Serotonin Transporter Gene Promoter Region Polymorphism Associated With Poststroke Major Depression
Abstract
The authors examined variations of serotonin transporter-linked promoter region (5-HTTLPR) functional polymorphism in 26 stroke patients with major depression and in 25 unrelated nondepressed stroke subjects of Caucasian descent. Findings indicate a significant association between 5-HTTLPR short variant genotype and poststroke major depression.
The elucidation of etiological mechanisms linking stroke and depression has significant clinical, treatment, and preventive implications. Over the last two decades, the etiological research in poststroke depression (PSD) (poststroke major and minor depression) has focused on the assessment of lesion location, psychosocial, and demographic risk factors as determinants of PSD.1 In recent years, the molecular genetic approach has been widely used to address the biological mechanisms contributing to interindividual differences in susceptibility to primary psychiatric disorders. It is increasingly believed that primary major depression (major depression not due to a medical condition) results from the interaction of predisposing genes and a hazardous environment. However, the genetic hypothesis of major depression secondary to stroke (poststroke major depression) has not been examined to date, although there is evidence that personal and familial predisposition to depression may be risk factors.2,3
Serotonin transporter gene-linked promoter region (5-HTTLPR) functional polymorphism may be a potential candidate gene for susceptibility to poststroke major depression for several reasons. First, 5HTT is the target of selective serotonin reuptake inhibitors that are effective in the treatment of poststroke major depression.4,5 Second, serotonin transporter gene regulates 5-HTT availability which is critical in maintaining the homeostasis of serotonin function6,7 and disturbances in serotonergic responsiveness have been implicated in poststroke major depression.8,9 Third, 5-HTTLPR polymorphism has shown to influence the individual variations in the development of major depression in response to life stress although there is no conclusive evidence for a direct association between 5-HTTLPR and primary major depression.10,11 Since stroke is a major life event and poststroke major depression is associated with severe physical and psychosocial consequences, it is possible that 5-HTTLPR may play a role in poststroke major depression. In this study, we investigated whether patients with poststroke major depression was significantly associated with variations in 5-HTTLPR compared to nondepressed stroke patients.
METHOD
The subjects were 26 stroke patients with mood disorders (mean age 59.5 [SD 13.3] range=37–82) who attended the poststroke psychiatric clinic from August 2002 to July 2003 at Foothills Hospital, which is affiliated with the University of Calgary School of Medicine. The comparison controls were 25 nondepressed stroke patients (mean age 60.4 [SD 12.4] range=33–80). Written informed consent was obtained from all participants. This study was approved by the local ethics review board. All subjects were unrelated Caucasians of both genders. The exclusion criteria were: absence of infarct as defined by MRI or CT scan, severe cognitive impairment (Mini Mental State Examination [MMSE ≤20]), communication deficits with severe dysphasia or dysarthria, non-English speaking patients, comorbid neurological conditions (epilepsy, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis and other neurodegenerative conditions) and a history of psychosis. Further, patients with prestroke depression that continued during poststroke period and patients who were taking antidepressants at the time of the evaluation were excluded.
The diagnosis of major depressive disorder was determined by using the Structured Clinical Interview for DSM–IV (SCID) and DSM–IV diagnostic criteria. In controls, a nondepressed status was ascertained by a depression screening instrument (CESD scores<15) and an interview using SCID. A positive family history was defined as the presence of one first- or second-degree relative with a history of depression or bipolar disorder. A personal history of depression and or anxiety disorder was identified through direct questioning of the patients and/or by collateral history obtained from relatives. MMSE was used to measure cognitive functioning and Barthel measures were used to measure physical functioning. Lesion characteristics and other medical and medication history were obtained from medical charts. 5-HTTLPR genotyping was performed using Touchdown Polymerase chain reaction conditions. We detected alleles with 14 or 16 repetitive elements corresponding to short (S) and long (L) polymorphic variants as reported by Heils et al.12 The chi-square test was used to compare genotypic frequencies and other categorical variables and Student’s t test was used to compare continuous variables between the depressed and nondepressed stroke groups. Logistic regression analysis was performed to study the effect of significant variables on depression diagnosis.
