A Reappraisal of Poststroke Depression, Intra- and Inter-Hemispheric Lesion Location Using Meta-Analysis
Abstract
A recent publication based on a meta-analysis concluded that there was no association between poststroke depression (PSD) and lesion location. This study, therefore, was undertaken to reappraise the hypothesis using meta-analysis of the correlation between severity of depression following stroke and proximity of the lesion to the frontal pole, an issue that was not examined in the prior meta-analysis. Results showed there was a significant inverse correlation between severity of depression and distance of the lesion from the frontal pole among 163 patients with left hemisphere stroke but not among 106 patients with right hemisphere stroke. This study supports the hypothesis that risk of poststroke depression is related to the location of brain injury.
In 1981 and 1984, we first reported that severity of depression, as measured by the standardized depression rating scale, in patients with stroke was significantly correlated with the proximity of the anterior border of the lesion on computed tomography scan (CT) to the frontal pole in the left hemisphere but not in the right hemisphere.1,2 These lateralized and intra-hemispheric associations between severity of depression on standardized rating scales and location of brain injury have been replicated by some,3–8 but not all researchers.9–12 This discrepancy, however, may have been the result of the time when patients were examined after stroke. In a 2-year longitudinal study of depressive symptoms and lesion location, we found that symptom severity, as measured by total score on the Present Status Examination, correlated with the proximity of the lesion to the frontal pole only in the left hemisphere during the acute stroke period, but this hemispheric asymmetry was not present in the longitudinal follow-ups.13 This finding suggests that there was a dynamic, time dependent, relationship between the severity of depressive symptoms and lesion location.
Based on these prior findings, we have hypothesized that: 1) frontal or basal ganglia lesions in the left hemisphere during the acute poststroke period (i.e., less than 2 months poststroke) are associated with a higher frequency of major depression than comparable lesions of the right hemisphere or parietal-temporal-thalamic lesions of the left hemisphere, and 2) during the first 6 months of poststroke, there is a significant inverse correlation between the severity of depressive symptoms and the distance of the anterior border of the lesion from the frontal pole in the left hemisphere.
A recent publication by Carson et al.14 reported on a meta-analysis of all studies of poststroke depression (PSD), which examined the association between prevalence of depression (sometimes determined by a cut off score on a severity rating scale and sometimes based on diagnostic criteria) and lesion location. The authors concluded that “there is no support for the hypothesis that the risk of depression after stroke is affected by the location of the brain lesion.” Our first hypothesis, however, was that patients with lesions involving the left frontal basal ganglia circuit would have a significantly greater frequency of depression compared to patients with similar lesions of the right frontal region or posterior left hemisphere region, but only during the first 1 or 2 months following stroke.13 Their analysis of studies conducted on patients within 1 month of poststroke included 10 studies. Only two of these studies, however, (Robinson et al.15 and Morris et al.16) examined anterior-posterior location. Both studies found a significantly greater frequency of depression following left anterior lesions compared with right anterior lesions. The authors, however, omitted other relevant studies that appear to have met their criteria (e.g., Astrom et al.,4 Robinson et al.17), and a reanalysis of all the relevant studies demonstrated that the pooled data relative risk of PSD between left anterior lesions and left posterior lesions was 2.17 (95% CI 1.57-3.00), and between left anterior and right anterior lesions, this risk was 2.28 (95% CI 1.60-3.24).18 The Carson et al. study, however, did not examine our second hypothesis suggesting that there is a significant correlation between the proximity of the lesion to the left frontal pole and severity of depressive symptoms during the first 6 months following stroke. The current study, therefore, examined the association of depressive symptoms and lesion location focusing for the first time on a meta-analysis of the severity of depressive symptoms and the proximity of the lesion to the frontal pole in each hemisphere during the first 6 months following stroke.
METHODS
Searching Strategy
All studies that examined the correlation between PSD and lesion location were initially eligible for inclusion. We screened journal articles that were published between January,1981 and December, 2000 as original research publication. We excluded abstracts, review articles, case reports, clinical observations, and unpublished data.
