Amount of HIV DNA in Peripheral Blood Mononuclear Cells is Proportional to the Severity of HIV-1-Associated Neurocognitive Disorders
Our group and others have previously reported HIV DNA levels to be potentially important in HIV disease prognosis, including the diagnosis of HIV-1-associated dementia. 18 – 22 One rationale for pursuing HIV DNA further as a marker for HIV-1-associated dementia is that currently available highly active antiretroviral therapy regimens have little effect on HIV DNA, allowing for persistence of viral DNA in reservoirs. 23 – 25 We previously demonstrated high HIV DNA copies in peripheral blood mononuclear cells from individuals with and those without HIV-1-associated dementia from two different cohorts from Hawaii (n=49) and Thailand (n=30). 22 , 26 In the study with the Hawaii Aging with HIV Cohort, we further demonstrated that specific neuropsychological deficits were associated with HIV DNA levels. 27 However both the studies from Hawaii and Thailand were limited to individuals diagnosed with HIV-1-associated dementia and those with normal cognition, and did not include subjects with milder forms of impairment (i.e., minor cognitive motor disorder) which potentially includes a significant proportion of HIV-1-infected individuals with associated neurocognitive disorders. 28 – 30 The purpose of the current study was to assess HIV DNA in peripheral blood mononuclear cells from a separate subset of patients from the Hawaii Aging with HIV Cohort (n=189) not previously analyzed, and to determine if patients with minor cognitive motor disorder demonstrate a continuum increase in peripheral blood mononuclear cell HIV DNA compared to those with HIV-1-associated dementia and those with normal cognition. Additionally, we hypothesized that HIV DNA would be associated with individual neurocognitive domains, as we previously reported in a small subset from the Hawaii Aging with HIV Cohort. 27 If positive association were found, then peripheral blood mononuclear cell HIV DNA may prove to be an important factor in the pathogenesis of neurocognitive dysfunction.
METHODS
The Hawaii Aging with HIV Cohort
The Hawaii Aging with HIV Cohort is a longitudinal cohort established to examine HIV-1-associated neurocognitive disorders in older (≥50 years old) and younger (20–39 years old) HIV-seropositive individuals. The Hawaii Aging with HIV Cohort is unique with less than 5% of the participants identified as intravenous drug users, which is lower than other cohorts. 31 – 35 Following informed consent guidelines established by the University of Hawaii Institutional Review Board, participants living in Hawaii were enrolled; we excluded those with a major psychiatric or neurologic disorder, a history of head injury with loss of consciousness greater than 1 hour, current or past opportunistic infection with brain involvement, a diagnosed learning disability, or delirium due to medications at the time of examination. For the current study, subjects who were included in the previous study were also excluded from this analysis. 36 Subjects were evaluated at entry into the cohort and yearly for 4 years. Subsequent follow-up visits were scheduled in advance at the patient’s convenience and ability to complete the neurocognitive testing in one visit in the absence of any acute medical crisis.
Participant evaluations included demographic information, medical history, neurologic examination including the United Parkinson’s Disease Rating Scale to examine for extrapyramidal signs, medication/adherence history, DSM-IV-based substance abuse/dependence inventory, immunologic and virologic laboratory tests, and neuropsychiatric testing; as previously reported. 27 The 80-minute neuropsychiatric test battery, adapted from the NorthEast AIDS Dementia Cohort, assessed multiple cognitive domains affected by HIV-1 and included the following: choice and sequential reaction time from the California Computerized Assessment Package, Rey Auditory Verbal Learning Test (RAVLT), Rey Osterreith Complex Figure (RCF) Copy and Recall, Trail Making tests A and B, WAIS-R Digit Symbol, Grooved Pegboard (dominant and nondominant hands), Verbal fluency test, Animal Naming, Boston Naming Test, the WAIS-R Digit Span (forward and backward), and Timed Gait. Depression symptomatology was assessed using the Beck Depression Inventory (BDI). 