Impairments of Attention and Effort Among Patients With Major Affective Disorders
Abstract
Impairments of attention are common among people with major affective disorders, yet the influence of effortful task demands on attentional performance in unipolar and bipolar illness has been little studied. The authors compared psychiatric inpatients with primary diagnoses of unipolar or bipolar affective disorder (n=27) and age-matched normal control subjects (n=20) on a battery of eight neuropsychological tasks designed to measure different attentional functions. There were low-effort and high-effort versions of each task. Significant group differences were consistently observed on tasks demanding sustained and focused attention, but not on tasks requiring visual selective attention. Although affective disorder patients showed impairments on most tasks regardless of level of task effort, group differences were greatest on high-effort conditions. Results indicate that patients with major affective disorders show significant attentional impairments on most measures of effortful attention, and the magnitude of these impairments increases as the effortful demands of the task increase.
Complaints of diminished cognitive capacity, particularly involving diminished ability to concentrate, are common among patients with major affective disorders.1 These symptoms were once thought to reflect primarily the subjective experience of psychiatric patients.2 It is now widely recognized that many patients with affective disorders show objective impairments of cognition and behavioral functioning secondary to neurophysiological disturbance.3–16 The neurocognitive manifestations of major affective illness have been the subject of considerable inquiry. Despite abundant evidence that certain functions tend to be impaired among patients with both unipolar and bipolar affective disorders, the neuropsychological bases of these impairments remains unresolved.
Early investigations suggested a link between nondominant hemispheric brain dysfunction and affective disorders, since patients with both major depression and bipolar illness were found to have problems with tactile perception, nondominant motor speed and dexterity, and performance on other “lateralizing tasks.”17–21 However, the spectrum of functional difficulties in major affective disorders cannot be fully explained by lateralized cerebral dysfunction; neurocognitive deficits are common that are not attributable solely to nondominant hemispheric brain dysfunction.4,5,10,22–31 Learning and memory deficits often coexist with problems in the areas of planning, initiation, generation, and organization,32 suggesting that impairments of attention and executive functioning may be key to many of the neuropsychological impairments caused by major affective illness.33–43
In fact, diminished attentional performance is one of the few consistent findings across neuropsychological studies of major affective illness.44 Although data regarding lateralized brain disturbances and other functional impairments have been equivocal, evidence of attentional dysfunction emerges from almost all studies that included measures to assess attention processes. Furthermore, existing evidence regarding the neurophysiological underpinnings of affective disorders provides compelling theoretical grounds for postulating that diminished “attentional capacity”44,45 is central to this symptomatology.40,42,44 Patients with major affective disorders seem to have particular difficulty when tasks require effortful processing with demand for focused and sustained attention.42–44,46–48 Yet few investigators have systematically examined the influence of attentional effort on the performance of patients with major affective disorders.
In the present investigation, the effect of increasing effortful attentional demands on the performance of patients with unipolar and bipolar affective illness relative to age-matched normal control subjects was examined. High-effort versions of a set of attentional tasks were developed. Effort level was increased on the task variable that was considered to be the defining characteristic of each task, enabling determination of which attentional processes were most affected when task demands for effortful processing were increased. We hypothesized there would be a significant between-group main effect with respect to overall attentional performance, in which patients with major affective illness would have greater impairment than control subjects. These impairments were expected to be greatest on tasks with high demand for focused and sustained attention, intention, and executive response control; group differences were not expected on tasks requiring primarily sensory selective attention. We also predicted a significant main effect for effort level, expecting that both patients and control subjects would perform more poorly on the high-effort than the low-effort conditions across measures of attention. Finally, we predicted an interaction effect, with affective disorder patients having greater difficulty on the high-effort conditions of these tasks. This interaction would indicate that among individuals with affective disorders, impairments of attention were magnified when effortful task demands were increased.
