Gender Differences in Poststroke Depression
Abstract
In stroke and other medical illnesses, secondary depression may be associated with different factors in women than in men. The authors examined 301 consecutive admissions for acute treatment of cerebrovascular accident for gender differences in depression, psychosocial factors, physical impairment, and lesion location. Women were twice as frequently diagnosed with major depression as men. Women with major depression had a greater frequency of left hemisphere lesions than men. In men, major depression was associated with greater impairment in activities of daily living, and greater severity of depression was associated with greater impairment in daily activities and social functioning. In women, greater severity of depression was associated with prior diagnosis of psychiatric disorder and cognitive impairment. These findings suggest a different nature of poststroke depression in men and women and may have implications for its treatment.
Gender differences in the frequency of affective disorders are well established in the psychiatric literature.1 Unipolar disorders occur more frequently in women than in men, and it has been proposed that this difference may be due to biological factors. Gender-based differences in brain functioning or organization that may contribute to differences in frequency of affective disorders have been found by some investigators.2–4 For example, Borod2 found that facial recognition of emotion is more bilaterally distributed in females than males; Gur et al.3 have shown that gender differences in resting glucose metabolism in the limbic system may be the basis of some cognitive and emotional differences between sexes; and Shaywitz et al.4 found that cerebral organization of language was more bilateral in its cerebral localization in females than males.
One might expect, given these gender-based differences in brain organization, that brain injury would affect men and women differently. A common psychopathological manifestation after stroke is mood disorder. Mood disorders following stroke have been proposed as a model to study affective illness in general. If women are biologically more vulnerable to developing a depressive disorder, one might expect that a precipitating factor common to men and women (such as a cerebrovascular accident) would provoke more depression in females than males. One would also predict poststroke depression to be associated with clinical variables indicating a greater biological predisposition in females. On the other hand, depression in males might be associated with nonbiological factors such as severity of physical and psychosocial impairment.
Morris et al.,5 examining a stroke population in a rehabilitation hospital, found that among men depression was associated with greater physical disability. Depressed males also tended to perceive their social support as less adequate compared with nondepressed men.5 This association of depression with impairment may also apply to other medically ill populations; in patients with myocardial infarction, who are predominantly males, depression was associated with severity of medical illnesses6 and social factors.7
Given these gender-related findings in other patients populations, we undertook a study to investigate the frequency and clinical correlates of acute poststroke depression in women compared with men. We hypothesized that females would have an increased frequency of depression that would be correlated with family and prior personal history of mood disorder, whereas males would have depressions that correlated with severity of physical impairment and social support. To test these hypotheses, we examined depression both as a categorical and a dimensional variable.
METHODS
Patients and Evaluations
Patients included in the study were 301 consecutive admissions to the University of Maryland Hospital, Baltimore, MD, for the treatment of acute cerebrovascular accident from 1980 to 1990. This population has previously been examined for anxiety disorders8 and cognitive impairment related to poststroke depression.9 Of the consecutive series of stroke patients admitted to the hospital, only patients with moderate or severe comprehension deficits or markedly decreased levels of consciousness were excluded. Such patients represented about one-third of all admissions and were not among the 301 patients studied.
About 2 weeks after the acute episode, and after giving informed consent following full explanation of all procedures, patients were administered a series of standardized quantitative measures of mood and impairment as part of an extensive neurological10 and psychiatric examination. These measures were previously shown to be reliable and valid in this stroke population.11 Examinations were administered in a private room between 11:00 a.m. and 2:00 p.m. to minimize the possible effect of diurnal variations of mood on interview response. The Hamilton Rating Scale for Depression12 (Ham-D), a 17-item interviewer-rated scale, was used to measure severity of psychological and physiological symptoms of depression. The modified Present State Examination13 (PSE), a structured psychiatric interview that elicits symptoms related to depression and anxiety, was administered by a psychiatrist. Based on symptoms elicited by the PSE, a diagnosis of mood disorder due to stroke with major depression–like episode or minor depression (research criteria) was made by using DSM-IV14 criteria. The Mini-Mental State Examination (MMSE) was also administered to each patient. The MMSE has been shown to be reliable and valid in assessing a limited range of cognitive functions in patients with stroke.15 Scores range from 0 to 30, and lower scores indicate greater impairment.
