Magical Ideation Modulates Spatial Behavior
Abstract
Previous research has found that animals as well as persons with psychotic disorders preferentially orient away from the cerebral hemisphere with the more active dopamine system. This study investigated the modulation of spatial behavior by a mode of thinking reminiscent of the positive symptoms of psychosis. In a non-treatment-seeking sample of healthy volunteers (20 women and 16 men), the authors assessed the lateral biases in turning and veering behavior and in line bisection as a function of their magical ideation, that is, a mild form of schizotypy. Across tasks, pronounced magical ideation was associated with reduced right-sided orientation preferences. This finding suggests a relative hyperdopaminergia of the right hemisphere as the biological basis of magical ideation.
It is likely that an abnormally functioning dopamine (DA) system is involved in the generation of positive psychotic symptoms. This association originates from the observation that neuroleptic medication (DA antagonists) improves psychotic symptoms, especially positive symptoms.1 Administration of levodopa and DA agonists can trigger psychotic relapse in patients with schizophrenia2,3 and induce hallucinatory and delusional episodes in nonpsychotic individuals.4,5 The relationship between DA and psychosis is also apparent from findings in patients with parkinsonism. When overtreated with DA agonists, these patients may develop psychotic symptoms.6
Turning behavior in animals is a reliable marker of DA activity. Animals turn preferentially in the direction of the cerebral hemisphere with less DA.7 Moreover, animals trained to turn in one direction were found to have increased DA concentrations in the contralateral hemisphere.8 The finding that patients with schizophrenia reveal a left-sided turning preference that is quantitatively related to the severity of psychotic symptoms9 suggests an asymmetrical DA system in this population, with the right hemisphere having a more active DA system than the left. Also, patients with hemiparkinsonism preferentially turn toward the hemisphere with the more severe dopaminergic cell degeneration.10 In bisection tasks, analogous symptom-related shifts in spatial attention were reported for both patients with psychosis11 and hemiparkinsonism.12
The relevance of DA for spatial awareness is further supported by patients with neglect. This population has an impressive tendency to direct their attention toward the left hemispace, a pathological condition most frequently associated with impairments in right-hemispheric parietal,13 temporal,14 and subcortical structures.15 A study by Heilman's group has shown that neglect in such patients can attenuate under DA-agonistic treatment,16 which is thought to restore the specific left-sided deficits in spatial attention. These findings have been replicated by an independent research group.17 In the case of schizophrenia, a baseline hyperdopaminergia of the right hemisphere would shift attention toward the left hemispace much in the same way as DA treatment would do in patients with neglect. Therefore, in schizophrenia, hemiparkinsonism, and neglect, hemispatial inattention seems to result from an asymmetrically organized DA system.
Recent data suggest that spatial awareness in healthy subjects may also be modulated by a psychotic-like thinking style, namely magical ideation (MI). Subjects who endorse magical beliefs evidence a right hemispatial inattention that is qualitatively similar to that of patients with schizophrenia.18–20 MI is conceived of as a mild analog to the positive symptoms reported by patients with schizophrenia. It primarily comprises a tendency to assume hidden meanings in random configurations and to insist in a causal determination of coincidences.21 Although the concept of MI was introduced as an indicator of schizotypy,22 subsequent work has unequivocally demonstrated that the continuum of MI is psychometrically relevant even within samples of healthy subjects scoring below what would be considered indicative of a schizotypal personality disorder by commonly accepted standards. Most importantly, even entirely healthy subjects with relatively high MI scores display neuropsychological abnormalities that are qualitatively similar to those displayed by patients with schizophrenia. These comprise, among other things, impairments of left-hemispheric temporal lobe functions,23 an enhanced reliance on right-hemisphere-mediated language functions,24 and deficient somatosensory abilities.25 Investigations of MI in healthy subjects thus may help to specify primary brain mechanisms underlying schizophrenia, especially with regard to positive symptoms.