METHODS
Participants
Twenty-seven patients (9 male, 18 female) who met DSM-III-R49 criteria for major affective disorder participated in the study. Patients were recruited from the inpatient psychiatric unit at the University of Massachusetts Medical Center in Worcester, MA. Fourteen patients were diagnosed with major depressive disorder (mean age [±SD]=8.6±8.1 years) and 13 were diagnosed with bipolar disorder (mean age=36.1±11.0 years). All of the unipolar patients were classified as severely depressed according to DSM-III-R criteria, and 3 had psychotic features. Of the bipolar patients, all had symptoms of major depression. At the time of their hospitalization and neurocognitive assessment, 8 of the bipolar patients were in a depressive episode; the remainder had mixed features, depression coexisting with either mania or hypomania. Bipolar patients who were experiencing an acute manic or hypomanic episode without depressive features were excluded from this analysis. All patients were receiving psychopharmacological treatment at the time of their assessment. Patients with current or past electroconvulsive therapy were excluded. Additional exclusion criteria included other major psychiatric diagnoses (e.g., schizophrenia), alcohol or drug abuse according to DSM-III-R criteria, and neurological disorder.
The control group consisted of 20 healthy participants (9 male, 11 female) recruited through local newspaper ads and posted advertisements at the hospital, local universities, and agencies. Volunteers who scored in the nondepressed range on the Inventory to Diagnose Depression (IDD)50 and did not have a past history of major affective disorder were asked to participate. Additional exclusion criteria were identical to those described for the patient group. The groups were matched in terms of years of education, socioeconomic status, marital status, and age.
Clinical Measures
All participants completed a demographic information form and the Interview to Diagnose Depression.50 The IDD is a 22-item self-report measure that quantifies depressive symptomatology and provides cutoff guidelines for diagnosing major affective disorder according to DSM-III-R criteria and determining severity of depression. The Shipley Vocabulary Test51 was administered to each participant to obtain an estimate of level of intellectual functioning.
Measurement of Attention
A battery of eight tests of attention, each containing two conditions that varied with regard to demand for effortful processing, was administered (Table 1). These tests were selected because they have been shown to be differentially sensitive to four primary attention factors: 1) sensory selective attention, 2) response selection and control, 3) attentional capacity and focus, and 4) sustained attention.44,52
Sensory selective attention involves the processes of filtering, selection, and focusing of sensory stimuli and features. Although most cognitive tasks require selective attention to sensory information, this attentional process is not central to all attention tasks. For example, the continuous performance test (CPT), Stroop Color-Word Interference test (Stroop), Symbol Coding, and Trail Making involve attention to, and selective processing of, sensory information. Yet sensory selective attention is not an essential process for these tasks. Typically, tests that measure sensory selective attention require the subject to detect a target stimulus from a larger array of distracters.
Response selection and control involves processes through which an “intent” to act is generated and control over response execution is established through processes of inhibition, facilitation, and switching. This attention function involves executive control processes.
Attention capacity and focus involves those processes that influence the quantity and complexity of information that can be attended to by an individual at any given point in time. This capacity ultimately governs the intensity of attentional focus that can be allocated to the task at hand. Attention capacity and focus is determined by energetic factors (e.g., arousal) and structural factors (e.g., central processing speed). It can be evaluated through tasks that divide attention between concurrent tasks, require rapid information processing, or involve working memory or other cognitive operations together with attentional focus.
Sustained attention and vigilance involves the allocation of attentional resources over time. Effort is required because the subject is required to maintain attention to stimuli and/or to responding over a prolonged period. The degree of change in attentional performance over time defines an individual's capacity for sustained attention.
Eight tests of attention were created that varied with respect to their demand for specific attentional processes. Each of the tests had two conditions (low effort, high effort). For each test, the “high effort” condition was created by modifying the task parameters previously found to most strongly associated with the attentional factor assessed by the test.44 This was accomplished by increasing the complexity, load, or processing requirements of the test—generally by accelerating stimulus presentation speed, increasing the number of stimuli, increasing the ratio of noise to signal, placing greater demand on working memory, or requiring divided attention and/or concurrent response production. For example, the test of sustained attention was made more effortful by increasing the number of trials, ratio of distractors to targets, and rate of stimulus presentation rate on an adaptive rate continuous performance test.44 The eight attention tests are grouped below according to the primary attention factor that each assesses.