37 Normative neuropsychiatric data for individuals with a high school or greater education were derived from the Multicenter AIDS Cohort Study consisting of 733 HIV-1-seronegative subjects with risk profiles similar to the Hawaii cohort. For individuals with less than a high school education, normative neuropsychological data from the AIDS Link to IV Experience study (n=150) was used. 38 These two normative sets have few individuals over 54 years old. Thus, for individuals over 54, alternative published normative data were used. 39 , 40 Normative data for the Rey Osterreith Complex Figure were taken from alternative published norms for individuals over 59 and the main Multicenter AIDS Cohort Study normative set for individuals under 60 years old. 41 A similar battery with a large overlap in norms was shown to be appropriate for HIV-1-infected individuals of similar ethnic diversity. 42 Various normative data were required due to the use of a comprehensive test battery and the inclusion of both younger and older subjects. The normative data were selected as the best possible dataset for this population with a long history of applications in HIV-1 research (e.g., Multicenter AIDS Cohort Study). Application of these norms was guided by the clinical neuropsychologists on the team. All test results were transformed to Z scores using appropriate age and education-matched normative data sets. Scores for cognitive domains (motor skills/motor speed; verbal memory; visual memory; working memory, attention, and concentration; learning; recognition memory; visuospatial abilities; executive functioning; language) were calculated by averaging the Z scores of the neuropsychiatric tests corresponding to the domains they were intended to measure, as previously published. 27 All patients who had research-based neurocognitive diagnoses using the American Academy of Neurology 1991 criteria (normal cognition, minor cognitive motor disorder, and HIV-1-associated dementia) without confounds (methamphetamine/cocaine use, stroke/transient ischemic attacks) who consented and donated blood for research studies were included in the analyses. 43
Specimens and Assessment of Peripheral Blood Mononuclear Cell HIV DNA Copies
Specimens were obtained and stored at the time of study entry. Plasma VL (Amplicor HIV-1 Monitor Ultra Sensitive Test, Roche Diagnostics, Switzerland) and CD4 cell counts were performed by a certified clinical laboratory. Genomic DNA was extracted from peripheral blood mononuclear cells as per manufacturer’s protocol (Qiagen, Valencia, Calif.). The quantity and quality of the DNA was assessed by UV spectrophotometry (DNA concentration and OD 260/280 nm absorbance ratio) and polymerase chain reaction (PCR) as previously described. 44 The assay for measuring HIV DNA, which was previously published, was used to calculate the number of copies of HIV-1 DNA per 10 6 cells. 22 , 26 , 27
Statistical Analyses
The statistical analyses were performed without division into the age groups of the parent study. All tests of hypotheses were used for participants classified as normal cognition, minor cognitive motor disorder, and HIV-1-associated dementia. The primary analysis was performed on specimens obtained at the earliest time of participation in which matching clinical laboratory data and neurocognitive status were available. The Mann-Whitney Rank Sum Test and one-way analysis of variance (ANOVA) were used to test for differences between groups. We examined the association between HIV DNA and each of the nine neuropsychiatric domains in a series of multilevel longitudinal regression models. The level one variable in the model included initial neuropsychiatric deficits and annual rates of change over 5 years. All analyses were conducted using SAS® v9.1 PROC MIXED specifying maximum likelihood as the estimation method and an unstructured covariance matrix. We examined the association between HIV DNA and baseline neuropsychiatric deficit and annual rate of change of each deficit, as indicated by the TYPE III fixed effect for HIV DNA after it was added to the unconditional growth model. To determine whether the obtained estimates were attributable to other patient characteristics we added age, ethnicity, CD4 cell count, and premorbid IQ.
RESULTS
The demographics of the 189 subjects from the Hawaii Aging with HIV Cohort are similar to what is reported in the state of Hawaii. The cohort, as initially designed, enrolled older (≥50 years old) and younger (20–39 years old) subjects; thus the mean age was 44 years old, SD=11 years (range=21–73 years). The mean years of education was 13.93 years (SD=2.25 years) with 51% Caucasian and 37% Asian-Pacific Islander; 15% were women and 85% were men. 32 The HIV RNA levels (VL) and CD4 cell counts among the three groups (normal cognition, minor cognitive motor disorder, and HIV-1-associated dementia) were not statistically significant (p=0.78 and p=0.36, respectively) ( Table 1 ).