First, we searched studies on the computerized database using the keyword “depression or poststroke depression,” “stroke or cerebrovascular disease,” and ”lesion location.” Our literature search used the PubMed version of MEDLINE on the Internet. Second, we checked all the references of the identified studies and reviews. Third, we hand-searched the latest key journals. Our selection protocol defined the criteria for relevant studies before we began searching.
Study Selection
By reading all the retrieved papers in detail, we found the studies that examined the correlation between depressive symptom severity and lesion location. Among these papers that assessed lesion location, we selected studies that examined the correlation coefficient (i.e., Pearson's R or Spearman's Rho) between the severity of depression and the distance of the anterior border of the lesion from the frontal pole.
The inclusion criteria required 1) the use of standardized measurement for severity of depression; 2) imaging using either CT or MR scanning; 3) exclusion of patients with comprehension deficits, which would have prohibited reliable assessment of the patient; 4) identification of the total number of subjects in the study; 5) clinical examination performed within 6 months following stroke; 6) publication in English; 7) the evaluation containing the largest number of patients if there were repeated evaluations over time (this was usually the initial evaluation); and 8) the availability of relevant raw data if no correlation coefficient was reported. If the data were available, we excluded patients with a history of depressive disorder prior to the stroke. As indicated in previous publications,19 we included only studies that used standardized measurements of depressive symptoms while trying to include the largest number of studies possible.
All studies were reviewed by one investigator (K.N.) with a structured form that recorded the correlation coefficient, the source of patients, the interval between stroke and assessment of depressive symptom severity, personal and family psychiatric history, employed imaging method and results, gender, age, diagnostic criteria, exclusion criteria or clinical judgment for excluding patients with severe comprehension deficits, and the number of patients in the study. Results were checked for accuracy by a second reviewer (R.G.R.), and any discrepancies were resolved by discussion and consensus.
Assessment of Quality of Studies
We assessed the methodological quality of the studies using a scoring system. Four items were evaluated for each study: 1) were standardized depression rating instruments used; 2) were CT or MRI imaging measurements made blind to the psychiatric data; 3) was the method for exclusion of receptive aphasia operationalized; and 4) were there less than 20 subjects or more than 30 subjects included? Methodological quality was graded for each of the four items on a scale of 0,1, or 2 (Maximum score = 8). We included studies that had more than 5 total points (Tables 1 and 2).
Statistical Analysis
While there are a variety of indices in common use for effect size measurements (e.g., Cohen's d, omega-squared, or eta-squared), we used the correlation coefficient. This index has been suggested by Rosenthal et al.20 for a number of reasons. For example, correlation coefficients are widely understood, generalizable to situations where one or both the independent and dependent variable are measured on a scale or grouping, and mathematically equivalent to other common effect size measures (e.g., Cohen's d).
The program Comprehensive Meta-Analysis21 was used for analyses. Both random and fixed models were fit, and assessment of statistical intertrial heterogeneity was made.22,23 In case the study reported results on more than one rating scale, we found the pooled correlation using Fisher's r-to-Z transformation to produce a single measure of the effect.
RESULTS
We examined 356 potentially eligible studies and 36 reviews and found 111 studies that referred to the correlation of our interest. Of these, 14 studies contained original data.2,7,8,11–13,17,24–30 Thirteen studies examined left-emisphere stroke patients, while 10 examined patients with right hemisphere stroke.
In the studies of Herrmann et al.,12,24 the study with the largest number of patients was chosen according to the inclusion criteria.24 Three studies included patients with past psychiatric history.7,8,17 We removed those patients in two studies.8,17 It was impossible, however, to remove patients with past psychiatric history in the third study7 due to lack of information (i.e., four out of 11 patients with left hemisphere stroke had a past psychiatric history). Data in the Sinyor et al. study29 was taken from the scatter plots.
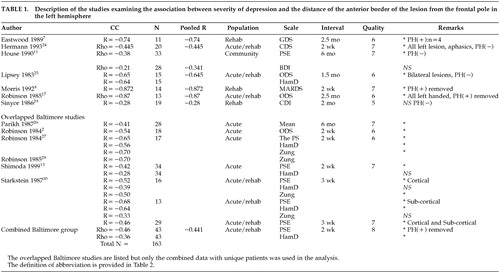
In a series of studies, our group has examined the association between depressive symptom severity and lesion location. Some studies employed unique samples while others examined overlapped samples. For the purpose of removing the overlap and utilizing as many patients as possible in this meta-analytic study, we combined six studies2,13,26–28,30 into one large group with no overlap (Tables 1 and 2). All patients with a past history of depression were removed, and the evaluation assessing the largest number of patients was used.
