Performance on the Mini-Mental State Examination and Mattis Dementia Rating Scale Among Older American Indians
The Mini-Mental State Examination (MMSE) 13 is widely used to screen for cognitive impairment in clinical practice and dementia studies. The Mattis Dementia Rating Scale (MDRS) 14 is also commonly employed and has often been used as an alternative to the MMSE by neuropsychologists in clinical settings. 15 Both measures are sensitive to the effects of age and education. 15 – 17 Consequently, age- and education-adjusted scores have been developed for these measures in order to improve their utility in the identification of cognitive impairment. 18 – 20
This study investigated cognitive performance on the MMSE and MDRS among 140 members of a Northern Plains American Indian tribe (the specific tribe is not identified here in accordance with tribal confidentiality agreements). In light of previously identified health and cultural differences between American Indians and the general U.S. population, we hypothesized that participants would perform below age- and education-adjusted performance expectations derived from currently available normative data sets for the MMSE 18 and the MDRS. 19 , 20 We were especially interested in the relationship between cognitive scores and age, education, and self-reported health conditions among older American Indians. This article describes the performance of a sample of older American Indians on two common cognitive measures, and examines correlates of test performance.
METHOD
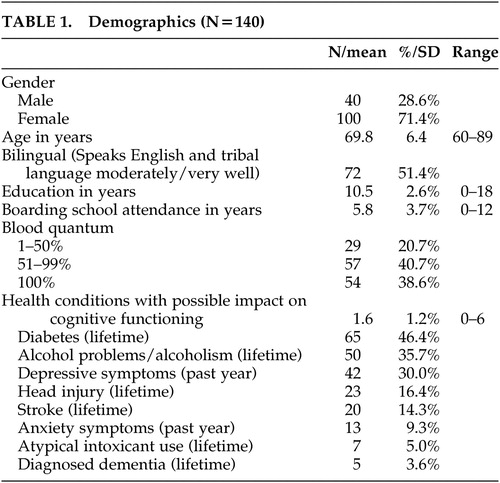
This study was approved by the tribe’s government, the Indian Health Service, and the Colorado Multiple Institutional Review Board. American Indians 60 years of age or older were recruited from 10 Administration on Aging-funded senior nutrition program sites throughout the reservation and in several off-reservation trust areas. One hundred thirty-seven participants completed the MMSE and 129 participants completed the MDRS ( Table 1 ). All participants signed approved consent forms. Criteria for utilizing the senior nutrition program included being age 60 or older, American Indian, and living within a 5-mile radius of a senior nutrition center. The program served the vast majority of the reservation’s older American Indians (83%), excluding mainly remote or uninterested elders.
 |
The MMSE and MDRS were selected for use in the study because of their familiarity to researchers and clinicians. Both measures are commonly used as screening assessments of cognitive health status and are regarded as relatively easy to administer. The measures differ with respect to their emphasis on specific cognitive domains: the MMSE heavily emphasizes memory, orientation, and language functions, while the MDRS is generally regarded as a more comprehensive screener.
Prior to data collection, focus groups conducted on the reservation identified some potential problems with the comprehensibility and cultural relevance of the measures, resulting in slight modifications to both measures (e.g., “room” was substituted for “floor” in the Orientation section of the MMSE since few buildings on the reservation exceed one floor) ( Table 2 ). Private interviews were conducted in a reservation-based field office that was affiliated with the University of Colorado at Denver and Health Sciences Center’s American Indian and Alaska Native programs. These interviews obtained demographic and health-related information; the MMSE and MDRS were administered at this time.
 |
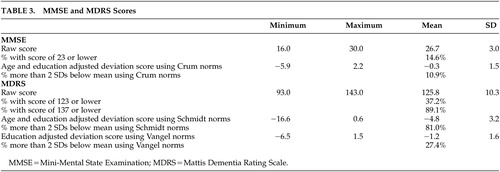
Raw MMSE scores among study participants were compared with a normative population-based database developed by Crum et al. 18 These norms were derived from a sample of over 18,000 community-dwelling individuals in the United States (ages 18 to 85+) who participated in the National Institute of Mental Health’s Epidemiologic Catchment Area Program survey. They provide normative performance data grouped by age over 5-year intervals, by education over 4-year intervals, and by both age and education. MDRS scores were compared with two sets of published norms with dissimilar ethnic compositions but fairly comparable age and education levels.
The first were age- and education-adjusted norms from an Austrian sample of 1,001 healthy adults who were 66.3 (SD=9.3) years old (range=50 to 80) with 10.8 (SD=2.4) years of education. 19 The second were age-adjusted norms derived from 36 Euro-American and 53 African American urban comparison subjects who were an average of 74 (SD=5.9) years old (range=62 to 95) with 10.5 (SD=3.6) years of education. 20 We considered comparison to a set of norms based on 133 rural white and African American comparison subjects ages 55 and older, 12 but that sample was limited to individuals with less than a 10th-grade education and hence lacked sufficient comparability to this study’s entire sample.
Possible cognitive impairment was defined as performance more than two standard deviations below each participant’s corresponding age- and education-matched cohort on each of these measures. In order to ascertain the rate of possible cognitive impairment, MMSE and MDRS scores were recoded using SPSS 21 to signify a dichotomous possible cognitive variable for each norming system.
Statistical analysis included descriptive statistics, chi-square tests and Fisher’s exact test (when an expected cell size was less than five) for categorical data, and t tests for continuous data. Both univariate and multivariate regression models investigated possible relationships between age, education, gender, and health problems with possible impact on cognitive functioning. Health problems with possible impact on cognitive functioning included a self-reported history of head injury, stroke, alcoholism, diagnosed dementia, or use of atypical intoxicants (i.e., alcohol-based mood altering substances not originally intended for consumption, such as Lysol, gasoline, hair-spray, or spray paint). Composite International Diagnostic Interview (CIDI) 22 screeners for symptoms of depression, anxiety, and alcohol abuse were also included.
Regression models used continuous age- and education-matched scores (according to the appropriate comparison study population). All tests were two tailed.
RESULTS
The vast majority (93%) of elders who were approached participated in the study. Nearly 72% of the 140 participants were women; elders had completed an average of nearly 11 years of education ( Table 1 ). Participants tended to be relatively young, from a geriatric perspective, with 52.9% of the sample 60 to 69 years of age, 38.6% was 70 to 79 years of age, and 8.6% was 80 to 89 years of age. This age distribution, however, was generally comparable to that of the greater reservation population. 23
The majority (79.3%) of elders reported a blood quantum (a crude measure of degree of Native heritage, often listed on one’s tribal membership card) of over 50%; fully 38.6% identified themselves as “full bloods” (i.e., 100% American Indian). All participants spoke English. Participants averaged two self-reported health condition with possible effects on cognitive functioning; of these, diabetes and past and/or present alcohol problems/alcoholism were present among more than one-third of the sample. Women were less likely than men to carry a self-reported diagnosis of dementia (chi-square=6.72, df=1, p=0.023), head injury (chi-square=10.54, df=1, p=0.001), or alcohol problems/alcoholism (chi-square=20.92, df=1, p<0.001). Women, however, were more likely to report depressive symptoms in the year preceding study participation (chi-square=4.17, df=1, p=0.041.)
When compared with the normative database for the MMSE developed by Crum et al., 18 10.9% of participants were classified as cognitively impaired ( Table 3 ). The prevalence of possible cognitive impairment in this sample, determined by the number of participants who scored greater than two standard deviations below age- and education-adjusted means, differed significantly from that found in the Crum et al. 18 study (chi-square=57.79, df=1, p<0.0001). For the MDRS, the proportions of possible cognitive impairment were 81.0% using the Schmidt et al. 19 age- and education-adjusted norms and 27.4% with the Vangel and Lichtenberg 20 age-adjusted norms.
 |
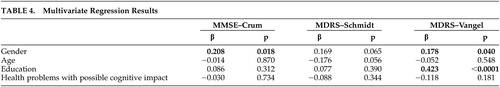
Univariate regression analysis demonstrated a relationship of female gender on adjusted MMSE scores (β=0.214, p=0.012), with women performing better than men (data not shown); multivariate regressions controlling for the other independent variable showed gender retained the only significant relationship to MMSE scores (β=0.208, p=0.018) ( Table 4 ). Similar to the results of the adjusted MMSE scores, univariate regression analysis demonstrated women performing better than men on Schmidt et al. 19 adjusted MDRS scores (β=0.190, p=0.037; data not shown). After controlling for other possible correlates, gender was not significant. Using the Vangel and Lichtenberg 20 norms, univariate regression analysis demonstrated individual relationships of both gender and education on adjusted MDRS scores (β =0.207, p=0.028, and β=0.422, p<0.0001; data not shown). Multivariate regressions ( Table 4 ) indicated both gender and education retained significant relationships with adjusted MDRS scores (β=0.178, p=0.040, and β =0.423, p<0.0001, respectively).
 |
As men were more likely to report a history of dementia, head injury, and alcohol problems, we tested for interaction for these health conditions with gender, age, and education. No significant interaction effects were found (data not shown).
DISCUSSION
Both the MMSE and MDRS identified many older American Indian participants as cognitively impaired when compared with normative samples of predominantly or completely non-American Indian populations with similar age and education levels. However, the frequency with which American Indians were classified as cognitively impaired varied according to the measure and the set of norms employed. The lowest prevalences of possible cognitive impairment were identified by the MMSE. At present, there are no data with which to determine whether “impaired” performance on these measures reflects a decline from prior levels of cognitive function and/or is associated with functionally significant impairments in daily living (i.e., whether they suggest the presence of a dementia) in this population. Given the types of medical and neurological problems observed in this sample (e.g., cerebrovascular disease, TBI, alcohol abuse), 5 , 24 a nontrivial but presently indeterminable frequency of cognitive impairment attributable to these problems would be expected. If the presently available norms for the MMSE are indeed applicable to the American Indian population, it is possible that the frequency of impairment observed here may be underestimated: the dominant effect of the types of medical and neurological problems experienced in this population would be on frontally mediated cognition (i.e., executive function) and the MMSE is relatively insensitive to impairments in this cognitive domain.
Interpretation of the subjects’ MDRS scores using either set of norms for this measure resulted in a greater number of subjects being classified as cognitively impaired than did the MMSE. It is possible that the assessment by the MDRS of cognitive domains not assessed by the MMSE, such as verbal conceptualization, 25 may improve its sensitivity to the types of cognitive impairment that would be expected in this population. However, the extremely high frequency of possible cognitive impairment using the Schmidt et al. 19 norms suggests that the application of these norms to the interpretation of MDRS performance among older American Indians is inappropriate. Although little is known about the frequency of dementia among older American Indians, it is likely considerably lower than the 81% frequency that might be suggested by these data. 3 – 5 It is probable that cultural and educational differences between the Austrian and American Indian contexts artificially inflate the rates of cognitive impairment among reservation-dwelling American Indians when employing the MDRS-Schmidt et al. 19 norms.
Interpretation of MDRS performance using the Vangel and Lichtenberg 20 norms identifies a much smaller number of older American Indians as cognitively impaired than do the Schmidt et al. 19 norms. As noted above, the MDRS appears to be more sensitive to impairments in the domains of cognition that would most likely be affected by the types of medical and neurological problems experienced in this population. However, the present study did not identify significant relationships between MDRS performance as interpreted using the Vangel and Lichtenberg 20 norms and any of these conditions, which may possibly be related to measurement issues with respect to the health variables. Additionally, an association between higher levels of education and better performance using the MDRS Vangel and Lichtenberg 20 norms was observed. It is likely that the lack of education adjustments in this set of norms accounts for this finding. Collectively, these observations suggest that additional investigation and/or modification of the MDRS and the norms used to interpret its scores are needed for use in the American Indian population.
The finding that gender was significant in almost all of the multivariate tests and norms (with the exception of the MDRS Schmidt model) might be explained by the fact that men in this sample were significantly more likely than women to self-report head injury, alcohol problems, or a dementia diagnosis. However, tests of interactions between gender and the three health conditions proved to be nonsignificant. These results underscore the probable complexity between gender and health conditions, with possible effects on cognitive functioning as assessed by the MMSE and MDRS. More comprehensive measures of health conditions might have yielded different results as well.
These findings should be interpreted with caution due to their preliminary nature, the use of a purposive rather than a random sample on one reservation, and the uncertain clinical significance of the cognitive assessment scores observed. To this latter point, this study employed a conservative statistical definition of cognitive impairment: performance was compared with normative data, and scores two standard deviation points below age- and education-adjusted performance expectations defined possible impairment. This definition does not address the functional significance of that impairment, nor does it attempt to place any impairments identified into a broader clinical/diagnostic context. In other words, cognitive impairment as defined in this manner is not synonymous with the presence of a dementia.
Additional studies are needed to refine these measures for use among older American Indians and to optimize interpretation of the data they yield. The widely disparate frequencies of cognitive impairment identified with available normative data suggest strongly that population-specific norms for these measures are needed. The present data indicate that these norms may require adjustment for not only age and education but also, possibly, gender. Investigation of the possible effects of ethnicity (perhaps as reflected by blood quantum), socioeconomic status, type/character of education, language status, and other variables on MMSE and MDRS performance is also needed in order to develop optimal normative data. Once valid and reliable normative data on these measures are established, the task of placing the “impairments” they identify into a sociocultural and clinical context can be undertaken. A comparison with American Indians’ performance on measures thought by some to be more culturally fair (e.g., Ravens Colored Progressive Matrices, Color Trails Tests, and the Culture Fair Test) might also be illuminating. Continuing work in these areas will help to determine optimal measurement tools for cognitive impairment and for the identification of dementia among American Indian elders.
1. Jervis LL, Manson SM: American Indians/Alaska Natives and dementia. Alzheimer Dis Assoc Disord 2002; Suppl2: S89–S95Google Scholar
2. Jervis LL, Cullum CM, Manson SM: American Indians, cognitive assessment, and dementia, in Ethnicity and the dementias, 2nd ed. Edited by Yeo G, Gallagher-Thompson D. New York, Taylor & Francis, 2006, pp 87–101Google Scholar
3. Rosenberg RN, Richter RW, Risser RC, et al: Genetic factors for the development of Alzheimer disease in the Cherokee Indian. Arch Neurol 1996; 54:997–1000Google Scholar
4. Henderson JN, Crook R, Crook J, et al: Apolipoprotein E4 and tau allele frequencies among Choctaw Indians. Neurosci Lett 2002; 324:77–79Google Scholar
5. Hendrie HC, Hall KS, Pillay N, et al: Alzheimer’s disease is rare in Cree. Int Psychogeriatr 1993; 5:5–14Google Scholar
6. Liao Y, Tucker P, Giles WH: Health status of American Indians compared with other racial/ethnic minority populations—selected states, 2001–2002. JAMA 2004; 291:935–937Google Scholar
7. Langlois JA, Kegler SR, Butler JA, et al. Traumatic Brain Injury-Related Hospital Discharges: Results From a 14-State Surveillance System, 1997. Department of Health and Human Services, Centers for Disease Control and Prevention, 2003Google Scholar
8. Denny CH, Holtzman D, Cobb N: Surveillance for health behaviors of American Indians and Alaska natives: findings from the behavioral risk factor surveillance system, 1997–2000. MMWR Surveillance Summaries 2003; 52:1–13Google Scholar
9. Ferarro FR, Bercier B: Boston Naming Test performance in a sample of Native American elderly adults. Clin Gerontol 1996; 17:58–60Google Scholar
10. Ferarro FR, Bercier BJ, Holm J, et al: Preliminary normative data from a brief neuropsychological test battery in a sample of Native American elderly, in Minority and Cross-Cultural Aspects of Neuropsychological Assessment. Edited by Ferarro FR. Lisse, Swets & Zeitlinger, 2002, pp 227–240Google Scholar
11. Whyte SR, Cullum CM, Hynan LS, et al: Performance of elderly Native Americans and caucasians on the CERAD neuropsychological battery. Alzheimer Dis Assoc Disord 2005; 19:74–78Google Scholar
12. Marcopulos BA, McLain CA, Giuliano AJ: Cognitive impairment or inadequate norms? a study of healthy, rural, older adults with limited education. Clin Neuropsychol 1997; 11:111–131Google Scholar
13. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
14. Mattis S: Mental status examination for organic mental syndrome in the elderly patient, in Geriatric Psychiatry. Edited by Bellack R, Karasu B. New York, Grune & Stratton, 1976, pp 77–121Google Scholar
15. Spreen O, Strauss E: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 2nd ed. New York, Oxford University Press, 1998Google Scholar
16. Uhlmann RF, Larson EB: Effects of education on the Mini-Mental State Examination as a screening test for dementia. JAGS 1991; 39:876–880Google Scholar
17. Freidl W, Schmidt R, Stronegger WJ, et al: Sociodemographic predictors and concurrent validity of the Mini-Mental State Examination and the Mattis Dementia Rating Scale. Eur Arch Psychiatry Clin Neurosci 1996; 246:317–319Google Scholar
18. Crum R, Anthony J, Bassett S, et al: Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269:2386–2391Google Scholar
19. Schmidt R, Friedl W, Fazekas F, et al: The Mattis Dementia Rating Scale: normative data from 1,001 health volunteers. Neurology 1994; 44:964–966Google Scholar
20. Vangel SJJ, Lichtenberg PA: Mattis Dementia Rating Scale: clinical utility and relationship with demographic variables. Clin Neuropsychol 1995; 9:209–213Google Scholar
21. SPSS [computer program]. Chicago, SPSS Inc, 2003Google Scholar
22. Robins LN, Wing J, Wittchen H, et al: The Composite International Diagnostic Interview: an epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry 1988; 45:1069–1077Google Scholar
23. U.S. Census Bureau: United States Census 2000. Available at http://www.census.gov/main/www/cen2000.htmlGoogle Scholar
24. Dubois B, Slachevsky A, Litvan I, et al: The FAB: a Frontal Assessment Battery at bedside. Neurology 2000; 55:1621–1626Google Scholar
25. Bobholz JH, Brandt J: Assessment of cognitive impairment: relationship of the dementia rating scale to the Mini-Mental State Examination. J Geriatr Psychiatry Neurol 1993; 6:210–213Google Scholar