This study used a within-subject design to investigate whether line bisection and axial deviations during whole-body movements are directed more strongly to the left hemispace in healthy subjects with high compared with low MI scores.
METHOD
Subjects
A total of 36 healthy subjects (20 women and 16 men) were recruited by personal contact and flyers posted at the University of Zurich and at the local university hospital, where the testing took place. All of them gave informed consent prior to participation in the study. The group had a mean age of 29.6 years (SD, 6.6 years; range, 23–48 years) and had a mean of 19.0 years (±3.5 years) of education. Potential participants who were currently taking any medications or had a history of drug abuse were not enrolled in the study. Absence of a neuropsychiatric history was assessed with an extended clinical interview.26 All subjects were right-handed according to a 13-item handedness questionnaire.27
Magical Ideation Scale
The Magical Ideation scale is a 30-item questionnaire that includes items such as “I sometimes have a feeling of gaining or losing energy when people look at me or touch me” (keyed “true”) and “Some people can make me aware of them just by thinking about me” (keyed “true”). Scores on the MI scale range from 0 to 30, with higher scores indicating more pronounced magical thinking. The scale is published in full in Eckblad and Chapman,22 and normative data can be found in Garety and Wessely.28
Spatial Tasks
Line Bisection: Six horizontal parallel lines (lengths: 13 cm, 16 cm, 18 cm, 20 cm, 24 cm, and 25 cm) were displayed twice on a single sheet of paper. The sheet was placed centrally in front of the subject, who was instructed to mark the center of each line, using a paper with a 29 cm × 2 cm window to suppress visual interference from the other lines. Two trials were conducted, one with the left hand and one with the right hand. The side of the starting hand was counterbalanced between subjects. For each hand separately, we calculated the number of lines that were bisected to the left or right, respectively, from the center. Possible scores thus ranged from 0 (all lines correctly bisected) to 12 (all 12 lines were bisected to one side; a minus point for each line left-bisected, a plus point for each line right-bisected).
Turning: Spontaneous turning preferences were measured with a rotometer, which is a lightweight belt-mounted device that consists of a position sensor, an electronic processing circuit, and a rechargeable battery that monitors changes in the orientation of the dorsal-ventral axis.9,10,29 Magnetic north is used as an external reference and is tracked by a compass. Full 360-degree turns to either side were measured for each subject over a period of 20 hours over 3 consecutive days.
Veering: Subjects were asked to walk blindfolded, with their ears plugged and their shoes off, along a straight line in the middle of a corridor (1.60 m wide and 20 m long). The experimenter walked in front of the subject and counted a veer (subjects were stopped) when the deviation from the line was larger than 20 cm for both feet. After a veer, the subjects' bearings were restored by instructing them to walk for a short distance along a metal strip placed on the line between subjects' feet. The strip was then removed and the subjects continued to walk. The start side of the corridor was counterbalanced between subjects. We assessed the side of the first veer and the number of veers to either side.
Procedure
At a first meeting, participants received the rotometer and instructions about its use. The instructions indicated that the device was to be worn all day and removed only for sports, sleep, or activities that could result in its damage. When it was removed, the subjects were instructed to lay the device down in such a way as to minimize any confounding non-body-related movements. At the second meeting, which took place at least 3 days later, subjects returned the device, filled in the questionnaires, and performed the line bisection and veering tasks, in that order.
Data Analyses
Shapiro-Wilk statistics revealed that most measures (displacements in the line bisection task with the left hand [W = 0.92, P = 0.02] and right hand [W = 0.93, P=0.04], full turns to the left side [W = 0.89, P<0.001], veers to the right [W = 0.90, P = 0.004] and left [W = 0.89, P = 0.002] side) were not normally distributed, so nonparametric statistics were calculated. Group comparisons were assessed with the Mann-Whitney U test and single comparisons between variables with the Wilcoxon test. Spearman rank-correlation coefficients, corrected for continuity, were calculated to compare continuous variables. All tests were two-tailed, and, except where otherwise noted, the alpha level was set at 0.05.