Physical independence was evaluated with the Johns Hopkins Functioning Inventory (JHFI), a 10-item scale that quantifies impairment in activities of daily living.11,16 Scores range from 0 to 27, with higher scores indicating a greater degree of impairment. Quantitative assessments of social functioning were made by using the Social Functioning Examination (SFE) and the Social Ties Checklist (STC).17 These instruments assess personal satisfaction with social functioning and quantity of available social supports, respectively. Higher scores indicate greater impairment.
Computed Tomographic (CT) Scan Examination
CT scans were read by a neurologist who was blind to the other measures. All CT scans, performed with GE 9900, AS&E 500, and EMI 1010 scanners, had consistent slice thickness and were performed without contrast at 0° angle from the canthomeatal line. CT scanning was performed on the day of admission, and if no lesions were visible, a repeat scan 2 days to 3 weeks later was obtained. The damaged area was localized in specific brain regions according to the procedure of Levine and Grek.18 The anteroposterior location of each lesion was defined as the mean distance of the anterior border of lesion from the frontal pole averaged over all the slices where the lesion was visible. This distance was expressed as a percentage of the maximum distance between the anterior and posterior poles on the CT slice in which the lesion was visible. The posterior border of the lesion was calculated with the same methods. Lesion volume (expressed as percentage of total brain volume) was calculated from the ratio of the largest cross-sectional area of the lesion on any CT scan slice to the cross-sectional area of the whole brain on the slice passing through the body of the lateral ventricles. Reliability of this procedure and correlation with other methods of determining lesion volume have been previously demonstrated.15,19
Statistical Analysis
Data concerning gender differences were analyzed by using means, standard deviations, and F tests. Frequency distributions were analyzed by using likelihood ratio chi-square. Discrete dependent variables were analyzed by using logistic regression models. Continuous dependent variables were analyzed by using stepwise (backward) regression models. Alpha level was set at 0.05, and P-values were two-tailed.
RESULTS
Frequency and Severity of Depression
Twenty-one of 170 males (12.3%) and 31 of 131 females (23.6%) had major depression (likelihood ratio chi-square=6.57, df=1, P=0.01). In this analysis, patients with minor depression (males, n=31, 18.2%; females, n=24, 18.3%) were included in the non–major depression group. If minor depressions were excluded, the increased frequency of major depression in females compared with males remained significant.
Male and female patients with major depression had similar mean depression severity scores as measured by the Ham-D scale (males, 16.5±5.93; females, 16.2±5.34).
CT Scan Examination
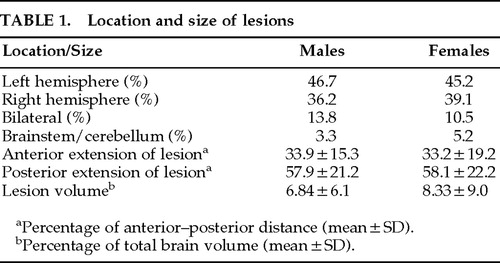
No significant difference was found between male and female patient groups in hemispheric side of lesion or the anterior or posterior extension of lesion along the anterior–posterior axis of the brain and lesion volume (Table 1). However, more depressed women had a left hemisphere lesion than depressed men (Figure 1). Nominal logistic regression using gender, left and right hemispheric side of lesion, and their interaction yielded a significant overall effect (χ2=11.8, df=3, P=0.008) and a significant interaction (Wald χ2=4.63, df=1, P=0.031). Models using anterior or posterior extension or volume of lesion and their interactions with gender did not show significant effects.