Tables 1 and 2 show all the studies that were included in the meta-analysis. There were 163 patients in the left hemisphere stroke group and 106 patients in the right hemisphere stroke group.
Patients With Left Cerebral Injury
Figure 1 shows the results of the meta-analysis among patients with left hemisphere lesions. There were eight unique study samples. Among them, six out of eight found a significant inverse correlation between the severity of depressive symptoms and the proximity of the lesion to the frontal pole.
The results of the meta-analysis showed that on both the fixed and random models, the inverse correlation between the severity of depressive symptoms and the distance of the anterior border of the lesion from the frontal pole was significant (Z-statistic; Z = −7.04, p < 0.001 on the fixed model, Z = −4.68, p < 0.001 on the random model). The heterogeneity among the eight study populations was significant (Q-statistic; Q = 17.65, df = 7.00, p = 0.01). The global estimate revealed that the correlation of our interest was moderately strong (Pooled correlation coefficient: −0.53 on the fixed model, −0.59 on the random model).
Patients With Right Cerebral Injury
Figure 2 shows the results among patients with right hemisphere lesions. There were five unique samples and four out of five studies showed a nonsignificant association. Only one study found an inverse correlation (i.e., negative correlation coefficient) between the severity of depressive symptoms and the distance of the lesion from the frontal pole, which was significant.12
The meta-analysis, both on the fixed and random models, found that the association of interest was not significant (p = 0.14, 0.17 respectively). There was no evidence of significant heterogeneity among the studies (p = 0.29). Pooled correlation coefficient was −0.15 on the fixed model and −0.17 on the random model.
We also compared the effect in the left hemisphere versus the right hemisphere using both the fixed and random models and Z transformed standard errors. The correlation in the left hemisphere was significantly greater than that of the right hemisphere (Z fixed = 3.43, p < .0001, Z random = 2.63, p < .008) indicating that the relationship between depression severity and lesion location was stronger in the left than the right hemisphere.
DISCUSSION
This study found that there is a moderately strong inverse correlation between the severity of depressive symptoms and the distance of the anterior border of the left hemisphere lesion from the frontal pole for the first 6 months following stroke. In contrast, the correlation among patients with right hemisphere strokes was more variable and not statistically significant. The difference between the correlations in the two hemispheres was also statistically significant.
Before discussing these findings, the limitations imposed by the methods used in the study should be acknowledged. Naturally, bias can be introduced into the process of searching and selecting studies and represents a characteristic shortcoming of meta-analysis. For instance, studies with significant results are more likely to be published. In addition, potentially confounding factors, which could affect the association of our interest, could not be controlled in this study due to the limited data and the relatively small number of eligible studies.
For example, the source of patients (e.g., outpatient, community, rehabilitation hospital, or acute hospital), handedness, gender, age, psychiatric measurement instruments (i.e., some studies used interviewer rated scales such as the Ham-D while others used self rated instruments such as the BDI, and these scales are not comparable), and the criteria for verbal comprehension deficits were often imprecise, and anatomical asymmetry of the brain, which has been shown to influence the diagnosis of PSD and lesion location, was not taken into account.19 Despite these limitations, the result of this meta-analysis has supported our second hypothesis that lesion location is significantly associated with the severity of depressive symptoms in the first 6 months following stroke. Further, this study is consistent with our first hypothesis that the pathophysiological changes mediated by left hemisphere injury are more important than those of the right hemisphere in the development of depressive symptoms during the acute poststroke period. Finally, one might speculate that the failure of some studies to show a significant correlation between the severity of depressive symptoms and the proximity of the lesion to the frontal pole may have been due to the examinations of patients who were more than 6 months poststroke.