RESULTS
Subjects
Age and education did not differ significantly between women and men (mean age for women, 29.0 ± 5.8 years, for men, 30.5 ± 7.5 years; Z = –0.10, P = 0.92; education for women, 19.6 ± 3.7 years, for men, 18.3 ± 3.1 years; Z = –0.93, P = 0.35).
MI Scale
The mean MI score was 9.4 (± 5.8); no significant difference was observed between men and women (men, 8.2 ± 4.6; women, 10.4 ± 6.6; Z = –0.81, P = 0.42). The range of MI scores was within the range reported for normal individuals (mostly college students).28 A preplanned split at the median scale score9 produced a low MI group (n = 17 subjects, 8 of them women) and a high MI group (n = 19 subjects, 12 of them women). The distribution of women and men in the high and low MI groups was not significantly different (χ2 = 0.94; df = 1, P = 0.33). Neither years of education nor subjects' age differed between groups (education, Z = –0.54, P = 0.59; age, Z = –1.16, P = 0.25). Since gender did not interact with age, education, or MI group, data for women and men were collapsed.
Spatial Tasks
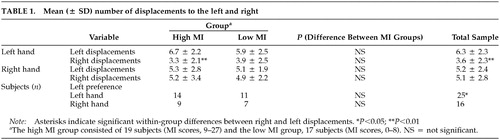
Line Bisection: Subjects made a significantly higher number of total left (right plus left hand, 11.5 ± 3.7) than right displacements (right plus left hand, 8.7 ± 4.1; Z = –2.34, P = 0.02). For the hands separately, this difference was significant for the left hand (Z = –3.14, P = 0.002) but not the right (Z = –0.43, P = 0.66) (Table 1). With the left hand, more subjects bisected lines to the left than to the right (χ2 = 6.43, df = 1, P = 0.01). With the right hand, neither side was preferred over the other (χ2 = 0.26, df = 1, P = 0.61).
Line Bisection Performance and MI: Across hands, the high MI group did not differ from the low MI group in the number of left (Z = –0.98, P = 0.33) or right (Z = –0.48, P = 0.63) displacements. Groups did not differ significantly in left displacements with the left (Z = –0.90, P = 0.37) or right hand (Z = –0.60, P = 0.55) and for right displacements with the left (Z = –0.74, P = 0.46) or right hand (Z = –0.22, P = 0.83). As illustrated in Figure 1A, the high MI group demonstrated a significant preference for left-sided displacements with the left hand (Z = –2.70, P = 0.007). For the right hand, the difference was in the same direction, but was note statistically significant (Z = –0.46, P = 0.65). The low MI group did not differ between left or right displacements for either hand (left hand, Z = –1.66, P = 0.10; right hand, Z = –0.06, P = 0.95).
The correlations between MI scores and number of left displacements with either hand (left hand, Spearman rank-correlation coefficient [Sr] = –0.15, P = 0.38; right hand, Sr = –0.04, P = 0.81) or right displacements with either hand (left hand, Sr = –0.05, P = 0.78; right hand, Sr = 0.11, P = 0.52) were not significant.
Two chi-square comparisons, one for each hand, between the number of subjects with a right- or left-side bias and MI groups were not significant (left hand, χ2 = 0.73, P = 0.39; right hand, χ2 = 0.27, P = 0.60). In both groups, slightly more subjects bisected lines to the left than to the right of the center (Table 1).
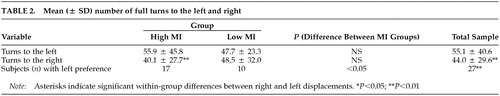
Turning: For the whole sample, the mean total number of turns (left plus right) was 99.2 (± 66.3). Significantly more turns were performed to the left than to the right (Z = –2.48, P = 0.01), and significantly more subjects demonstrated a preference for turning to the left than to the right (χ2 = 9.0, P = 0.003) (Table 2).