RESULTS
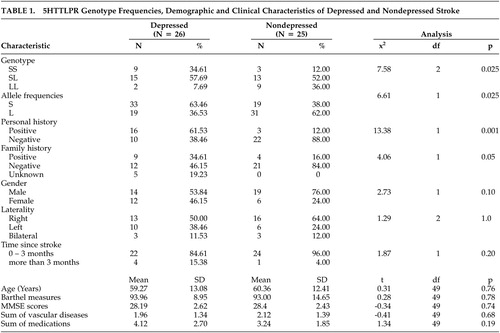
The data analysis showed that there was a significant difference in the frequencies of 5-HTTLPR genotypes (SS, SL, LL) between the experimental and control groups (Table 1). In particular, the homozygosity of short alleles was significantly associated with poststroke major depression. Since S-allele may have dominant negative effect on basal transcriptional activity,6 we combined SS and SL into one group and compared with LL between depressed and nondepressed stroke patients. The results remained statistically significant (χ2=6.04, df=1, p=0.025). Furthermore, the depressed group had significantly higher rate of personal history of MDD and or anxiety disorder and family history of MDD and or bipolar disorder than the nondepressed group. There were no differences between case and control groups in age, gender, laterality of lesions, Barthel measures, MMSE scores, sum of vascular diseases and medications, and time since stroke. There were no differences in genotype frequencies between stroke subjects with and without personal history (χ2= 1.33, df=2, p=0.51) and family history (χ2= 2.59, df=4, p=0.63). Due to small sample size, we performed two logistic regression analyses separately for personal and family history in order to examine the independent effect of genotypes on depression. The regression of genotype and personal history on depression diagnosis yielded no significant effect of genotype (Wald χ2=5.63, df=2, p=0.06). Similarly regression of genotype and family history on depression failed to show any significant effect of genotype (Wald χ2=3.08, df=2, p=0.22). In an attempt to account for the higher frequency of women in the depressed group (46.2%) than in the nondepressed group (24%), we examined the effect of gender on genotypic frequencies and on the association between poststroke major depression and genotypes. There were no gender differences in genotypic frequencies (χ2= 3.65, df=2, p=0.16). The effect of genotype on depression remained significant even after controlling for gender (Wald χ2= 7.2649, df=2, p=0.027).
DISCUSSION
Our results show that 5-HTTLPR genotype is associated with the expression of major depression after stroke, and the liability is significantly increased when the S-allele is in the homozygous state. Conversely the homozygosity of L-allele provides a protective effect. Our findings also indicate that the personal and familial predisposition to depressive disorder may influence the association between 5-HTTLPR genotype and poststroke major depression. Taken together these preliminary results suggest that variations in 5-HTTLPR may mediate the genetic risk of depression vulnerability in poststroke major depression.
There are several possible mechanisms by which S-allele might influence susceptibility to poststroke major depression. The observed direct association between the SS genotype and poststroke major depression may suggest that the risk of depression that S-allele confers in stroke patients may be mediated by decreased expression of 5-HTT resulting in the reduction of 5-HT reuptake and the 5-HT neurotransmitter pool. This is in line with the evidence of diminished serotonergic responsiveness in poststroke major depression.9 However, since brain vascular lesions may directly contribute to diminished central serotonergic functioning,8,13 it is likely that the depressogenic effect of S-allele in poststroke major depression could also be influenced indirectly through interaction with stroke lesions.
Another alternative mechanism is that stroke patients with short 5-HTTLPR alleles rather than long alleles may be more vulnerable to stress reaction in response to stroke deficits resulting in depression. This interaction between gene and stress hypothesis is indirectly supported by several lines of evidence. The short allele of 5-HTTLPR polymorphism seems to have a reliable effect on neuroticism,14 a personality pattern of hypersensitivity to stress, which has been related to PSD.15 Major life events and severe disability were found to be associated with an increased risk of PSD1,2 and the short allele of 5-HTTLPR seems to influence the individual variations in the development of major depression to environmental insults.10 Furthermore, poststroke major depression has been reported to be more reactive and less endogenous in nature.16
When interpreting these results, one should consider several caveats. The expression of 5-HTTLPR on 5-HTT transcription in the brain may be modified by nongenetic factors, including stress,17 and probably by stroke lesions. 5-HTTLPR may have linkage disequilibrium with another functional polymorphism of the 5-HTT (17 bp) variable number of tandem repeats in the second intron [VNTR-2].18 Furthermore, similar to functional major depression, it is possible that multiple genes and complex gene interactions might be associated with poststroke major depression.
There are several limitations in this study. Contrary to previous studies,1 our results show no group differences in physical functioning and cognitive performance which might be due to sampling bias or small sample size. Although the gene/environment interaction hypothesis is appealing, it cannot be evaluated appropriately in the present study because we did not measure personality traits, psychosocial stress, or lesion volume, and our sample size is small and heterogeneous in lesion locations. Another limitation is related to gender differences in PSD. Previous studies reported that women were twice as frequently diagnosed with poststroke major depression as men.19 Although our data did not show any significant group differences in gender or gender differences in genotypes, there was a higher frequency of women in the depressed (46.5%) than in the nondepressed group (24%). The influence of gender on the association between poststroke major depression and 5-HTTLPR variations need to be investigated in larger samples.
The family history method used in this study to determine MDD in family members was limited by poor sensitivity.20 The observed influence of personal and familial predisposition to depressive disorder on the association between 5-HTTLPR genotypes and poststroke major depression requires further investigation. Additionally, we did not involve consecutive assessment of stroke patients, and some acute stroke cases included in the nondepressed group may ultimately develop depression at a later stage. Furthermore, this sample was drawn from an outpatient clinic and the majority was ambulatory and relatively well functioning, which limits generalization.