One might wonder why this meta-analysis found a significant association between depressive symptoms and lesion location, while the Carson et al. study14 mentioned in the introduction found no association. First, it should be emphasized that the Carson et al. study examined the association of inter or intrahemispheric lesion location and the frequency of diagnosed depression, while this study examined the correlation between the proximity of the lesion to the frontal pole and severity of depressive symptoms. The correlation of the proximity of the lesion to the left frontal pole and the severity of depressive symptoms appears to last for approximately 6 months, while the association between left frontal or left basal ganglia lesion location and increased frequency of depression appears to last only 1 to 2 months.13
Secondly, we discussed the shortcomings of the Carson analysis and why their data did not allow them to examine the specific (our first) hypothesis regarding the frequency of PSD and lesion location. Ultimately, validation of the hypothesis that depression is more frequently provoked by left frontal or left basal ganglia lesions than comparable lesions of the right hemisphere is dependent upon the generalizability of this finding to depression associated with other disorders. For example, hyperintensities seen on MRI scans of the left basal ganglia have been shown to be associated with geriatric depression significantly more frequently than any other location for subcortical hyperintensities.31 Major depression following acute traumatic brain injury has been specifically associated with left frontal and left basal ganglia lesions based on logistic regression analysis.32 Major depression in the early stages of Parkinson's disease (PD)33 and following focal brain stimulation in the subthalamic nucleus34 for treatment of PD has been associated with dysfunction of the left nigro-striatal but not the right nigro-striatal or subthalamic structures. In addition, positron emission tomography (PET) studies of regional brain dysfunction among depressed patients without known brain injury have shown changes in metabolic activity or blood flow in numerous limbic connected brain structures, including the left frontal cortex or left basal ganglia.35 Finally, a large recently published MRI study of 3,236 participants in a cardiovascular disease study found that the occurrence of depressive symptoms was significantly associated with small lesions in the basal ganglia.36 This was a similar finding to that found in the Stroke Data Bank.37
Another relevant question raised by this study is why there is a linear correlation between the proximity of the lesion to the frontal pole and the severity of depressive symptoms? Laboratory experiments in rats have shown that identical size suction lesions of the lateral cortex produce a linear decrease in the concentrations of cortical and brainstem norepinephrine, as the lesion is closer to the frontal pole.38 Ascending noradrenergic and serotonergic fibers from the brainstem enter the deep layers of frontal cortex and run anterior to posterior with arborizing terminals branching into superficial cortical layers. Thus, anterior lesions would interrupt these pathways closer to their origin and cause greater depletion of norepinephrine and presumably serotonin than more posterior lesions.39 Using PET, subsequent studies in humans showed that the amount of depletion of serotonin (S2) receptors in the left temporal cortex was significantly correlated with the severity of depressive symptoms.40 Thus, the phenomenon of correlation between lesion location and the severity of depressive symptoms may result from greater biogenic amine depletion associated with more proximal lesions. Other hypotheses might also be proposed.
Although this study has focused on the role of lesion location in the etiology of acute PSD, it is important to emphasize that this is only one of several factors that have been associated with the frequency or severity of PSD. Other risk factors that have been associated with an increased prevalence of poststroke depression are: subcortical atrophy,41 structural brain asymmetries,42 lesion volume,9,13 female gender,43–45 family or previous history of mood disorder,46,47 neuroticism trait,48 younger age,45 greater impairment in activities of daily life,7 impaired social support (especially support from spouse),49–51 and negative life events.48 Some of them independently increase the prevalence of diagnosed depression following stroke, while others have been shown to have an additive effect.48 Thus, the cause of PSD probably includes several mechanisms that vary with premorbid as well as poststroke factors.
In summary, this reappraisal of lesion factors and severity of depressive symptoms sheds a different light on the statement by Carson et al. that “there is no support for the hypothesis that the risk of depression after stroke is affected by the location of the brain lesion.” We believe that if future studies carefully control for time-since-stroke as well as premorbid and family psychiatric history, use standardized interviews and established diagnostic criteria, and ascertain lesion location using the most sensitive imaging techniques6, then the literature on severity of depressive symptoms or the frequency of diagnosed depression and lesion location will be clearer and more consistent.