Sensory Selective Attention:
The Letter Search task,53 a computerized visual search task, was administered by using an IBM-compatible personal computer, with letter stimuli presented on a 15-inch monitor. The high- and low-effort conditions varied with respect to the number of letter stimuli in the array to be detected and the ratio of targets to total stimuli. On each trial, a random string of 12 letters appeared on the screen with either two (low effort) or six (high effort) target letters positioned above the string. Participants were required to search the letter array and to determine if all of the target letters were contained within the string. They were instructed to press one of two response keys to indicate either ”yes“ or ”no“ as to the presence of the complete set of target letters. The dependent measures for both conditions were total errors and mean response times across trials.
Response Selection and Control:
The Trail Making Test54 was used to assess this attention factor (i.e., intention). A standard administration was used. The low-effort condition (Trails A) requires simple tracking of a 25-number sequence using a pencil on paper. The high-effort condition (Trails B) requires sequential alternation between 25 numbers and letters. On both tasks, the subject's speed of processing is determined as time for completion of the sequence. Total number of errors was also recorded as a dependent measure.
Attentional Focus and Capacity:
Several of the tests were most sensitive to attention focus and capacity: Concurrent Response Production (COWAT55+Tapping56), Symbol Coding,57 the Stroop task53,58 (computerized) and the Levels of Processing (LOP) test of working memory.44,59 These measures also placed significant demand on other attentional processes, including both sensory and response selection and control, although in the context of this experiment, demands for attentional focus and capacity were manipulated. For example, the two tests comprising the Concurrent Response Production task require executive response production and control. However, the requirement of concurrent response production taps most strongly into attentional capacity. Similarly, Symbol Coding and the Stroop Task require both sensory and response selection and control and are often considered as measures of executive functioning. Increased task demand was created on these tasks by employing modifications that either increased demand for working memory (Symbol Coding) or freedom from interference (Stroop). The parameters for each task are described below.
Concurrent Response Production44—This task consisted of two tests (Finger Tapping and COWAT), administered separately for the low-effort conditions and as a combined task of Tapping+COWAT for the high-effort condition. The Controlled Oral Word Association test (COWAT)55 required participants to quickly name words beginning with the letters C, F, H, and L for 60 seconds. The dependent measure was total number of words generated. For the other task, the Finger Tapping test56 was modified44 (Sustained Finger Tapping) to provide a measure of motor persistence: the basic response was identical to the standard Finger Tapping test (i.e., tapping a key mounted on a board with an attached counting device with the index finger), except that they tapped for 60 seconds instead of 10 seconds on each trial. Participants were instructed to tap the key as quickly as possible with their dominant hand until they were told to stop. On the low-effort conditions, word production and sustained finger tapping were assessed during separate tasks with no concurrent response demand. On the high-effort condition, participants performed sustained finger tapping for 1 minute simultaneous with verbal response production (COWAT), so that the high-effort task required concurrent response demands.
Symbol Coding57—The low-effort condition of the test consisted of a sheet of paper on top of which was printed a series of nine symbols paired with the digits 1–9. Four rows of 110 divided boxes were printed underneath the symbol-digit key. The nine symbols were randomly distributed and repeated in the upper boxes; the lower boxes were blank. Participants were required to write the numbers associated with each symbol in the lower boxes. Ninety seconds were allotted to fill in as many numbers as possible. On the high-effort condition, subjects were required to substitute symbols for symbols, using a key of symbol–symbol pairings held constantly in view. Like the low-effort condition, the high-effort condition required subjects to substitute the symbols as quickly as possible for 90 seconds. The dependent measure for each task was the total number of correct substitutions.
Stroop Task53,58—A computerized modification of the Stroop interference procedure was administered to all participants. Color words (i.e., ”red,“ ”blue,“ ”green“) printed in an incongruent color (e.g., the word ”red“ printed in blue) were presented one at a time on a computer monitor. In the low-effort condition, participants were required to name the color that the word was printed in while ignoring the actual word. On some trials, noncolor words were presented (e.g., ”dog“ printed in red) and participants likewise were required to name the color the word was printed in. The high-effort condition was identical to the low-effort condition, with the addition of auditory distraction stimuli being presented to both ears. While they performed the Stroop as described above, participants heard color names that were inconsistent with the correct response through a set of headphones. The dependent measure for each condition was the total number of errors.