The mean total number of turns was not different between the high MI (96.0 ± 72.5) and low MI (102.7 ± 60.5) groups (Z = –0.56, P = 0.59). The number of left turns was significantly higher than the number of right turns for the subjects in the high MI (Z = –2.90, P = 0.004) but not the low MI group (Z = –0.85, P = 0.39) (see Figure 1B and Table 2). However, the two groups did not differ from each other in the number of left (Z = –0.56, P = 0.58) or right turns (Z = –0.84, P = 0.40). No relationship was found between raw MI score and the number of turns to the left (Sr = –0.02, P = 0.93) or to the right (Sr = –0.05, P = 0.77).
The number of subjects preferring right or left turns differed between groups (χ2 = 4.5, P = 0.03). In the high MI group, the number of subjects with a left turning preference was higher than in the low MI group (see Table 2).
Veering: For the whole group, the mean total number of veers (left plus right) was 3.6 (± 2.2). The number of veers to the right did not differ significantly from the number of veers to the left (Z = –0.53, P = 0.60). The number of subjects veering to the left was comparable to those veering to the right (χ2 = 1.50, df = 2, P = 0.47) (Table 3). With respect to the first veer, however, only three subjects walked straight ahead without any veer, and the remainder veered in almost equal numbers to the left or the right (χ2 = 10.50, df = 2, P = 0.005) (Table 3).
The high and low MI groups did not differ in mean total number of veers (high MI group, 3.1 ± 2.3; and low MI group, 4.1 ± 2.0; Z = –1.44, P = 0.15) or in number of veers to one side or the other (high MI group, Z = –1.00, P = 0.32; low MI group, Z = –1.64, P = 0.10). Figure 1C shows that subjects in the high MI group deviated less to the right than those in the low MI group (Z = –2.43, P = 0.02). The difference between MI groups for veers to the left was not significant (Z = –0.29, P = 0.77). Likewise, correlation analyses confirmed that subjects deviated significantly less to the right the higher their MI scores were (Sr = –0.43, P = 0.01). By contrast, veers to the left were independent of MI scores (Sr =–0.01, P = 0.97).
The number of subjects with a left, right, or no side preference did not differ between MI groups (χ2 = 3.90, df = 2, P = 0.14). With regard to the first veer, however, subjects in the high MI group showed a preference for walking either straight or veering to the left, whereas the subjects in the low MI group had a preference for veering to the right (χ2 = 8.18, df = 2, P = 0.02).
DISCUSSION
This study investigated whether MI, a thinking style found in the normal population but originally introduced as an indicator of schizotypy,22 is related to an enhanced right-sided spatial inattention similar to that reported for patients with psychosis.9,11 Three types of spatial behavior were investigated, namely, line bisection, whole-body turns, and veers while attempting to walk blindfolded in a straight line. MI was assessed with a well-validated 30-item scale22 previously used to demonstrate associations between magical beliefs and hemispheric processing, both in our own lab18,20,23–24,30 and by other research groups.19,31–32
In the line bisection task, the high MI group (those with scores above the median) evidenced a hand difference in bisection performance. Subjects showed a “pseudoneglect”33 with their left hand but not with their right. The occurrence of pseudoneglect restricted to the left hand is known from previous investigations,33 as is the association between MI and right-sided inattention.18–20 However, the finding of a left-hand pseudoneglect exclusively for the high MI group but not the low MI group is new. We should note that line bisection performance critically depends on task conditions.33,34 Our procedure involved a paper-and-pencil version and differed from that used in previous research. Brugger and Graves,18 for instance, used a tactile rod bisection task, and Taylor et al.20 measured lateral deviations in subjects' recollections of a complex figure from memory. These procedural differences likely explain the lack of a pseudoneglect for the right hand in our experiment. Our finding suggests that the association between MI and manual space exploration may be more clear if the focus is on left-hand rather than right-hand performance. In any case, the association between MI and spatial attention adds to current discussions about the involvement of the parietal lobes in the genesis of psychotic-like symptoms.25
Lateralized whole-body movements (turning and veering) both showed that relatively high MI scores are associated with a greater degree of inattention to the right hemispace. On the group level, we found a left-sided turning preference. Crucially, as in the line bisection task, this bias remained significant for the high MI but not the low MI group. We found that high MI subjects exhibited not an increased left-sided but a decreased right-sided preference relative to the low MI group. This pattern of hemispatial preferences was also seen in the veering task, in which high MI subjects veered less frequently to the right than low MI subjects (see Figure 1).
