patients with focal lesions in other brain areas (4.1 ± 4.0 versus 1.4 ± 2.7, t = 2.78, df = 45, P = 0.008).
DISCUSSION
In this study, we used a valid and reliable quantitative scale to formulate an operational definition of aggression following traumatic brain injury. We found that 33.7% of the TBI patients demonstrated significant aggressive behavior during the first 6 months after their injury. Aggression was significantly more frequent among patients with traumatic brain injury than patients in a comparable group with traumatic injury that did not involve the brain. Aggressive behavior was significantly associated with the presence of major depression, a history of alcohol or drug abuse, and frontal lobe lesions. In addition, aggressive patients evidenced poorer social functioning than the nonaggressive group. Compared with nonaggressive TBI patients, aggressive patients had a significantly higher frequency of legal interventions for aggressive behavior prior to the traumatic event.
Before discussing the implications of this study, we should acknowledge its methodological limitations. First, most of our subjects were young male Caucasian patients. Thus, our findings may not pertain to other populations of TBI patients. Second, patients with a decreased level of consciousness or severe comprehension deficits were excluded from the study. It is uncertain whether our findings would change if these patients were included. Finally, we did not assess patients within the PTA period. The pathophysiology and clinical correlates of aggressive behavior may certainly differ in this context.
Given these limitations, how may we construe these findings? Aggressive behavior was significantly associated with the occurrence of major depression during the first 6 months after the traumatic brain injury. The most obvious explanation of this association is that major depression causes aggressive behaviors and that aggression is one of the clinical features of the depressive disorders that follow TBI. However, the onset of major depression was independent of the onset of severely aggressive behavior in the majority of our patients. This suggests that other factors contribute to both behavioral disturbances. What would these factors be?
The association of violent and impulsive behavior with abnormalities of the serotonergic system is one of the findings most consistently replicated in neuropsychiatric research. Furthermore, a specific subtype of major depressive disorder characterized by aggressive behavior and anger attacks has been reported to be associated with a dysfunctional serotonergic system.8,32
Aggressive behaviors have also been related to the presence of brain lesions in specific locations such as the hypothalamus,33 paralimbic areas of the temporal lobe,34 and the prefrontal cortex.35 On the other hand, we have previously reported an association between major depression and left prefrontal lesions during the early recovery period from TBI.36 It is conceivable that frontal lobe damage, including lesions of the ascending serotonergic pathways, contributes to the pathophysiology of both depression and violent behavior. Interestingly, compared with patients who suffered diffuse axonal injury, patients with frontal contusions were found to have lower levels of CSF 5-hydroxyindoleacetic acid, a metabolite of serotonin.37 In addition, treatment with a selective serotonin reuptake inhibitor improved depression and reduced aggressive behaviors following TBI.38
The frequency of a history of alcohol and substance abuse was significantly higher among aggressive patients than among nonaggressive patients. This finding is consistent with previous reports17,18 and indicates that alcohol and substance abuse may contribute to aggressive behavior, independently of withdrawal effects. Frontal lobe dysfunction as well as abnormal serotonergic modulation has been observed in patients with addictive disorders.39,40 The interaction or independent effects of these abnormalities in the etiology of aggression need to be clarified in future studies.
Finally, our studies have shown that in TBI patients, both aggression and depressive disorder are associated with poor social functioning. Previous studies reported that aggression was associated with disruption of family relationships35 and poor occupational performance.41 Social integration decreases impulsive behavior and lessens the vulnerability to developing depressive disorders. Furthermore, it has been suggested that social behavior is influenced by the same biological factors as impulsiveness and aggression (i.e., prefrontal modulation and serotonergic function).42
In summary, aggression following TBI is associated with multiple biological and psychosocial factors, including major depression, substance abuse, and impaired social function as well as the presence of brain injury involving the frontal lobe. These findings suggest that interventions aimed at treating major depression or substance abuse and improving social function may help reduce episodes of aggression in patients who have suffered traumatic brain injury.