Working Memory-Levels of Processing (LOP) Task44,59—Thirty-six word stimuli with a mean frequency of 1/ 1,000 words in the English language60 were randomly divided into three blocks of 12 items. Individual word stimuli were presented on a computer monitor and participants were required to respond by providing verbal associates based on three separate task demands: 1) Phonemic Association (low effort), 2) Semantic Association (medium effort), and 3) Multiple Semantic Associations (high effort). For the Phonemic Association task, participants were required to say a single word aloud that rhymed with the stimulus (e.g., rock-clock). In the high-effort condition, they were required to produce a single semantic associate (e.g., table-chair), while in the higher-effort condition participants produced five semantic associates to the stimulus word (e.g., tree: trunk, bark, leaf, plant, flower). Following each wordlist presentation, participants were asked to freely recall the word stimuli. The dependent measure was the number of words correctly recalled.
Sustained Attention:
The primary test used to assess sustained attention was the Continuous Performance Test (CPT-VIGIL).61 This computerized vigilance test was administered on an IBM-compatible PC and involved visual presentation of a sequence of individual letters in the center of a 15-inch computer monitor. The task was to detect the particular target letter (or letters) and to respond by pressing a response key. The low- and high-effort conditions of the CPT differed with respect to total number of stimuli, ratio of targets to distracter stimuli, and conditional response demand. On the low-effort CPT condition, participants were instructed to respond when they saw the letter A (low conditional demand) on the screen. A total of 200 stimuli were presented (4 successive blocks of 50 stimuli) with a target-to-distracter ratio of 1:1. The high-effort condition required greater sustained attention and vigilance: the total number of stimuli was increased to 1,000 (4 blocks of 250 stimuli) with a target-to-distracter ratio of 1:8, and subjects responded only when they saw the letter A followed by the letter X (conditional demand). The dependent measures for both conditions were discrimination coefficient (a′) and mean response time on correct trials.
Data Analysis
Descriptive statistics were derived for all clinical and demographic indices. Shipley Vocabulary score was treated as a covariate to control for estimated verbal intellectual ability. A mixed multiple analysis of covariance (MANCOVA) procedure (one between-group and one within-group level) was conducted to compare the performance of the three groups (unipolar, bipolar, control) between the two effort conditions (low effort, high effort). The eight dependent measures described above were entered into this MANCOVA as dependent measures. Between-group contrasts revealed that the unipolar and bipolar patients did not differ significantly in their overall attention performance, or with respect to the group-by-conditions interaction, so that these groups were subsequently pooled. Reported results are based on between-group MANCOVA comparing affective disorder patients and control subjects. Univariate comparisons were subsequently derived for the main effects and interaction for each of the dependent measures. Stepwise linear regression analyses were conducted to examine the relationship between the eight attention indices for both the low-effort and high-effort conditions among affective disorder patients.
RESULTS
Demographic and Clinical Characteristics
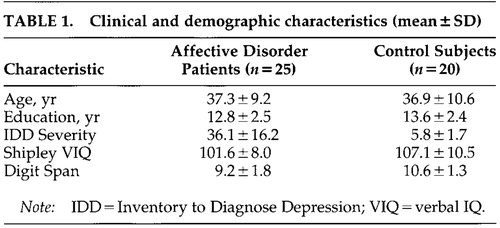
No statistically significant differences between the patient and the control groups were found on most of the background information measures. The groups did not differ in terms of their age, years of education, gender distribution, level of alcohol use, or incidence of prior learning disability. The groups also did not differ with respect to Shipley Vocabulary scores, nor was there a significant interaction of Verbal IQ by effort condition. Clinical and demographic characteristics of each group are summarized in Table 1.
A statistically significant difference in Inventory to Diagnose Depression total score between the unipolar, bipolar, and control subjects was found (F=27.40, df=2,44, P<0.001). Tukey's test revealed that both unipolar (IDD=40.61, SD=12.66) and bipolar (IDD=34.46, SD=20.98) patients were severely depressed compared with control subjects (IDD=7.10, SD=8.56). Unipolar patients did not differ significantly from bipolar patients with respect to total IDD score (P<0.05). The patients and control subjects differed in their responses on specific IDD items of potential relevance to their attentional performance. Affective disorder patients rated themselves with lower energy levels, greater restlessness and psychomotor retardation, poorer ability to concentrate and to make decisions, and greater sleep disturbance in comparison to the control subjects (P<0.05).
Attention Performance Between Groups
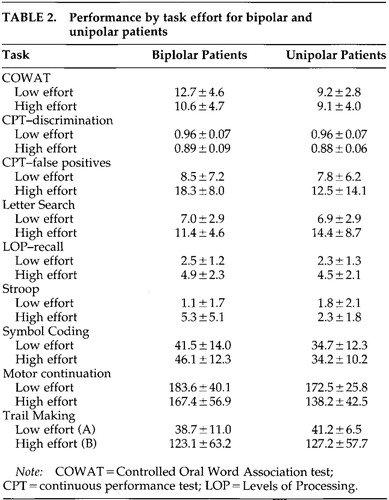
Unipolar and bipolar patients showed weaker overall performance than normal control subjects across the tests of attention as measured by the between-group main effect on multivariate analysis of variance (MANOVA; Wilks' lambda=0.34, F=2.79, df=18,70, P<0.01). The between-group effect size was very large (eta=0.50, power=0.99). There was no significant difference in performance between the unipolar and bipolar patients (Table 2), so these groups were pooled for subsequent univariate analyses.
Performances on the specific attention measures across attention factors are summarized in Table 3 for the affective disorder patients and control subjects. Affective disorder patients had weaker performance than control subjects on all tasks across attention domains.
Sensory Selective Attention:
Response time for correct detection of visual targets on Letter Search was slower for affective disorder than control subjects (F=7.06, df=1,43, P<0.05).
Response Selection and Control:
Time for completion of the Trail Making tasks was greater for affective disorder than control subjects (F=24.45, df=1,43, P<0.001).
Attention Capacity and Focus:
Affective disorder patients produced fewer correct responses on the Symbol Coding tasks than control subjects (F=32.68, df=1,43, P<0.001). Response times on the interference trials of the Stroop task were greater for affective disorder patients than control subjects (F=15.38, df=1,43, P<0.001). Finger tapping rate was slower on the motor persistence task (F=8.24, df=1,43, P<0.05). Affective disorder patients produced fewer words than control subjects on the verbal fluency tasks (F=23.39, df=1,43, P<0.001). Affective disorder patients also recalled fewer word stimuli on the Levels of Processing tasks than control subjects (F=17.79, df=1,43, P<0.001).
Sustained Attention:
Discrimination (d′) performance on the CPT was weaker for affective disorder patients (F=15.19, df=1,43, P<0.001), who made a greater percentage of errors of all types over time compared with control subjects.
Effect of Effortful Task Demand
Collapsed across groups, there was a highly significant within-subject main effect for task demand (effort) on MANOVA (Wilks' lambda=0.61, F=2.90, df=8,36, P<0.05). The effect size was very large (eta=0.94, power=1.00). Overall performance was stronger on the low-effort than the high-effort task conditions across tasks. There was no significant interaction of effort by Shipley Verbal IQ.
Sensory Selective Attention:
Response time for correct detection of visual targets on Letter Search was slower on the low-effort than the high-effort task (F=7.39, df=1,43, P<0.01).
Response Selection and Control:
Time for completion of the Trail Making tasks was greater on the high (Trails B) than low-effort (Trails A) task (F=19.01, df=1,43, P<0.001).
Attention Capacity and Focus:
On the Symbol Coding tasks, fewer symbols were coded in the allotted time during the high-effort (symbol–symbol) condition than on the low-effort (digit symbol) condition (F=15.43, df=1,43, P<0.001). Response times on the Stroop task were slower on the high-effort (dual interference) condition than on the low-effort (standard interference) condition (F=15.02, df=1,43, P<0.001). Performance was stronger on the high-effort (Semantic) condition of the Levels of Processing memory task than the low-effort (Phonemic) condition (F=33.70, df=1,43, P<0.001).
Performance was weaker on the high-effort conditions of both indices of the concurrent response production task. Tapping rate was slower for the motor persistence task on the high-effort condition with concurrent response demand (Tapping+COWAT) than on the low- effort condition when no concurrent response demand was present (F=15.02, df=1,43, P<0.001). Similarly, fewer words were generated on the high-effort verbal fluency condition (COWAT+Tapping) than the low-effort verbal fluency condition (F=8.22, df=1,43, P<0.05).
Sustained Attention:
Discrimination (d′) performance was much stronger on the low-effort (short duration) condition than on the high-effort (long duration) condition of the CPT (F=16.94, df=1,43, P<0.001).
Effect of Effortful Task Demands Among Persons With Major Affective Disorders
A significant group-by–effortful condition interaction on MANCOVA was found (Wilks' lambda=0.52, F=5.54, df=8,36, P<0.001), indicating that the neurocognitive performance of the affective disorder patients and the control subjects differed across conditions as a function of effortful demand on the attention tasks. Again the effect size was very large (eta=0.47, power=0.98). Patients with major affective disorders showed greater overall impairment relative to the control subjects on the high-effort conditions than the low-effort conditions.
Sensory Selective Attention:
Group differences did not vary as a function of effort condition on the Letter Search task. The extent to which affective disorder patients performed more poorly than control subjects was consistent across conditions.
Response Selection and Control:
The magnitude of the difference in time for completion of Trails B versus Trails A varied as a function of group (F=14.19, df=1,43, P<0.001).
Attention Capacity and Focus:
Significant group-by–effort condition interactions were found across most measures in this domain. Affective disorder patients coded fewer symbols in the allotted time than control subjects during the high-effort (Symbol-Symbol) condition than on the low-effort (Digit Symbol) condition (F=5.89, df=1,43, P<0.05). The magnitude of difference in response time between the high-effort (dual interference) and low-effort (standard interference) conditions of the Stroop task also varied between groups (F=4.53, df=1,43, P<0.05). Patients with major affective disorders showed much greater slowing on the high-effort condition than the low-effort condition compared with control subjects. Similarly, recall performance on the Levels of Processing task varied as a function of the interaction of group by condition (F=4.48, df=1,43, P<0.05). Patients with affective disorders did not show as much benefit from semantic processing during the high-effort condition relative to phonemic processing on the low-effort condition when compared with control subjects. There was no significant group-by-effort interaction on the concurrent response production paradigm for sustained finger tapping, although there was a small but statistically significant interaction for the verbal fluency condition (F=4.25, df=1,43, P<0.05). Affective disorder patients showed a greater decline in words generated on the COWAT during the concurrent task condition than did control subjects.
Sustained Attention:
A highly significant group-by–effort condition interaction was found with regard to discrimination (d′) performance on the CPT task (F=23.16, df=1,43, P<0.001). Affective disorder patients performed much more poorly on the high-effort (long duration) task than the low-effort (short duration) task compared with control subjects.
Attention, Effort, and Depression Severity
The indices from the low-effort condition of eight attention tasks were entered, along with IDD total score, into an initial stepwise multiple linear regression analysis. A significant relationship between attention performance and Inventory to Diagnose Depression (IDD) total score was found (R=0.63, F=6.47, df=3,20, P<0.01). Response rate on the Sustained Finger Tapping test (beta=−0.48), Trail Making A (beta=0.42), and time for completion of the Symbol Coding task (beta=−0.32) were most strongly associated with depression severity.
Next, the indices from the “high-effort” condition of eight attention tests were entered along with IDD total score into a similar regression analysis. Again, a strong relationship between attention performance and IDD total score was found (R=0.68, F=8.33, df=4,19, P<0.01). Time completion of Trail Making B (beta=0.46), Symbol Coding (beta=−0.40), CPT-mean response time (beta=0.36), and LOP-Recall (beta=−0.27) were the variables most strongly associated with IDD score.
In a final analysis, all 16 variables from the low-effort and high-effort conditions of the eight tests were simultaneously entered into a similar regression analysis. (R=0.61, F=8.33, df=3,20, P<0.01). Trail Making B (beta=0.46), Symbol Coding (beta=0.40), and LOP-recall (beta=−0.27) were the indices most strongly associated with IDD total score.