Overall, the results of the spatial performance tasks resemble those reported from unmedicated patients with positive psychotic symptoms.9,11 In animals, the preferred side for spontaneous turning has been related to an asymmetrical DA system, specifically to a greater striatal DA receptor stimulation in the hemisphere contralateral to the observed turning bias.7 This well-established relationship has also been demonstrated in patients,10,12 in particular in persons with tardive dyskinesia and asymmetrical neuroleptic-induced parkinsonism,35 which supports the postulated relationship between right-hemispheric hyperdopaminergia on the one hand and right-sided inattention and psychosis on the other.
This study extends the literature linking psychotic-like thinking in healthy subjects to a neurotransmitter, namely, dopamine. While previous studies have found evidence for conceptual similarities between MI and schizophrenia,18–20,23–25,30–32 DA, the major neurotransmitter linked to schizophrenia, has remained largely neglected. Although Davis et al.36 argued that comparative studies of schizophrenia and its spectrum disorders are useful for generating major ideas about effective treatments and tools for differentiating between diagnoses, few studies have investigated the DA system within the broader spectrum.37 The experiments described here provide indirect evidence for a common dopaminergic mediation of lateral spatial preferences and the susceptibility to unfounded referential thinking—that is, MI. In analogy to previous findings from studies with patients and with animals, we interpret the observed effects as a consequence of hemispheric asymmetries in dopaminergic activity. We emphasize, however, that the relationship we found between MI and right-sided spatial inattention suggests that a mildly hyperactive DA system in the right hemisphere may reflect a normal neurochemical asymmetry rather than a secondary consequence of psychopathology. This neurochemical asymmetry, a property of the normal brain, may be accentuated in people with schizotypal personality disorders or schizophrenia, in whom lateral biases in spatial attention are reportedly associated with symptom severity.9,11
In conclusion, in this report we have described a modulation of normal subjects' spatial behavior by a mode of thinking reminiscent of the positive symptoms of psychotic patients. Overall, the results of the spatial performance tasks resemble those previously reported in studies of unmedicated patients with positive psychotic symptoms. Qualitatively, the observed effects were consistent across three different tasks. However, given the simplicity and cost-effectiveness of the veering task, the assessment of veering behavior may be especially recommended for future use in psychiatric patients in a wide variety of both research and clinical settings.
ACKNOWLEDGMENTS
Preliminary results of this study were presented at the Fourteenth International Congress on Parkinson's Disease, Helsinki, Finland, July 27–August 1, 2001. This research was supported by the Institut für Grenzgebiete der Psychologie und Psychohygiene, Freiburg im Breisgau (No. 690610) to Dr. Mohr and Dr. Brugger. This material is also based on work supported in part by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and by NIMH grant MH-43537 to Dr. Bracha. The authors are grateful to Theodor Landis for his technical and editorial advice.
 |
 |
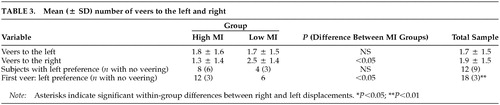 |
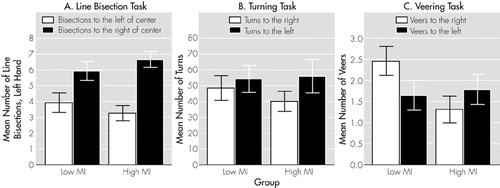
FIGURE 1. Hemispatial biases in three different spatial behavioral measures. Low MI group = subjects who scored below the median on the Magical Ideation scale; high MI group = subjects who scored above the median.