Relationship to Background and Clinical Characteristics
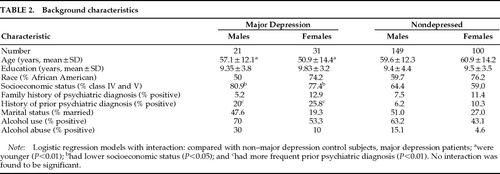
Background characteristics of subjects are shown in Table 2. Because the association of female gender was with major and not with minor depression, background characteristics were compared between major depression and non–major depression patients. Logistic regression analyses of gender and each background characteristic and their interaction on major depression diagnosis (categorical variable) were performed with and without inclusion of minor depression patients in the non–major depression group. Major depression patients were younger (age effect, F=6.87, df=1,287, P=0.008), had a greater frequency of lower socioeconomic status (Wald χ2=5.25, df=1, P=0.021), and had a greater frequency of prior psychiatric diagnosis (Wald χ2=8.29, df=1, P=0.004). A trend effect for alcohol abuse was also found (Wald χ2=3.17, df=1, P=0.07). No background characteristics×gender interaction showed a significant effect. When minor depression patients were excluded from the analysis, results were essentially the same.
We then investigated the unique predictors of depressive symptom severity (depression as a dimensional variable). We used age, race, education, prior and family psychiatric history, socioeconomic status, marital status, alcohol use, and alcohol abuse as the independent variables and the Ham-D as the dependent variable in two separate stepwise (backward) regression analyses for male and female patients. Analyses revealed that younger age in males (F=6.07, df=9,85, P=0.016) and prior psychiatric diagnosis in females (F=5.80, df=9,60, P=0.019) predicted severity of depressive symptoms. In females, younger age showed a nonsignificant trend (F=2.73, df=9,60, P=0.10).
Cognitive Impairment, Activities of Daily Living, and Psychosocial Functioning
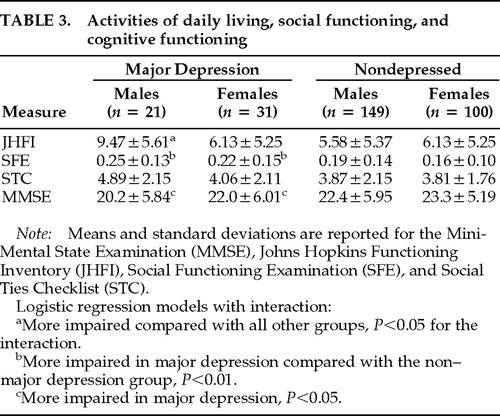
Data concerning activities of daily living, social impairment, and cognitive impairment are shown in Table 3. Nominal logistic regression of gender and JHFI on major depression diagnosis yielded a significant overall effect (χ2=14.25, df=3, P=0.002). There were significant gender (Wald χ2=9.12, df=1, P=0.002), JHFI (Wald χ2=4.10, df=1, P=0.042), and interaction effects (Wald χ2=4.10, df=1, P=0.042). Regression of gender and STC on major depression diagnosis yielded a significant overall effect (χ2=11.93, df=3, P=0.007) and a significant gender effect (Wald χ2=3.98, df=1, P=0.046), but no STC (Wald χ2=3.19, df=1, P=0.07) or interaction effects (Wald χ2=0.73, df=1, P=0.4). Regression of gender and SFE on major depression diagnosis yielded a significant overall effect (χ2=11.61, df=3, P=0.008) and a significant SFE effect (Wald χ2=7.45, df=1, P=0.006), but no gender (Wald χ2=1.06, df=1, P=0.3) or interaction effects (Wald χ2=0.05, df=1, P=0.8). Regression of gender and MMSE on major depression diagnosis yielded a significant overall effect (χ2=8.27, df=3, P=0.040) and a significant MMSE effect (Wald χ2=3.87, df=1, P=0.049), but no gender (Wald χ2=0.11, df=1, P=0.7) or interaction effects (Wald χ2=0.07, df=1, P=0.7). Hence, an increased risk of depression was associated with female gender interacting with impairment in activities of daily living performance, so that among males, those with the greatest physical impairment were the most depressed. Depression in both males and females was associated with impaired social functioning and greater cognitive impairment.
We then used depression as a dimensional variable (Ham-D) and conducted stepwise (backward) multiple regressions. The analyses revealed that activities of daily living (JHFI: F ratio=13.4, df=4,112, P=0.0004) and social functioning (SFE: F ratio=4.29, df=4,112, P=0.040) predicted severity of depressive symptoms in males. Among the measures of cognitive and social functioning, the only predictor for severity of depression in females was MMSE (F ratio=4.63, df=4,87, P=0.034).
In summary, activities of daily living and social impairment predicted severity of depression in males. Prior psychiatric diagnosis and cognitive impairment predicted severity of depressive symptoms in females.
DISCUSSION
Several interesting findings emerged from this study. First, major depressive disorder after stroke was twice as frequent among females as among males. Second, depression was significantly associated with left hemisphere lesion location only among women. Third, among women the risk factors for depression were younger age, personal history of psychiatric disorder, and cognitive impairment, whereas among men, the risk factors for depression were younger age and impairment in activities of daily living and social functioning.
Before we discuss the implications of these findings, several limitations of the study should be acknowledged. First, most patients enrolled in this study were African Americans of lower socioeconomic status. Thus, our conclusion about gender differences may apply only to this population. Second, patients with significant comprehension deficits were excluded. Thus, we do not know if comprehension impairment affected depression frequency differentially in males and females. Third, this study examined patients during the acute poststroke period. We do not know if gender differences with regard to depression changed over time.
Results from this study show that, as in primary depression, there is greater frequency of poststroke depression in females compared with males. The most fundamental and important question raised by this study is why major depression is more common in women than in men. Although this study is unable to definitively answer this question (the question remains unanswered for functional depression as well), the association of increased depression with prior psychiatric history suggests a predisposition for depression in females. These results are in agreement with those of Burvill et al.,20 who reported that poststroke depression in females was frequently associated with a history of depressive disorder prior to stroke. A biological vulnerability for depression in females is suggested also by the finding that only women showed a significant association of major depression with left hemisphere lesion. A cerebrovascular injury may have acted either as a stressor or a biological trigger for major depression.
Over the last 15 years, we19,21 and other groups22,23 have shown that major depression following brain injury is more frequent after left anterior lesions. The results of this study may help to explain some of the inconsistent past results24–26 with regard to poststroke depression and lesion location. Major depression has also been shown to be associated with cognitive impairment,15,19 but this finding has not always been replicated.27 The results from this study may also shed some light on these inconsistencies. However, since the cognitive impairment in this study was ascertained by using the MMSE, a screening test that assesses predominantly left hemisphere cognitive function, we are not able to say whether in females, cognitive impairment is directly associated with greater depression or is a consequence of the greater frequency of left hemispheric lesions in females with major depression. The latter interpretation, however, contrasts with the report that cognitive impairment assessed by using a large neuropsychological battery in subjects with left hemispheric stroke and depression encompasses functions not restricted to the left hemisphere.28 These issues will require further studies.
Males appeared to be more susceptible to developing a depressive response to physical and social impairment. This finding replicates the results of Morris et al.5 They found a greater frequency of depression among men with greater physical disability than among men with less severe impairment. Other studies29–31 reported an association of depression and functional impairment, but no gender-based analyses were performed. Our findings also confirm those of Morris et al.,5 who reported that depressed men tended to perceive their social support as less adequate compared with nondepressed men. Åström et al.23 showed that “living alone” was associated with depression at initial evaluation, but they did not perform gender-based analyses.