DISCUSSION
As expected, patients with major affective disorders (unipolar and bipolar illness) had clinically significant attention impairments compared with age- and education-matched normal control subjects. Not only did the affective disorder patients show weaker performance across most attention tasks compared with the control subjects, but their performance on many of the measures was more than one standard deviation below established norms, with very large effect size. Thus, the affective disorder patients had robust and clinically significant impairments. This result is consistent with the results of other investigations that have demonstrated that impairments of attention are among the most pronounced deficits among patients with major affective disorders.33–48 The robust and pervasive nature of these impairments reinforces the claim that the symptoms of diminished concentration and thinking ability, common among people with affective disorders, reflect objective deficits rather than simply the subjective experience associated with these disorders. These findings provide additional support for the hypothesis that attention impairment is at the core of the neurocognitive syndrome associated with major affective disorders.44
Because the unipolar and bipolar patients did not differ in their overall performance across the eight attention tests on MANCOVA, the unipolar and bipolar groups were pooled into a single “affective” group in subsequent analyses to simplify interpretation of the results. For the most part, the univariate between-group comparisons also indicated that the unipolar and bipolar patients were quite similar in their quantitative performance across the attention tasks. This finding was not expected. Given past studies that have shown neurocognitive differences between unipolar and bipolar patients, we suspected that bipolar illness would be associated with either more severe attention or qualitatively different impairments of attention compared with unipolar illness. The absence of such differences between the unipolar and bipolar patients indicates that these disorders do not necessarily result in different neurocognitive syndromes, at least with respect to attention performance. The fact that the bipolar group consisted of patients who were experiencing either bipolar depression or mixed symptoms (depression and mania or hypomania) may explain this finding. Apparently, bipolar patients who have significant depressive symptoms function in a way that is similar to the functioning of patients with major unipolar depression with regard to attention. However, given the relatively small size of the two patient groups, firm conclusions regarding distinctions between unipolar and bipolar affective disorders requires further inquiry.
Although affective disorder patients showed overall attention impairment compared with control subjects, the magnitude of these impairments differed considerably across tasks. Large between-group differences were evident on measures of sustained attention (CPT), response selection and control (Trail Making), and certain measures of attentional capacity and focus (Symbol Coding, Stroop task, LOP-Recall). Discriminant function analysis performance on Trail Making, Symbol Coding, and the CPT accounted for most of the difference between the groups. Letter Search and Finger Tapping were the variables that contributed the least to group differences. These findings suggest that attention processes are not all affected equally by affective illness. Sustained attention on the CPT, which was severely impaired, seems to be strongly affected by affective illness. Attention capacity and focus were also impaired on most, but not all tasks. Those tasks that placed greatest demand on response selection and control, working memory, and speed of processing appeared most affected.
Intentional processes of response selection and control were significantly impaired, whereas sensory selective attention was largely unaffected. This conclusion is supported by the fact that patients with affective disorders were markedly impaired on Trail Making, but relatively intact on Letter Search. Trail Making requires the maintenance of rapid response production, sequencing, and alternation, whereas Letter Search requires visual selective attention for detection of target stimuli. The affective disorder patients were almost twice as slow as the normal control subjects on Trails B. In contrast, the groups showed minimal differences in mean response time to targets on Letter Search, and affective patients did not exhibit clinically significant impairment. This finding suggests a potentially important dissociation between these two attention factors. Several current theories of attention posit that different processes and brain mechanisms underlie sensory selective attention and response selection and control.44,52 There is also considerable theoretical and empirical support for this distinction.62,63 Sensory selective attention, which can often be performed automatically with little effort, is largely under the influence of posterior brain systems acting in parallel. Response selection and control tends to be much less automatic, usually requiring controlled effortful serial processing organized an “intention” to act. Anterior brain systems, including the frontal cortex, anterior cingulate region, and associated subcortical systems, play a much greater role in the formation of response intention and subsequent control processes.44,52,64 The current findings regarding this dissociation also fit well with a growing body of functional neuroimaging data from brain positron emission tomography and functional magnetic resonance imaging demonstrating frontal hypoperfusion among patients with affective disorders patients.64–67
Effects of Effortful Demand
In light of the issues just discussed, the distinction between controlled-effortful and automatic processing may be a critical factor when accounting for the attention deficits that were found. This hypothesis was tested by comparing the performance of patients and control subjects on each of the attention tasks on two effort conditions: low versus high effortful demand. Before this comparison could be made, however, it was first necessary to ensure that the “high effort” condition of each task was in fact more demanding. Collapsing across groups, a significant main effect of task effort was found on MANCOVA, and significant differences between the low- and high-effort conditions were found on every attention task. Subjects showed weaker performance on the high-effort than low-effort conditions of all but one task. The one exception was the working memory-LOP test, on which high-effort semantic processing actually resulted in greater subsequent incidental recall than the low-effort phonemic processing. This finding, however, was theoretically consistent with the results; the semantic task was expected to elicit greater incidental recall because of the deeper level of processing it evoked.44,59,68 The overwhelming significance of the main effect for effort condition justified examination of how performance differed between groups as a function of effortful task demand.
