SPECT Imaging of Body Dysmorphic Disorder
Abstract
[99mTc]Hexamethylpropylene amine oxime (HMPAO) single photon emission computed tomography (SPECT) brain scans were undertaken in six subjects with body dysmorphic disorder (BDD). The scans showed a broad range of discrepant findings that do not immediately support a view of BDD as resting on either an obsessive-compulsive or affective disorder spectrum. Nevertheless, involvement of parietal regions is consistent with the characteristic altered body perception of BDD. These preliminary data highlight the need for further systematic functional imaging studies of this condition.
Body dysmorphic disorder (BDD) is a somatoform disorder characterized by excessive preoccupation with an imagined or mild defect in appearance. This preoccupation is either clinically distressing or functionally impairing.1 The prevalence of BDD has been estimated at 1.9% in a community sample2 and 3.2% in psychiatric outpatients.3 There are high levels of coexistence with obsessive-compulsive disorder (OCD)4 and depression,5 but unfortunately diagnosis is often delayed.3
The phenomenology of BDD, its family history, and comorbidity with OCD support conceptualizing it on the obsessive-compulsive spectrum (aversive, risk avoiding). However, there are also arguments indicating that BDD falls on a broader affective spectrum of disorders.6
Neuroanatomical hypotheses link the affective disorders to the temporolimbic regions7 and OCDs to dysfunction in the orbitofrontal subcortical-thalamic pathways.8 Normal perception of body image is disturbed by localized lesions of the posterior nondominant parietal lobe as well as the temporal lobe.9 Positron emission tomography in anorexia nervosa (one of the core features being a disturbed body image) has implicated frontal and parietal regions through reduced glucose uptake.10,11 To date, we are not aware of any studies on the functional neuroimaging of BDD.
Owing to the clinical overlap with OCD and the high comorbidity with depression, we hypothesized that single photon emission computed tomography (SPECT) would show changes in brain regions that encompass changes seen in both of these disorders, but that the core disturbance of body image would be represented by changes specific to the parietal lobes, supporting its categorization as a unique disorder.
METHODS
Six subjects with BDD diagnosed by using the Structured Clinical Interview for DSM-IV,12 following written informed consent underwent [99mTc]hexamethylpropylene amine oxime (HMPAO) SPECT. The age range was 19–63 years and excluded any substance abusers. Subjects with comorbid depression and OCD were not excluded. All subjects had been free of psychotropic medication for a minimum of a month and had normal physical and neurological examinations.
Subjects were injected following 30 minutes of rest and images were obtained from a dual detector gamma camera (Elsinct Helix) with fan-beam collimators. Image reconstruction was performed with standard protocols, standardized to whole brain counts. Region of interest analysis was performed with region of interest templates. Perfusion alterations are expressed relative to the contralateral hemisphere.
RESULTS
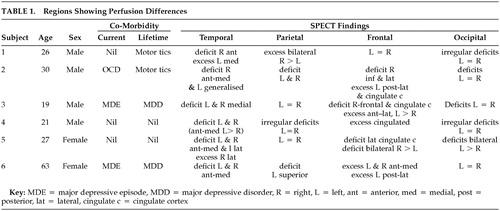
Our sample of six subjects included four males and two females ranging in age from 19 to 63 years. One subject had comorbid OCD and another two had major depression at the time of the scan. Motor tics were untreated and in full remission at time of study. No perfusion change/asymmetry was noted in the thalamus or cerebellar regions. Perfusion to the basal ganglia was symmetrically increased in two of six and reduced in one of six subjects. The more consistent regions showing perfusion differences are tabulated (Table 1).
DISCUSSION
This small series of cases provides preliminary evidence that BDD may be mediated by widespread neurocircuitry in parieto-occipital, temporal, and frontal areas. Indeed, the scans here showed a broad range of discrepant findings and so do not immediately support a view of BDD as resting on either an obsessive-compulsive or affective disorder spectrum.
Parietal changes (one increased, two decreased, one irregular) and occipital perfusion deficits (four decreased, one irregular) are noteworthy. Neither of these areas has been implicated in either depression or in OCD neurocircuitry. Abnormalities in parietal circuits are, however, consistent with the core feature of disturbed perception of body form or appearance in BDD.
Perfusion deficits were noted in the temporal regions of all six subjects. These however were not consistently localized and predominantly involved the anterior and medial areas but in two subjects included the lateral areas. Perfusion deficits also occurred in the case with comorbid OCD. While these findings may have been partly influenced by comorbidity with depression, four subjects did not have comorbid depression.
The findings in the frontal regions and cingulate cortex in this series are inconsistent. Similarly, there are no consistent changes in the thalamus and basal ganglia (two increased, one reduced). Thus the data here do not fully support the notion of an overlap with the functional neuroanatomy of OCD, in which fairly consistent hyperactivation in frontostriatal circuits has been reported.
Small sample size, comorbidity, absence of structural imaging data, and the lack of healthy subjects limit the conclusions that can be drawn from this pilot study. Given the preliminary finding of parietal and occipital perfusion alterations, the results may be useful in generating hypotheses for future research on this disorder. In particular, future controlled studies on the functional anatomy of BDD are warranted to further develop understanding of this chronic and disabling disorder.
 |
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, APA, 1994Google Scholar
2 Rich N, Rosen JC, Orosan PG, et al: Prevalence of body dysmorphic disorder in non-clinical populations. Presented at the Annual Meeting of the Association for the Advancement of Behavior Therapy, Boston, November 1992Google Scholar
3 Zimmerman M, Mattia JI: Screening for body dysmorphic disorder in psychiatric outpatients: recognition, prevalence, co-morbidity, demographic, and clinical correlates. Compr Psychiatry 1998; 39:265–270Crossref, Medline, Google Scholar
4 Simeon D, Hollander E, Stein DJ, Cohen L, Aronowitz B: Body dysmorphic disorder in the DSM-IV field trial for obsessive-compulsive disorder. Am J Psychiatry 1995; 152:1207–1209Crossref, Medline, Google Scholar
5 Nierenberg AA, Phillips KA, Petersen TJ, et al: Body dysmorphic disorder in outpatients with major depression. J Affect Disord 2002; 69:141–148Crossref, Medline, Google Scholar
6 Phillips KA, McElroy SL, Hudson JI, et al: Body dysmorphic disorder: an obsessive-compulsive spectrum disorder, a form of affective spectrum disorder, or both? J Clin Psychiatry 1995; 56(suppl 4):41–51Google Scholar
7 Post RM, Weiss SRB, Ketter TA, et al: The temporal lobes and affective disorders, in The Temporal Lobe and the Limbic System. Edited by Trimble MR, Bolwick A. Petersfield, UK, Wrightson Biomedical Publishing, 1992, pp 247–265Google Scholar
8 Saxena S, Brody AL, Schwartz JM, et al: Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl 1998; 35:26–37Medline, Google Scholar
9 Trimble MR: Body image and the temporal lobes. Br J Psychiatry Suppl 1988; 2:12–14Medline, Google Scholar
10 Delvenne V, Goldman S, De Maertelaer V, et al: Brain glucose metabolism in eating disorders assessed by positron emission tomography. Int J Eat Disord 1999; 25:29–37Crossref, Medline, Google Scholar
11 Nozoe S, Naruo T, Yonekura R, et al: Comparison of regional cerebral blood flow in patients with eating disorders. Brain Res Bull 1995; 36:251–255Crossref, Medline, Google Scholar
12 First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar