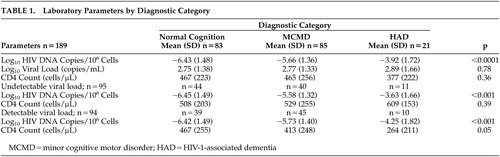 |
The analysis comparing HIV DNA in individuals with normal cognition (NC), minor cognitive motor disorder (MCMD) and HIV-1-associated dementia (HAD) showed a significant effect among all three groups (p<0.001; NC=83, MCMD=85, HAD=21; Table 1 ). To examine whether the HIV DNA association was due to HIV RNA levels, we repeated the analysis among participants with undetectable plasma HIV RNA viral load (N=95) and found that the significance was upheld in those with HIV-1-associated dementia (n=11) relative to those with minor cognitive motor disorder (n=40) and normal cognition (n=44) (p<0.001, Table 1 ); with similar CD4 cell counts (p=0.39). Similarly, in subjects with detectable plasma HIV RNA viral load (n=94), HIV DNA was also higher in those with HIV-1-associated dementia (n=10) relative to those with minor cognitive motor disorder (n=45) and normal cognition (n=39) (p<0.001, Table 1 ). The results from this relatively large separate cohort from Hawaii Aging with HIV Cohort showed a positive correlation; therefore the data were reanalyzed to include the smaller subset from Hawaii Aging with HIV Cohort previously reported. The combined data, using a univariate logistic regression model with a generalized logits link function, demonstrated a strong probability of cognitive diagnosis (normal cognition, minor cognitive motor disorder, or HIV-1-associated dementia) with HIV DNA ( Figure 1 ).
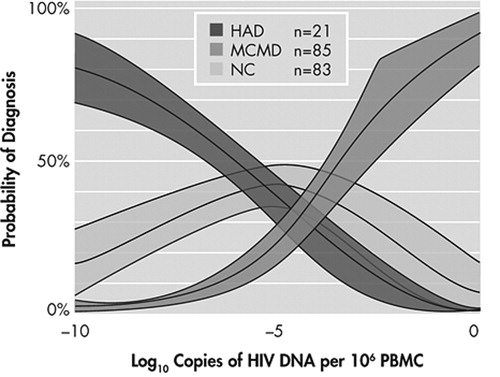
Shaded areas represent the 95% confidence limit around the predicted probability. Estimates are from a univariate logistic regression model with a generalized logits link function. HAD=HIV-1-associated dementia; MCMD=minor cognitive motor disorder; NC=normal cognition
The decrement in baseline of each neuropsychiatric deficit which is associated with HIV DNA is plotted in Figure 2 , adjusted for age, ethnicity, CD4 cell count, and estimated premorbid IQ, where ethnicity and IQ are not confounded with cognitive diagnosis. For each unit increase in log of HIV DNA, there is a decrease in neuropsychiatric deficit. Neuropsychological deficits were significantly associated with entry HIV DNA in all deficits ( Table 2 ) (regression coefficients range from −0.24 to −0.07, p<0.05). The effect of HIV DNA on neuropsychiatric deficits remained significant after adjusting for age, ethnicity, and estimated premorbid IQ.

Error bars=standard error for regression coefficient in a multilevel longitudinal model. Effects and standard errors are adjusted for age, ethnicity, CD4 cell count, and estimated premorbid IQ.
 |
DISCUSSION
This is the first time that the amount of HIV DNA in peripheral blood mononuclear cells is shown to be proportional to the level of HIV-1-associated neurocognitive disorder status across all three levels of neurocognitive function. Of significance is that individual deficits in neurocognitive domains are also associated with HIV DNA levels. The current study used previously unanalyzed specimens from subjects in Hawaii Aging with HIV Cohort and showed that there is a stepwise increase in peripheral HIV DNA levels from subjects with normal cognition to minor cognitive motor disorder to HIV-1-associated dementia. One possible explanation for the findings could be that those with worse deficits in cognitive domains or diagnosis were due to progression of their HIV-disease as suggested by high HIV RNA levels. Therefore, we performed the analyses separately among those with undetectable and detectable plasma HIV RNA viral load. In both situations, the magnitude differences in HIV DNA in subjects with HIV-1-associated dementia remained significantly higher compared to those with normal cognition and minor cognitive motor disorder regardless of the status of plasma HIV RNA levels. Among the 189 subjects in the cohort, the majority were on antiretroviral therapy with no differences in CNS-penetrating class of drugs.
While the neuropathogenic mechanisms leading to HIV-1-associated neurocognitive disorders remain unclear, we believe that our finding of HIV DNA levels falling within a continuum among individuals with varying degrees of neurocognition contributes to the importance of HIV DNA in the neuropathogenesis. The current study complements our earlier reports suggesting that HIV DNA may be important in the pathogenesis of HIV-1-associated dementia by showing in this unique group of patients that HIV DNA is associated with neurocognitive status and neurocognitive domains. 22 , 26 Previous studies did not include individuals with minor cognitive motor disorder and the significance of the results lies in reevaluating the paradigm of how HIV impacts the CNS.
A limitation in interpreting our findings is that the cellular subset(s) responsible for the assayed HIV DNA has not been clearly identified. We hypothesize that monocytes/macrophages may be an important cellular subset housing the HIV DNA based on preliminary findings, however further studies are still necessary to unconditionally determine specifically which cell or cells are involved. 22 , 26
1 . Sacktor N, McDermott MP, Marder K, et al: HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002; 8:136–142Google Scholar
2 . Anderson E, Zink W, Xiong H, et al: HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr 2002; 31(suppl 2):S43–54Google Scholar
3 . Gartner S: HIV infection and dementia. Science 2000; 287:602–604Google Scholar
4 . Major EO, Rausch D, Marra C, et al: HIV-associated dementia. Science 2000; 288:440–442Google Scholar
5 . McArthur JC, Sacktor N, Selnes O: Human immunodeficiency virus-associated dementia. Semin Neurol 1999; 19:129–150Google Scholar
6 . Neuenburg JK, Brodt HR, Herndier BG, et al: HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 31:171–177Google Scholar
7 . Pulliam L, Gascon R, Stubblebine M, et al: Unique monocyte subset in patients with aids dementia. Lancet 1997; 349:692–695Google Scholar
8 . Fischer-Smith T, Croul S, Sverstiuk AE, et al: CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 2001; 7:528–541Google Scholar
9 . Robertson K, Fiscus S, Kapoor C, et al: CSF, plasma viral load and HIV associated dementia. J Neurovirol 1998; 4:90–94Google Scholar
10 . De Luca A, Ciancio BC, Larussa D, et al: Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology 2002; 59:342–347Google Scholar
11 . Ellis RJ, Hsia K, Spector SA, et al: Cerebrospinal fluid human immunodeficiency virus type 1. RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Res Center Group Ann Neurol 1997; 42:679–688Google Scholar
12 . Reger MA, Martin DJ, Cole SL, et al: The relationship between plasma viral load and neuropsychological functioning in HIV-1 infection. Arch Clin Neuropsychol 2005; 20:137–143Google Scholar
13 . Cysique LA, Maruff P, Brew BJ: Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol 2004; 10:350–357Google Scholar
14 . Langford TD, Letendre SL, Larrea GJ, et al: Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol 2003; 13:195–210Google Scholar
15 . Albright AV, Soldan SS, Gonzalez-Scarano F: Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol 2003; 9:222–227Google Scholar
16 . McArthur JC, Haughey N, Gartner S, et al: Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol 2003; 9:205–221Google Scholar
17 . Lawrence DM, Major EO: HIV-1 and the brain: connections between HIV-1-associated dementia, neuropathology, and neuroimmunology. Microbes Infect 2002; 4:301–308Google Scholar
18 . Donovan RM, Dickover RE, Goldstein E, et al: HIV-1 proviral copy number in blood mononuclear cells from aids patients on zidovudine therapy. J Acquir Immune Defic Syndr 1991; 4:766–769Google Scholar
19 . Russell RR, Bowmer MI, Nguyen C, et al: HIV-1. DNA burden in peripheral blood CD4+ cells influences disease progression, antiretroviral efficacy, and CD4+ T-cell restoration. Viral Immunol 2001; 14:379–389Google Scholar
20 . Zhao Y, Yu M, Miller JW, et al: Quantification of human immunodeficiency virus type 1 proviral DNA by using taqman technology. J Clin Microbiol 2002; 40:675–678Google Scholar