The association of social impairment with depression in males may apply to other medically ill populations.6,7 In a sample composed predominantly of men with traumatic brain injury, we found that poor social functioning was the clinical variable that was most consistently associated with major depression throughout 1-year follow-up.32,33
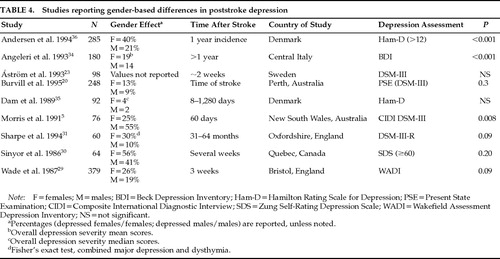
Previous studies examining the prevalence of major depression among males and females have not found consistent results (Table 4). There are several possible explanations for this lack of consistency, including differences in diagnosis, selection criteria, time of evaluation, ethnicity, and socioeconomic status. As shown in Table 4, it may be any single factor or, more likely, a combination of them that contributes to the lack of consistency among studies.
One of the reasons that studies may differ in the prevalence of gender-related major depression is the method of diagnosis. The criteria used to identify subjects with poststroke depression often differ from study to study. Use of depression scales and a depression cutoff score versus semistructured interviews and diagnostic criteria, or self-rating versus observer-rated scales, is likely to bear a significant impact on results. Similarly, since the increased frequency of depression in females is a phenomenon that is restricted to major depression, grouping minor and major depression under a common rubric may also affect the results.
Selection criteria may be another important cause of differences in findings concerning frequencies of males versus females with major depression. Excluding patients with a previous history of psychiatric disorder may mask gender differences in the prevalence of major depression.30 Morris et al.,5 in the only study that reported increased prevalence of depressed males, included patients only if they were able to “nominate a person they could turn to for support following the stroke.”
Time of evaluation is another reason why some studies may have failed to identify differences in the frequency of major depression in males versus females. Burvill at al.20 reported that 13% of female patients and 9% of males were depressed at the time of stroke but that the prevalence of depression at 1 year was almost equal between sexes (about 24%). Among those patients who were depressed at 4 months, significantly more women (39%) than men (16%) had been depressed at the time of the index episode.20 This example shows how time from index stroke to evaluation, as well as setting (hospital versus rehabilitation facility or nursing home), may also be a cause for discrepant results.
Population differences in ethnicity and socioeconomic status may also account for result inconsistencies in gender-related differences in poststroke depression.5,20 A longitudinal study conducted in Sweden23 did not find female gender to be associated with major depression. Two studies conducted in England showed an association of major depression with female gender (when other clinical and pathological variables were controlled)31 and an association of female gender with greater depression (assessed with the Wakefield Assessment Depression Inventory).29 Angeleri et al.,34 studying 180 consecutive patients in central Italy, also reported greater severity of depression (assessed with the Beck Depression Inventory) in females. Although Dam et al.,35 studying a sample of young and only slightly disabled patients in Glostrup, Denmark, found a similar trend of twice as severe depression measured by the Ham-D among women, this result did not reach statistical significance. Another study conducted in Denmark (Aalborg/Farsø) reported that the 1-year incidence of depression in an unselected stroke population was 85/209 (41%), with 60% of the depressed patients being female.36 The percentage of depressed females that the authors report is exactly comparable to ours (depressed males=20, 39%; depressed females=31, 61%).
Further investigations of these interesting gender-related differences are clearly needed.
In summary, major depressive disorder in the acute poststroke period is more frequent in females (at least within the type of population studied here) than males. In addition, poststroke depression has different correlates and predictors in males compared with females. This result may be the effect of psychosocial factors, different biological predisposition, or both. These findings might also have important therapeutic implications. Perhaps men would be more likely to respond to physical therapy for activities of daily living or social intervention, while women might be more likely to respond to psychological or somatic therapy.
ACKNOWLEDGMENTS
This research was supported in part by Research Scientist Award MH00163 (R.G.R.) and National Institute of Mental Health Grant MH40355. The findings were presented at the 149th annual meeting of the American Psychiatric Association, May 4–9, 1996, New York, NY.