ACKNOWLEDGMENTS
This work was supported in part by NIMH grants MH-40355, MH-52879, and MH-53592 to Dr. Jorge and Dr. Robinson and a grant from Nippon Medical School to Dr. Tateno. The authors thank Russell Hansen for image analysis and Stephanie Rosazza and Teresa Kopel for gathering data.
 |
 |
 |
 |
1 Guerrero JL, Thurman DJ, Sniezek JE: Emergency department visits associated with traumatic brain injury: United States, 1995-1996. Brain Inj 2000; 14:181-186Crossref, Medline, Google Scholar
2 Thurman D, Guerrero J: Trends in hospitalization associated with traumatic brain injury. JAMA 1999; 282:954-957Crossref, Medline, Google Scholar
3 Sosin DM, Sniezek JE, Waxweiler RJ: Trends in death associated with traumatic brain injury, 1979 through 1992. JAMA 1995; 273:1778-1780Crossref, Medline, Google Scholar
4 Morton MV, Wehman P: Psychosocial and emotional sequelae of individuals with traumatic brain injury: a literature review and recommendations. Brain Inj 1995; 9:81-92Crossref, Medline, Google Scholar
5 Bruke WH, Wesolowski MD, Lane I: A positive approach to the treatment of aggressive brain injured clients. Int J Rehabil Res 1988; 11:235-241Crossref, Medline, Google Scholar
6 Kim SH, Manes F, Kosier T, et al: Irritability following traumatic brain injury. J Nerv Ment Dis 1999; 187:327-335Crossref, Medline, Google Scholar
7 Sandel ME, Mysiw WJ: The agitated brain injured patient. I: definitions, differential diagnosis, and assessment. Arch Phys Med Rehabil 1996; 77:617-623Crossref, Medline, Google Scholar
8 Mysiw WJ, Sandel ME: The agitated brain injured patient. II: pathophysiology and treatment. Arch Phys Med Rehabil 1997; 78:213-220Crossref, Medline, Google Scholar
9 Levin HS, Grossman RG: Behavioral sequelae of closed head injury. Arch Neurol 1978; 35:720-727Crossref, Medline, Google Scholar
10 Rao N, Jellinek HM, Woolston DC: Agitation in closed head injury: haloperidol effects on rehabilitation outcome. Arch Phys Med Rehabil 1985; 66:30-34Medline, Google Scholar
11 Brooke MM, Questad KA, Patterson DR, et al: Agitation and restlessness after closed head injury: A prospective study of 100 consecutive admissions. Arch Phys Med Rehabil 1992; 73:320-323Crossref, Medline, Google Scholar
12 Yudofsky SC, Silver JM, Jackson W, et al: The overt aggression scale for the objective rating of verbal and physical aggression. Am J Psychiatry 1986; 143:35-39Crossref, Medline, Google Scholar
13 Silver JM, Yudofsky SC: The overt aggression scale: overview and guiding principles. J Neuropsychiatry Clin Neurosci 1991; 3: (suppl)S22-S29Google Scholar
14 Alderman N, Davies JA, Jones CY, et al: Reduction of severe aggressive behaviour in acquired brain injury: case studies illustrating clinical use of the OAS-MNR in the management of challenging behaviours. Brain Inj 1999; 13:669-704Crossref, Medline, Google Scholar
15 Paradiso S, Robinson RG, Arndt S: Self-reported aggressive behavior in patients with stroke. J Nerv Ment Dis 1996; 184:746-753Crossref, Medline, Google Scholar
16 Evans RW: The postconcussion syndrome and the sequelae of mild head injury. Neurol Clin 1992; 10:815-847Crossref, Medline, Google Scholar
17 Sparadeo FR, Gill D: Effects of prior alcohol use on head injury recovery. J Head Trauma Rehabil 1989; 4:75-82Crossref, Google Scholar
18 Swanson JW, Holzer III CE, Ganju VK, et al: Violence and psychiatric disorder in the community: evidence from the epidemiologic catchment area surveys. Hosp Community Psychiatry 1990; 41:761-770Medline, Google Scholar
