Psychiatric Presentation of Sporadic Creutzfeldt-Jakob Disease: A Challenge to Current Diagnostic Criteria
Abstract
Pathological diagnosis remains the gold standard for the diagnosis of sporadic Creutzfeldt-Jakob disease (sCJD), but being able to differentiate between CJD and non-prion diseases clinically is important because many of the non-prion, rapidly progressive dementias are treatable. Diagnostic criteria need both high sensitivity and specificity while remaining applicable to clinical practice. Despite extensive updates to the clinical criteria for sCJD, there remains a heavy emphasis on neurological signs. We describe a psychiatric presentation of sCJD that did not fulfill the diagnostic criteria until very late in a prolonged disease course and required biopsy for diagnosis.
Creutzfeldt-Jakob disease is one of the transmissible spongiform encephalopathies that are characterized clinically by a fatal neurological course and histologically by spongiform change, astrocytosis, and deposition of prion protein.
The sporadic variant accounts for about 85% of CJD cases and has a reported incidence of 1 per million. Clinical diagnostic criteria for sCJD were initially devised in 1979, using a combination of clinical features and supporting electroencephalography (EEG).1 These original criteria were adapted2 and subsequently updated3 to include magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) surrogate biomarkers.
Phenotypic variability of sCJD has been associated with various molecular subtypes,3,4 with the ancillary investigations having varying utility. Early psychiatric features are well recognized in variant Creutzfeldt-Jakob disease (vCJD), but there have now been studies demonstrating that psychiatric features also occur in sCJD, and usually before formal diagnosis.5 The following case report highlights a prolonged psychiatric presentation of sCJD, with few neurological signs, which would not have met the existing diagnostic criteria without neuropathological confirmation.
Case Report
A previously-healthy, 71-year-old woman initially complained of memory difficulties in December 2008 with the family becoming aware of them in March 2009. She had begun to recount the same conversations, had trouble recalling her bank PIN numbers, and forgot the often-used house alarm code. Other symptoms included poor sleep, early morning wakening, poor appetite, weight loss, tearfulness, and anhedonia over a 10-month period, in marked contrast to her previous involvement in many social activities. There was a suggestion of an early dysexecutive syndrome as she began to struggle to choose items from the supermarket shelves. However, in June 2009, her primary-care physician made a diagnosis of depression (subsequently confirmed at a psychiatric evaluation) and initiated citalopram. Despite this, her condition continued to deteriorate. Unable to look after herself at home despite support, she was admitted to a psychiatric hospital in November 2009. Although she had nonfluent aphasia, demonstrated mild utilization behavior, and had a Mini-Mental State Exam (MMSE) score of 25/30, there was no evidence of myoclonus, gait ataxia, extrapyramidal signs, or visual disturbances at this point. Her antidepressant was changed to mirtazapine, which was well tolerated and slightly reduced the anxiety, but her cognitive performance continued to deteriorate. Brain MRI was normal, and EEG revealed a normal posterior alpha rhythm, with only occasional nonspecific slow-wave abnormalities over both fronto-temporal regions. On the Test Your Memory (TYM) test, she scored 16/50 with assistance from her children (dementia cut-off in our clinic is ≤30/50).6
Extensive neurological investigations including thyroid peroxidase antibodies, VGKC, VGCC, and NMDA receptor antibodies, tumor markers, anti-neuronal antibodies, and CSF constituents and cytology were all normal or negative.
The MRI, repeated in January 2010, was now abnormal, showing multiple bilateral areas of high signal abnormality on DWI and FLAIR sequences, which progressed over the next month to bilateral cortical hyperintensities, most prominent over the left frontal and parietal regions, and also involving the right frontal, parietal, and bitemporal regions. The repeat CSF exam was positive for 14-3-3 protein, and S100b was raised (1.05; NR <0.41 ng/ml).
Although the CSF and MRI findings were consistent with a diagnosis of sCJD, a brain biopsy was performed because of the prolonged disease course and psychiatric presentation; this was consistent with sCJD (Figure 1).
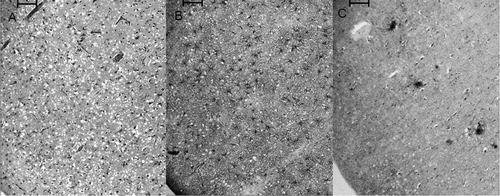
The figure shows [A]: spongiform degeneration with marked neuronal loss; [B]: diffuse GFAP-positive astrocytosis; and [C]: KG9-positive synaptic or granular prion protein deposits within the same area of the left frontal lobe biopsy (bar=100 microns).
Over the next 3 months, she became mute and required assistance with all personal care. However, no myoclonus, cerebellar signs, or primitive reflexes were observed (by April 2010) despite a disease course of over a year, although, by June 2010, very infrequent myoclonic jerks were reported by nursing home staff, other signs were still notably absent.