1 Seeman P: Dopamine receptor sequences: therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology 1992; 7:261-284Medline, Google Scholar
2 Angrist B, Peselow E, Rubinstein M, et al: Amphetamine response and relapse risk after depot neuroleptic discontinuation. Psychopharmacology 1985; 85:277-283Crossref, Medline, Google Scholar
3 Davidson M, Keefe RS, Mohs RC, et al: L-dopa challenge and relapse in schizophrenia. Am J Psychiatry 1987; 144:934-938Crossref, Medline, Google Scholar
4 Janowsky DS, Risch C: Amphetamine psychosis and psychotic symptoms. Psychopharmacology 1979; 65:73-77Crossref, Medline, Google Scholar
5 Sekine Y, Iyo M, Ouchi Y, et al: Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry 2001; 158:1206-1214Crossref, Medline, Google Scholar
6 Horvath TB, Meares RA: L-dopa and arousal. J Neurol Neurosurg Psychiatry 1974; 37:416-420Crossref, Medline, Google Scholar
7 Glick SD, Jerussi TP, Fleisher LN: Turning in circles: the neuropharmacology of rotation. Life Sci 1976; 18:889-896Crossref, Medline, Google Scholar
8 Yamamoto BK, Freed CR: The trained circling rat: a model for inducing unilateral caudate dopamine metabolism. Nature 1982; 298:467-468Crossref, Medline, Google Scholar
9 Bracha HS, Livingston RL, Clothier J, et al: Correlation of severity of psychiatric patients' delusions with right hemispatial inattention (left-turning behavior). Am J Psychiatry 1993; 150:330-332Crossref, Medline, Google Scholar
10 Bracha HS, Shults C, Glick SD, et al: Spontaneous asymmetric circling behavior in hemi-parkinsonism; a human equivalent of the lesioned-circling rodent behavior. Life Sci 1987; 40:1127-1130Crossref, Medline, Google Scholar
11 Harvey SA, Nelson E, Haller JW, et al: Lateralized attentional abnormality in schizophrenia is correlated with severity of symptoms. Biol Psychiatry 1993; 33:93-99Crossref, Medline, Google Scholar
12 Lee AC, Harris JP, Atkinson EA, et al: Evidence from a line bisection task for visuospatial neglect in left hemi-Parkinson's disease. Vision Res 2001; 41:2677-2686Crossref, Medline, Google Scholar
13 Landis T: Disruption of space perception due to cortical lesions. Spat Vis 2000; 13:179-191Crossref, Medline, Google Scholar
14 Karnath HO, Ferber S, Himmelbach M: Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature 2001; 411:950-953Crossref, Medline, Google Scholar
15 Fimm B, Zahn R, Mull M, et al: Asymmetries of visual attention after circumscribed subcortical vascular lesions. J Neurol Neurosurg Psychiatry 2001; 71:652-657Crossref, Medline, Google Scholar
16 Fleet WS, Valenstein E, Watson RT, et al: Dopamine agonist therapy for neglect in humans. Neurology 1987; 37:1765-1770Crossref, Medline, Google Scholar
17 Geminiani G, Bottini G, Sterzi R: Dopaminergic stimulation in unilateral neglect J Neurol Neurosurg Psychiatry 1998; 65:344-347Google Scholar
18 Brugger P, Graves RE: Right hemispatial inattention and magical ideation. Eur Arch Psychiatry Clin Neurosci 1997; 247:55-57Crossref, Medline, Google Scholar
19 Kalaycioglu C, Nalcaci E, Budanur OE, et al: The effect of familial sinistrality on the relation between schizophrenialike thinking and pseudoneglect. Brain Cogn 2000; 44:564-576Crossref, Medline, Google Scholar