ACKNOWLEDGMENTS
The authors would like to thank Stephan Arndt, Ph.D. for assistance with the statistical analysis. The authors also thank Toru Nishikawa, M.D., Ph.D., and Eisuke Matsushima, M.D., Ph.D. This work was supported in part by National Institute of Mental Health R01 grants MH-40355, MH-52879, MH-53592, MH63405 and Research Scientist Award MH-00163 (R.G.R.).
A part of the paper has been presented as a poster at the 2002 American Neuropsychiatric Association Annual Meeting, March 9-12, 2002, San Diego, California.
 |
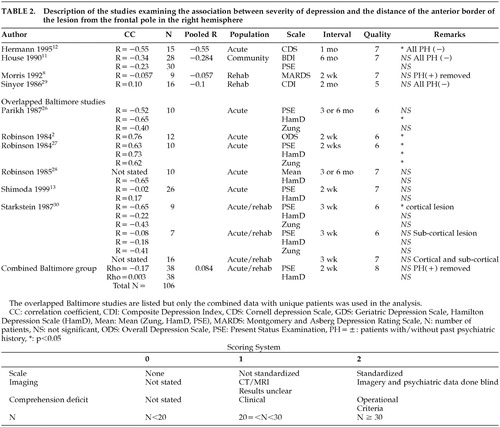 |
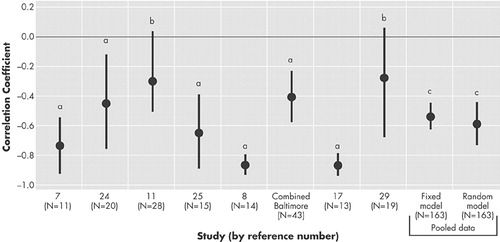
FIGURE 1. Study specific correlation coefficient between the severity of depression and the proximity of the lesion location to the frontal pole among patients with left-hemisphere lesions
a= p < .05
b = Not significant
c = p < .001
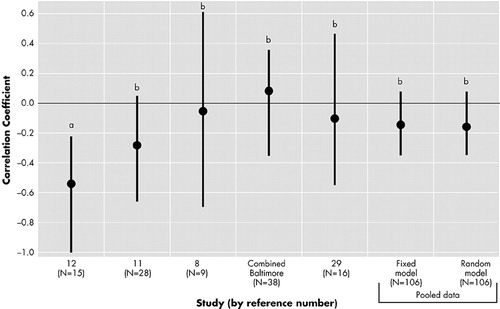
FIGURE 2. Study specific correlation coefficient between the severity of depression and the proximity of the lesion location to the frontal pole among patients with right-hemisphere lesions
a = p < .05
b = Not significant
1 Robinson RG, Szetela B: Mood change following left hemispheric brain injury. Ann Neurol 1981; 9:447–453Crossref, Medline, Google Scholar
2 Robinson RG, Kubos KL, Starr LB, et al: Mood disorders in stroke patients: importance of location of lesion. Brain. 1984; 107:81–93Google Scholar
3 Yamaguchi S, Kobayashi S, Koide H, et al: Longitudinal study of regional cerebral blood flow changes in depression after stroke. Stroke 1992; 1716–1722Google Scholar
4 Astrom M, Adolfsson R, Asplund K: Major depression in stroke patients: a 3-year longitudinal study. Stroke 1993; 24:976–982Crossref, Medline, Google Scholar
5 Iacoboni M, Padovani A, Di Piero V, et al: Post-stroke depression relationships with morphological damage and cognition over time. Ital J Neurol Sci 1995; 16(4):209–216Crossref, Medline, Google Scholar
6 Vataja R, Pohjasvaara T, Leppavuori A, et al. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. 2001; 58(10):925–931Google Scholar
7 Eastwood MR, Rifat SL, Nobbs H, et al: Mood disorder following cerebrovascular accident. Br. J. Psychiatry 1989; 154:195–200Crossref, Google Scholar
8 Morris PLP, Robinson RG, Raphael B: Lesion location and depression in hospitalized stroke patients: evidence supporting a specific relationship in the left hemisphere. Neuropsychiatry Neuropsychol Behav Neurol 1992; 3:75–82Google Scholar
9 Sharpe M, Hawton K, House A, et al. Mood disorders in long-term survivors of stroke: associations with brain lesion location and volume. Psychol Med 1990; 20:815–828Crossref, Medline, Google Scholar
10 Ng KC, Chan KL, Straughan PT: A study of post-stroke depression in a rehabilitative center. Acta Psychiatr Scand 1995; 92(1):75–79Crossref, Medline, Google Scholar
11 House A, Dennis M, Warlow C, et al: Mood disorders after stroke and their relation to lesion location. A CT scan study. Brain 1990; 113:1113–1130Crossref, Medline, Google Scholar
12 Herrmann M, Bartels C, Schumacher M, et al: Poststroke depression: is there a pathoanatomic correlate for depression in the postacute stage of stroke? Stroke 1995; 26:850–856Crossref, Medline, Google Scholar
13 Shimoda K, Robinson RG: The relationship between post-stroke depression and lesion location in long-term follow-up. Biol Psychiatry 1999; 45:187–192Crossref, Medline, Google Scholar