21 . Wood R, Dong H, Katzenstein DA, et al: Quantification and comparison of HIV-1 proviral load in peripheral blood mononuclear cells and isolated CD4+ T cells. J Acquir Immune Defic Syndr 1993; 6:237–240Google Scholar
22 . Shiramizu B, Gartner S, Williams A, et al: Circulating proviral HIV DNA and HIV-associated dementia. Aids 2005; 19:45–52Google Scholar
23 . Bruisten SM, Reiss P, Loeliger AE, et al: Cellular proviral HIV type 1. DNA load persists after long-term RT-inhibitor therapy in HIV type 1 infected persons. AIDS Res Hum Retroviruses 1998; 14:1053–1058Google Scholar
24 . Delobel P, Sandres-Saune K, Cazabat M, et al: Persistence of distinct HIV-1 populations in blood monocytes and naive and memory CD4. T cells during prolonged suppressive HAART. Aids 2005; 19:1739–1750Google Scholar
25 . Dickover RE, Donovan RM, Goldstein E, et al: Decreases in unintegrated HIV DNA are associated with antiretroviral therapy in aids patients. J Acquir Immune Defic Syndr 1992; 5:31–36Google Scholar
26 . Shiramizu B, Ratto-Kim S, Sithinamsuwan P, et al: HIV DNA and dementia in treatment-naive HIV-1-infected individuals in Bangkok, Thailand. Int J Med Sci 2006; 4:13–18Google Scholar
27 . Shiramizu B, Paul R, Williams A, et al: HIV proviral DNA associated with decreased neuropsychological function. J Neuropsychiatry Clin Neurosci 2007; 19:157–163Google Scholar
28 . Stern Y, McDermott MP, Albert S, et al: Factors associated with incident human immunodeficiency virus-dementia. Arch Neurol 2001; 58:473–479Google Scholar
29 . Larussa D, Lorenzini P, Cingolani A, et al: Highly active antiretroviral therapy reduces the age-associated risk of dementia in a cohort of older HIV-1-infected patients. AIDS Res Hum Retroviruses 2006; 22:386–392Google Scholar
30 . Valcour VG, Sacktor NC, Paul RH, et al: Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging with HIV cohort. J Acquir Immune Defic Syndr 2006; 43:405–410Google Scholar
31 . Valcour VG, Shikuma CM, Watters MR, et al: Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS 2004; 18:S79–86Google Scholar
32 . Valcour V, Shikuma C, Shiramizu B, et al: Higher frequency of dementia in older HIV+ individuals. The Hawaii Aging with HIV cohort. Neurol 2004; 63:822–827Google Scholar
33 . Grassi MP, Perin C, Clerici F, et al: Neuropsychological performance in HIV-1-infected drug abusers. Acta Neurol Scand 1993; 88:119–122Google Scholar
34 . Portegies P, Enting RH, de Gans J, et al: Presentation and course of AIDS dementia complex: 10 years of follow-up in Amsterdam. Aids 1993; 7:669–675Google Scholar
35 . Dougherty RH, Skolasky RL Jr, McArthur JC: Progression of HIV-associated dementia treated with HAART. AIDS Read 2002; 12:69–74Google Scholar
36 . Shiramizu B, Gartner S, Williams A, et al: Correlation of Circulating Proviral HIV DNA and with HIV-Associated Dementia. AIDS (in press)Google Scholar
37 . Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology 1996; 47:1247–1253Google Scholar
38 . Concha M, Selnes OA, McArthur JC, et al: Normative data for a brief neuropsychologic test battery in a cohort of injecting drug users. Int J Addict 1995; 30:823–841Google Scholar
39 . Heaton RK, Avitable N, Grant I, et al: Further crossvalidation of regression-based neuropsychological norms with an update for the Boston naming test. J Clin Exp Neuropsychol 1999; 21:572–582Google Scholar
40 . Tombaugh TN, Kozak J, Rees L: Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999; 14:167–177Google Scholar
41 . Chiulli S, Haaland K, LaRue A, et al: Impact of age on drawing the Rey-Osterrieth figure. The Clin Neuropsychologist 1995; 9:219–224Google Scholar
42 . Robbins R, Rivera Mindt M, Heaton RK, et al: Are there ethnicity differences in the ability of neuropsychological test performance to predict functional impairment in HIV infection? in Thirty-Second Annual International Neuropsychological Society Conference. Baltimore, 2004Google Scholar
43 . Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 1991; 41:778–785Google Scholar
44 . Shiramizu B, Gartner S, Cho M, et al: Assessment of HIV-1. DNA copies per cell by real-time polymerase chain reaction. Frontiers in Bioscience 2004; 9:255–261Google Scholar