20 Taylor KI, Zäch P, Brugger P: Why is magical ideation related to leftward deviation on an implicit line bisection task? Cortex, 2002, 38:247-252.Google Scholar
21 Brugger P: From haunted brain to haunted science: a cognitive neuroscience view of paranormal and pseudoscientific thought, in Hauntings and Poltergeists: Multidisciplinary Perspectives. Edited by Houran J, Lange R. Jefferson, NC, McFarland, 2001, pp 195-213Google Scholar
22 Eckblad M, Chapman LJ: Magical ideation as an indicator of schizotypy. J Consult Clin Psychol 1983; 51:215-225Crossref, Medline, Google Scholar
23 Mohr C, Röhrenbach CM, Laska M, et al: Unilateral olfactory perception and magical ideation. Schizophr Res 2001; 47:255-264Crossref, Medline, Google Scholar
24 Pizzagalli D, Lehmann D, Brugger P: Lateralized direct and indirect semantic priming effects in subjects with paranormal experiences and beliefs. Psychopathology 2001; 34:75-80Crossref, Medline, Google Scholar
25 Lenzenweger MF: Two-point discrimination thresholds and schizotypy: illuminating a somatosensory dysfunction. Schizophr Res 2000; 42:111-124Crossref, Medline, Google Scholar
26 Campbell JJ: Neuropsychiatric assessment, in Textbook of Geriatric Neuropsychiatry, 2nd edition. Edited by Coffey CE, Cummings JL. Washington, DC, American Psychiatric Press, 2000, pp 109-124Google Scholar
27 Chapman LJ, Chapman JP: The measurement of handedness. Brain Cogn 1987; 6:175-183Crossref, Medline, Google Scholar
28 Garety P, Wessely S: The assessment of positive symptoms, in The Assessment of Psychoses: A Practical Handbook. Edited by Barnes TRE, Nelson HE. London, Chapman and Hall, 1994, pp 21-39Google Scholar
29 Bracha HS, Seitz DJ, Otemaa J, et al: Rotational movement (circling) in normal humans: sex difference and relationship to hand, foot, and eye preference. Brain Res 1987; 411:231-235Crossref, Medline, Google Scholar
30 Leonhard D, Brugger P: Creative, paranormal, and delusional thought: a consequence of right hemisphere semantic activation? Neuropsychiatry Neuropsychol Behav Neurol 1998; 11:177-183Medline, Google Scholar
31 Luh KE, Gooding DC: Perceptual biases in psychosis-prone individuals. J Abnorm Psychol 1999; 108:283-289Crossref, Medline, Google Scholar
32 Weinstein S, Graves RE: Creativity, schizotypy, and laterality. Cogn Neuropsychiatry 2001; 6:131-146Crossref, Google Scholar
33 Jewell G, McCourt ME: Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000; 38:93-110Crossref, Medline, Google Scholar
34 Fischer MH: Cognition in the bisection task. Trends Cogn Sci 2001; 5:460-462Crossref, Medline, Google Scholar
35 Bracha HS: Is there a right hemi-hyper-dopaminergic psychosis? Schizophr Res 1989; 2:317-324Crossref, Medline, Google Scholar
36 Davis KL, Kahn RS, Ko G, et al: Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148:1474-1486Crossref, Medline, Google Scholar
37 Siegel BV Jr, Trestman RL, O'Flaithbheartaigh S, et al: D-amphetamine challenge effects on Wisconsin Card Sort Test: performance in schizotypal personality disorder. Schizophr Res 1996; 20:29-32Crossref, Medline, Google Scholar