14 Carson AJ, MacHale S, Allen K, et al: Depression after stroke and lesion location: a systematic review. Lancet 2000; 356(9224):122–126Crossref, Medline, Google Scholar
15 Robinson RG, Kubos KL, Starr LB, et al: Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry 1983; 24:555–566Crossref, Medline, Google Scholar
16 Morris PLP, Robinson RG, Raphael B, et al: Lesion location and post-stroke depression. J Neuropsychiatry Clin Neurosci 1996; 8:399–403Link, Google Scholar
17 Robinson RG, Lipsey JR, Bolla-Wilson K, et al: Mood disorders in left handed stroke patients. Am. J. Psychiatry 1985; 142:1424–1429Google Scholar
18 Robinson RG: Psychiatric management of stroke. Psychiatr Ann 2002; 3292:121–127Crossref, Google Scholar
19 Narushima K, Paradiso S, Robinson RG: The relationship between post-stroke depression and lesion location in follow-up studies: a review of the confounding factors. Essential Psychopharmacology 2002; 5(1)Google Scholar
20 Rosenthal R, Rosnow RL, Rubin DB: Contrasts and Effect Sizes in Behavioral Research: A Correlation Approach. New York: Camridge Unviersity Press; 2000Google Scholar
21 Comprehensive Meta Analysis [computer program]. Version 1.0. Englewood: Biostat; 2001Google Scholar
22 Hedges IV, Olkin I: Statistical Methods for Meta-Analysis, New York: Academic Press; 1985Google Scholar
23 Glass GV, McGaw B, Smith ML: Meta-Analysis in Social Research, Beverly Hills: Sage Publications; 1981Google Scholar
24 Herrmann M, Bartles C, Wallesch C-W: Depression in acute and chronic aphasia: symptoms, pathoanatomical-clinical correlations and functional implications. J Neurol Neurosurg Psychiatry 1993; 56:672–678Crossref, Medline, Google Scholar
25 Lipsey JR, Robinson RG, Pearlson GD, et al: Mood change following bilateral hemisphere brain injury. Br. J. Psychiatry 1983; 143:266–273Crossref, Google Scholar
26 Parikh RM, Lipsey JR, Robinson RG, et al: Two-year longitudinal study of post-stroke mood disorders: dynamic changes in correlates of depression at one and two years. Stroke 1987; 18:579–584Crossref, Medline, Google Scholar
27 Robinson RG, Starr LB, Price TR: A two-year longitudinal study of post-stroke mood disorders: dynamic changes in associated variables over the first six months of follow-up. Stroke 1984; 15:510–517Crossref, Medline, Google Scholar
28 Robinson RG, Starr LB, Lipsey JR, Rao K, et al: A two year longitudinal study of post-stroke mood disorders: in-hospital prognostic factors associated with six month outcome. J Nerv Ment Dis 1985; 173:221–226Crossref, Medline, Google Scholar
29 Sinyor D, Jacques P, Kaloupek DG, et al: Post-stroke depression and lesion location: an attempted replication. Brain 1986; 109:539–546Crossref, Google Scholar
30 Starkstein SE, Robinson RG, Price TR: Comparison of cortical and subcortical lesions in the production of post-stroke mood disorders. Brain 1987; 110:1045–1059Crossref, Medline, Google Scholar
31 Greenwald BS, Kramer-Ginsberg E, Krishnan RR, et al: Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke 1998; 29:613–617Crossref, Medline, Google Scholar
32 Fedoroff JP, Starkstein SE, Forrester AW, et al: Depression in patients with acute traumatic brain injury. Am J Psychiatry 1992; 149:918–923Crossref, Medline, Google Scholar
33 Starkstein SE, Robinson RG, Preziosi TJ: Depression in Parkinson's disease. J Nerv Ment Dis 1990; 178:27–31Crossref, Medline, Google Scholar