Discussion
Sporadic CJD is characterized by prominent neurological symptoms, including a rapidly-progressing dementia, myoclonus, ataxia, and a median disease duration of 4.5 months, whereas the variant form often presents with psychiatric features, nonspecific sensory complaints, and a longer median disease duration of 14 months.1 However, psychiatric features may also occur in sCJD. A retrospective review of 126 patients at the Mayo Clinic with definite (neuropathologically confirmed) or probable CJD (clinical dementia, diagnostic EEG changes, with at least one other clinical finding compatible with CJD [e.g., myoclonus, ataxia, extrapyramidal symptoms, or focal cortical syndromes]) found that 92% had at least one psychiatric manifestation during the disease course, of whom 101 (80%) had the first psychiatric symptom (depression, anxiety, psychosis, behavior dyscontrol) within 100 days of CJD onset, suggesting that psychiatric symptoms often occur before clinical diagnosis.5 Of the 83 cases who received inpatient care, 6 had been treated at some point in a psychiatric hospital. A retrospective audit at a regional neuroscience center revealed that 80% of patients presented initially to non-neurologists, and, indeed, 3 patients never saw a neurologist.7 Another study, identifying 114 sCJD patients in whom the very first symptom was well documented, showed that behavioral symptoms occurred in 20%,8 similar to the 26% with psychiatric symptoms at presentation recorded in the Mayo Clinic study.5
The original diagnostic criteria for sCJD used a combination of clinical and EEG features,1 but psychiatric symptoms were not specifically mentioned. These diagnostic criteria were amended as appreciation of the phenotypic heterogeneity of sCJD grew and progress was made in the development of other diagnostic investigations,3 particularly CSF biomarkers9 and brain MRI.4 However, psychiatric symptoms were again absent from the revised criteria.3 This omission may affect the sensitivity of the criteria, since psychiatric features may be the presenting feature in around 20% of sCJD patients.5,8 Certainly, in the absence of typical neurological signs other than progressive dementia, our patient did not fulfill criteria until the CSF and second MRI findings, over 1 year into the course of illness.
Sporadic CJD may not enter differential diagnosis when neurological features are absent or less prominent than psychiatric ones and, indeed, only about half of all suspected cases in the U.K. fulfill the criteria for definite or probable CJD. The initial diagnosis in the 26 MV2 subtype of sporadic CJD included depression, somatization, psycho-organic syndrome, and “hysterical neurosis.”10 Including psychiatric features in diagnostic criteria might raise sensitivity, albeit at the risk of lowering specificity, but this may be justified in order to facilitate early diagnosis and intervention, particularly if therapies become available.
In this case report, we highlight the importance of not misdiagnosing sCJD as depression solely on the basis of absence of the neurological features. A thorough review of the history is vital, as is the context—these psychiatric features developed in a patient who had previously been socially active in the community and with no obvious reason to be depressed or tearful, who then developed clear cognitive and language difficulties. Lack of recognition of the psychiatric features in the wider psychiatric and neurological community will mean that these patients will continue to be inappropriately admitted to psychiatric units, resulting in possible distress to both patients and their families; it continues to be reported in the medical literature that disease duration beyond 12 months makes a non-CJD neurodegenerative disease more likely.11 Diagnosis of sCJD allows appropriate psychosocial care to be implemented; this should include the use of antidepressants or anxiolytics (as in this case) if psychiatric symptoms are present. In the U.K., the National CJD Surveillance Unit in Edinburgh provides advice and support to patients, their families, and healthcare professionals.12 Although disease-modifying therapies for sCJD do not yet exist, clinical trials have tested a number of compounds, including quinacrine and pentosan polysulfate, and there is ongoing work to manufacture anti-prion antibodies (such as ICSM18 and ICSM35) for use in clinical trials—enrolling patients relatively “early” in the course of the disease would seem logical if one is to see any treatment benefit.
This case therefore emphasizes the need for systematic follow-up of patients who do not readily fulfill existing diagnostic criteria.
1 : Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann Neurol 1979; 5:177–188Crossref, Medline, Google Scholar
2 : Diagnosis of Creutzfeldt-Jakob disease: effect of clinical criteria on incidence estimates. Neurology 2000; 54:1095–1099Crossref, Medline, Google Scholar
3 : Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132:2659–2668Crossref, Medline, Google Scholar
4 : MRI lesion profiles in sporadic Creutzfeldt-Jakob disease. Neurology 2009; 72:1994–2001Crossref, Medline, Google Scholar
5 : Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci 2005; 17:489–495Link, Google Scholar
6 : Test Your Memory test: diagnostic utility in a memory clinic population. Int J Geriatr Psychiatry 2011; 26:976–980Crossref, Medline, Google Scholar
7 : Prion disease at a regional neuroscience centre: retrospective audit. J Neurol Neurosurg Psychiatry 2004; 75:1789–1790Crossref, Medline, Google Scholar
8 : First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 2006; 66:286–287Crossref, Medline, Google Scholar
9 : How to improve the clinical diagnosis of Creutzfeldt-Jakob disease. Brain 1999; 122:2345–2351Crossref, Medline, Google Scholar
10 : Clinical findings and diagnostic tests in the MV2 subtype of sporadic CJD. Brain 2006; 129:2288–2296Crossref, Medline, Google Scholar
11 : Rapidly progressive neurodegenerative dementias. Arch Neurol 2009; 66:201–207Crossref, Medline, Google Scholar
12 CJD Support Network Information sheet for sporadic CJD, Jan. 2008Google Scholar