19 De Renzi E, Vignolo LA: The token test: a sensitive test to detect receptive disturbances in aphasics. Brain 1962; 85:665-678Crossref, Medline, Google Scholar
20 Teasdale G, Jennett B: Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2:81-84Crossref, Medline, Google Scholar
21 Levin HS, Amparo E, Einsenberg HM, et al: Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J Neurosurg 1987; 66:706-713Crossref, Medline, Google Scholar
22 Russell WR, Smith A: Post-traumatic amnesia in closed head injury. Arch Neurol 1961; 5:4-17Crossref, Medline, Google Scholar
23 Marshall LF, Bowers SA, Klauber MR, et al: A new classification of head injury based on computerized tomography. J Neurosurg 1991; 75:S14-S20Google Scholar
24 Wing JK, Cooper JE, Sartorius N: Measurement and Classification of Psychiatric Syndromes: An Instruction Manual for the PSE and CATEGO Program. New York, Cambridge University Press, 1974Google Scholar
25 Spitzer RL, Williams JBW, Gibbon M, et al: The structured clinical interview for DSM-III-R (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
26 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
27 Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50-55Crossref, Medline, Google Scholar
28 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975; 12:189-198Crossref, Medline, Google Scholar
29 Granger CV, Hamilton BB, Keith RA, et al: Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil 1986; 1:59-74Crossref, Google Scholar
30 Starr LB, Robinson RG, Price TR: Reliability, validity, and clinical utility of the social functioning exam in the assessment of stroke patients. Exp Aging Res 1983; 9:101-106Crossref, Medline, Google Scholar
31 Robinson RG, Lipsey LR, Bolla-Wilson K, et al: Mood disorders in left-handed stroke patients. Am J Psychiatry 1985; 142:1424-1429Crossref, Medline, Google Scholar
32 Fava M, Vuolo RD, Wright EC, et al: Fenfluramine challenge in unipolar depression with and without anger attacks. Psychiatry Res 2000; 94:9-18Crossref, Medline, Google Scholar
33 Anderson K, Silver JM: Modulation of anger and aggression. Semin Clin Neuropsychiatry 1998; 3:232-242Medline, Google Scholar
34 Tonkonogy JM: Violence and temporal lobe lesion: head CT and MRI data. J Neuropsychiatry Clin Neurosci 1991; 3:189-196Link, Google Scholar
35 Grafman J, Schwab K, Warden D, et al: Frontal lobe injuries, violence, and aggression: a report of the Vietnam head injury study. Neurology 1996; 46:1231-1238Crossref, Medline, Google Scholar
36 Jorge RE, Robinson RG, Arndt SV, et al: Depression following traumatic brain injury: a 1 year longitudinal study. J Affect Disord 1993; 27:233-243Crossref, Medline, Google Scholar
37 Van Woerkom TC, Teelken AW, Minderhoud JM: Difference in neurotransmitter metabolism in frontotemporal-lobe contusion and diffuse cerebral contusion. Lancet 1977; 1:812-813Crossref, Medline, Google Scholar
38 Fann JR, Uomoto JM, Katon WJ: Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 2000; 12:226-232Link, Google Scholar
39 London ED, Ernst M, Grant S, et al: Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex 2000; 10:334-342Crossref, Medline, Google Scholar
40 Bailly D, Vignau J, Racadot N, et al: Platelet serotonin levels in alcoholic patients: changes related to physiological and pathological factors. Psychiatry Res 1993; 47:57-68Crossref, Medline, Google Scholar
41 Herzberg JL, Fenwick PBC: The aetiology of aggression in temporal-lobe epilepsy. Br J Psychiatry 1988; 153:50-55Crossref, Medline, Google Scholar
42 Higley JD, Suomi SJ, Linnoila M: CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology 1991; 103:551-556Crossref, Medline, Google Scholar