34 Bejjani BP, Damer P, Arnulf I, et al: Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med 1999; 340(19):1476–1480Crossref, Medline, Google Scholar
35 Baxter LR, Jr., Schwartz JM, Phelps ME, et al: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243–250Crossref, Medline, Google Scholar
36 Steffens DC, Krishnan KR, Crump C: Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke 2002; 33(6):1636–1644Crossref, Medline, Google Scholar
37 Morris PLP, Robinson RG, Carvalho ML, et al: Lesion characteristics and depressed mood in the stroke data bank study. J Neuropsychiatry Clin Neurosci 1996; 8:153–159Link, Google Scholar
38 Pearlson GD, Robinson RG: Effect of anterior-posterior lesion location on the asymmetrical behavioral and biochemical response to cortical suction ablations in the rat. Brain Res 1984; 293:241–250Crossref, Medline, Google Scholar
39 Morrison JH, Molliver ME, Grzanna R: Noradrenergic innervation of the cerebral cortex: widespread effects of local cortical lesions. Science 1979; 205:313–316Crossref, Medline, Google Scholar
40 Mayberg HS, Robinson RG, Wong DF, et al: PET imaging of cortical S2–serotonin receptors after stroke: lateralized changes and relationship to depression. Am J Psychiatry 1988; 145:937–943Crossref, Medline, Google Scholar
41 Starkstein SE, Robinson RG, Price TR: Comparison of patients with and without post-stroke major depression matched for size and location of lesion. Arch Gen Psychiatry 1988; 45:247–252Crossref, Medline, Google Scholar
42 Starkstein SE, Bryer JB, Berthier ML, et al: Depression after stroke: the importance of cerebral hemisphere asymmetries. J Neuropsychaitry Clin Neurosci 1991; 3(3):276–285Link, Google Scholar
43 Andersen G, Vestergaard K, Riis JO, Lauritzen L: Incidence of post-stroke depression during the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatr Scand 1994; 90:190–195Crossref, Medline, Google Scholar
44 Angeleri F, Angeleri VA, Foschi N, et al: The influence of depression, social activity, and family stress on functional outcome after stroke. Stroke 1993; 24(20):1478–1483Crossref, Medline, Google Scholar
45 Paradiso S, Robinson RG: Gender differences in post-stroke depression. J Neuropsychiatry Clin Neurosci 1998; 10:41–47Link, Google Scholar
46 Morris PLP, Robinson RG, Raphael B: Prevalence and course of depressive disorders in hospitalized stroke patients Intl J Psychiatr Med 1990; 20:349–364Google Scholar
47 Starkstein SE, Robinson RG, Honig MA, et al: Mood changes after right hemisphere lesion Br J Psychiatry 1989; 155:79–85Google Scholar
48 Morris PLP, Robinson RG, Raphael B, et al: The relationship between risk factors for affective disorder and post-stroke depression in hospitalized stroke patients. Aust N Z J Psychiatry 1992; 26:208–217Crossref, Medline, Google Scholar
49 Robinson RG, Bolduc PL, Kubos KL, et al: Social functioning assessment in stroke patients. Arch Phys Med Rehabil 1985; 66:496–500Medline, Google Scholar
50 Thompson SC, Sobolew-Shobin A, Graham MA, et al: Psychosocial adjustment following stroke. Soc Sci Med 1989; 28:239–247Crossref, Medline, Google Scholar
51 Morris PLP, Robinson RG, Raphael B, et al: The relationship between the perception of social support and post-stroke depression in hospitalized patients. Psychiatry 1991; 54:306–316.Crossref, Medline, Google Scholar