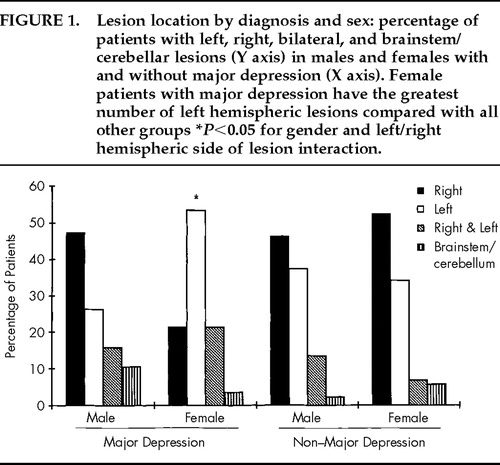
FIGURE 1. Lesion location by diagnosis and sex: percentage of patients with left, right, bilateral, and brainstem/cerebellar lesions (Y axis) in males and females with and without major depression (X axis). Female patients with major depression have the greatest number of left hemispheric lesions compared with all other groups *P<0.05 for gender and left/right hemispheric side of lesion interaction.
 |
 |
 |
 |
1. Weissman MM, Olfson M: Depression in women: implications for health care research. Science 1995; 269:799–801Crossref, Medline, Google Scholar
2. Borod J: Interhemispheric and intrahemispheric control of emotions: a focus on unilateral brain damage. J Consult Clin Psychol 1992; 60:339–348Crossref, Medline, Google Scholar
3. Gur R, Mozley L, Mozley P, et al: Sex differences in regional cerebral glucose metabolism during a resting state. Science 1995; 267:528–531Crossref, Medline, Google Scholar
4. Shaywitz B, Shaywitz S, Pugh K, et al: Sex differences in the functional organization of the brain for language. Nature 1995; 373:607–609Crossref, Medline, Google Scholar
5. Morris PLP, Robinson RG, Raphael B, et al: The relationship between perception of social support and post-stroke depression in hospitalized patients. Psychiatry 1991; 54:306–315Crossref, Medline, Google Scholar
6. Schleifer SJ, Macari-Hinson MM, Coyle DA, et al: The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789Crossref, Medline, Google Scholar
7. Travella JI, Forrester AW, Schultz SK, et al: Depression following myocardial infarction: a one year longitudinal study. Int J Psychiatry Med 1994; 24:357–369Crossref, Medline, Google Scholar
8. Castillo CS, Starkstein SE, Fedoroff JP, et al: Generalized anxiety disorder after stroke. J Nerv Ment Dis 1993; 181:100–106Crossref, Medline, Google Scholar
9. Downhill JE, Robinson RG: Longitudinal assessment of depression and cognitive impairment following stroke. J Nerv Ment Dis 1994; 182:425–431Crossref, Medline, Google Scholar
10. Kunitz S, Gross C, Heyman A, et al: The pilot stroke data bank: definition, design, and data. Stroke 1984; 15:740–746Crossref, Medline, Google Scholar
11. Robinson R, Benson D: Depression in aphasic patients: frequency, severity, and clinical pathological correlations. Brain Lang 1981; 14:282–291Crossref, Medline, Google Scholar
12. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
13. Wing J, Cooper E, Sartorius N (eds): Measurement and classification of psychiatric symptoms. Cambridge, England, Cambridge University Press, 1974Google Scholar
14. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
15. Robinson RG, Bolla-Wilson K, Kaplan E, et al: Depression influences intellectual impairment in stroke patients. Br J Psychiatry 1986; 148:541–547Crossref, Medline, Google Scholar
16. Robinson RG, Seztela B: Mood change following left hemisphere brain injury. Ann Neurol 1981; 9:447–453Crossref, Medline, Google Scholar
17. Starr LB, Robinson RG, Price TR: Reliability, validity, and clinical utility of the social functioning exam in the assessment of stroke patients. Exp Aging Res 1983; 9:101–106Crossref, Medline, Google Scholar
18. Levine D, Grek A: The anatomic basis of delusion after right cerebral infarction. Neurology 1984; 34:577–582Crossref, Medline, Google Scholar