Despite these limitations, this is the first report, to date and to our knowledge, suggesting that the genotype of 5-HTTLPR with low active short alleles may contribute to the susceptibility to poststroke major depression. This finding in independent samples gives our observation clinical and preventive implications.
ACKNOWLEDGMENTS
This study was supported by a faculty research grant (# 75-4559) to Dr. Ramasubbu from the Office of the Vice-President (Research) University of Calgary and a Roy and Joan Allen Professorship to Dr. N.T. Bech-Hansen, Ph.D. in the Faculty of Medicine, University of Calgary Calgary, Alberta, Canada.
The authors thank Dr. Biddle, Ph.D. for his comments and Dr. Faris, Ph.D., for statistical consultation and stroke neurologists at the Foothills Hospital for referring patients.
This article was presented as a poster at the Society of Biological Psychiatry 60th Annual Convention and Scientific Program, Atlanta, GA, May 19–21, 2005.
 |
1 White EM, Mulsant BH: Post-stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002; 52:253–264Crossref, Medline, Google Scholar
2 Andersen G, Vestergaard K, Ingemann-Nielsen et al: Risk factors for post-stroke depression. Acta Psychiatr Scand 1995; 92:193–198Crossref, Medline, Google Scholar
3 Morris P, Robinson R, Raphael et al: The relationship between risk factors for affective disorder and post-stroke depression in hospitalized stroke patients. Aust N Z J Psychiatry 1992b; 26:208-221Google Scholar
4 Wiart L, Petit H, Joesph P et al: Fluoxetine in early poststroke depression. A double blind placebo-controlled study. Stroke 2000; 31:1829–1832Crossref, Medline, Google Scholar
5 Andersen G, Vestergaard K, Lauritzen L: Effective treatment of post stroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 1994; 25:1099–1104Crossref, Medline, Google Scholar
6 Lesch KP, Bengel D, Heils A, et al: Association of anxiety-related traits with polymorphism in serotonin transporter gene regulatory region. Science 1996; 274:1527–151Crossref, Medline, Google Scholar
7 Torres GE, Gainetdinov RR, Caron MG: Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci 2003; 4:13–25Crossref, Medline, Google Scholar
8 Ramasubbu R, Flint A, Brown et al: Diminished serotonin mediated prolactin responses in non-depressed stroke patients compared with healthy normal subjects. Stroke 1998; 29:1293–1298Crossref, Medline, Google Scholar
9 Morris P, Hopwood M, Maguire et al: Blunted prolactin response to D-fenfluramine in post-stroke major depression. J Affect Disord 2003; 76:273–278Crossref, Medline, Google Scholar
10 Caspi A, Sugden K, Moffitt TE et al: Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389Crossref, Medline, Google Scholar
11 Angnelova M, Benkelfat C, Turecki G: A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: Mol Psychiatry 2003; 8:574–591Google Scholar
12 Heils A, Teufel A, Petri S, et al: Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66:1–4Crossref, Medline, Google Scholar
13 Ramasubbu R, Flint A, Brown G, et al: A neuroendocrine study of serotonin function in depressed stroke patients compared to non-depressed stroke patients and healthy controls. J Affect Dis 2000; 52:121–133Crossref, Google Scholar
14 Schinka JA, Busch RM, Robichaux-Keche N: A meta-analysis of the association between the serotonin transporter gene poylmorphism (5-httlpr) and trait anxiety. Mol Psychiatry 2004; 9:197–202Crossref, Medline, Google Scholar
15 Morris P, Robinson R: Personality neuroticism and depression after stroke. Intl J Psychiatry Med 1995; 25:93–102Crossref, Medline, Google Scholar
16 Gainotti G, Azzoni A, Marra C: Frequency, phenomenology and anatomical-clinical correlates of major post-stroke depression. Br J Psychiatry 1999; 175:163–167Crossref, Medline, Google Scholar
17 Mann JJ, Huang YY, Underwood MD, et al: A serotonin transporter gene promoter polymorphism (5HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 2000; 57:729–738Crossref, Medline, Google Scholar
18 Gelernter J, Cubells JF, Kidd JR, et al: Population studies of polymorphisms of the serotonin transporter protein gene. Am J Med Genet 1999; 88:61–66Crossref, Medline, Google Scholar
19 Paradiso S, Robinson R: Gender differences in post stroke depression. J Neuropsychiatry Clin Neurosci 1998; 10:41–47Link, Google Scholar
20 Andreason NC, Endicott J, Spitzen RL, et al: The family history method using diagnostic criteria. reliability and validity. Arch Gen Psychiatry 1977; 34:1229–1235Crossref, Medline, Google Scholar