19. Starkstein SE, Robinson RG, Price TR: Comparison of cortical and subcortical lesions in the production of post stroke mood disorders. Brain 1987; 110:1045–1059Crossref, Medline, Google Scholar
20. Burvill PW, Johnson GA, Jamrozik KD, et al: Prevalence of depression after stroke: the Perth community stroke study. Br J Psychiatry 1995; 166:320–327Crossref, Medline, Google Scholar
21. Robinson RG, Kubos KL, Starr LB, et al: Mood disorders in stroke patients: importance of lesion location. Brain 1984; 107:81–93Crossref, Medline, Google Scholar
22. Herrmann M, Bartels C, Schumacher M, et al: Post-stroke depression: is there a pathoanatomic correlate for depression in the post-acute stage of stroke? Stroke 1995; 26:850–856Google Scholar
23. Åström M, Adolfsonn R, Asplund K: Major depression in stroke patients: a 3-year longitudinal study. Stroke 1993; 24:976–982Crossref, Medline, Google Scholar
24. House A, Dennis M, Warlow C, et al: Mood disorders after stroke and their relation to lesion location. Brain 1990; 113:1113–1129Crossref, Medline, Google Scholar
25. Agrell B, Dehlin O: Depression in stroke patients with left and right hemisphere lesions: a study of geriatric rehabilitation in-patients. Aging Clin Exp Res 1994; 6:49–56Crossref, Google Scholar
26. Schwartz JA, Speed NM, Brunberg JA, et al: Depression in stroke rehabilitation. Biol Psychiatry 1993; 33:694–699Crossref, Medline, Google Scholar
27. House A, Dennis M, Warlow C, et al: The relationship between intellectual impairment and mood disorder in the first year after stroke. Psychol Med 1990; 20:805–814Crossref, Medline, Google Scholar
28. Bolla-Wilson K, Robinson RG, Starkstein SE, et al: Lateralization of dementia of depression in stroke patients. Am J Psychiatry 1989; 146:627–634Crossref, Medline, Google Scholar
29. Wade DT, Legh-Smith J, Hewer RA: Depressed mood after stroke. Br J Psychiatry 1987; 151:200–205Crossref, Medline, Google Scholar
30. Sinyor D, Amato P, Kaloupek DG, et al: Post-stroke depression: relationship to functional impairment, coping strategies, and rehabilitation outcome. Stroke 1986; 17:1102–1107Crossref, Medline, Google Scholar
31. Sharpe M, Hawton K, Seagroatt V, et al: Depressive disorders in long-term survivors of stroke: association with demographic and social factors, functional status, and brain lesion volume. Br J Psychiatry 1994; 164:380–386Crossref, Medline, Google Scholar
32. Fedoroff JP, Starkstein SE, Forrester AW, et al: Depression in patients with acute traumatic brain injury. Am J Psychiatry 1992; 149:918–923Crossref, Medline, Google Scholar
33. Jorge RE, Robinson RG, Arndt SV, et al: Depression following traumatic brain injury: a 1 year longitudinal study. J Affect Disord 1993; 27:233–243Crossref, Medline, Google Scholar
34. Angeleri F, Angeleri VT, Foschi N, et al: The influence of depression, social activity, and familial stress on functional outcome after stroke. Stroke 1993; 24:1478–1483Crossref, Medline, Google Scholar
35. Dam H, Pedersen HE, Ahigen P: Depression among patients with stroke. Acta Psychiatr Scand 1989; 80:118–124Crossref, Medline, Google Scholar
36. Andersen G, Vestergaard K, Riis JO, et al: Incidence of post-stroke depression during the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatr Scand 1994; 90:190–195Crossref, Medline, Google Scholar