Comparison of the performance of affective disorder patients and control subjects between the high- and low-effort conditions strongly supported the hypothesis that impairments in the allocation and/or engagement of effortful processing are central to the attention impairments associated with major affective disorders. Supporting this hypothesis, a significant overall group-by-effort interaction was found: affective disorder patients had much weaker performance on high-effort than low-effort conditions, whereas control subjects showed much smaller differences in performance between the high- and low-effort conditions.
The groups differed in performance as a function of effort condition on many, but not all, tasks. The greatest discrepancies between the high- and low-effort conditions of the CPT, Trail Making, Symbol Coding, and Stroop tasks were found among the affective disorder patients. Each of these tasks place considerable demand on sustained and focused attention. These tasks also require high levels of executive response selection and control for successful performance.
Paralleling the previously described results regarding the main between-group effect for the Letter Search task, no significant group-by-effort condition interaction was found on this test of sensory selective attention. Increasing the size of the string of targets to be detected from the letter array did not have significantly greater impact on the affective disorder patients than the control subjects. This result provides further evidence that attention impairments associated with major affective illness vary according to relative demand for automatic versus controlled-effortful processing. Furthermore, the effect of effortful task demand was not uniform across the component processes that underlie attention.44 Effortful task demands produced significant impact on tasks that load on Attention Capacity and Focus, Sustained Attention, and Response Selection and Control, but had little effect on Sensory Selective Attention.
Somewhat surprising was the relatively small impact of the concurrent response production paradigm on the performance of the affective disorder patients. Although COWAT performance of affective disorder patients was weaker when concurrent task demands were present (COWAT+Tapping), only a moderate group-by-effort condition interaction was found. No interaction effect was found for Sustained Finger Tapping. The affective disorder patients did not show a worsening of performance relative to control subjects when they performed the concurrent response production task (Tapping+COWAT) compared with performance on Sustained Finger Tapping alone. Perhaps one of the reasons for the absence of a stronger effect for this paradigm was that it was very difficult for both groups. Verbal fluency tended to decline substantially when simultaneous tapping was performed, among both affective disorder patients and control subjects. Conversely, although affective disorder patients were slower on Sustained Finger Tapping and Tapping+COWAT compared with the control subjects, their tapping rate remained relatively stable across the two levels of effort. The results suggest that motor production was preserved, but at the cost of reduced output on the COWAT task for both groups.
Affective disorder patients also benefited less than did the control subjects from higher-effort semantic processing on the LOP task. Besides showing less incidental recall on the semantic than the phonemic condition, they also produced fewer words on the semantic processing task than did the control subjects. That the quantity of response production was reduced along with subsequent incidental recall further illustrates the impact of affective illness on attention capacity and focus.
Attention and Depression Severity
A significant relationship between attention, effort, and severity of affective illness (as measured by IDD total score) was found in the final regression analyses. The stepwise regression analysis conducted with the eight attention indices from the low-effort condition revealed that Trail Making, Symbol Coding, and Sustained Finger Tapping performance were most strongly associated with severity of depression symptoms, these being the same variables that discriminated the affective disorder patients from the control subjects. When eight attention indices from the high-effort conditions were entered in the second regression analysis, a similar result occurred: the Trails B, Symbol Coding, CPT, and COWAT-Concurrent Response Demand measures were most strongly associated with depression severity. When the both the high- and low-effort conditions of the indices were entered into a final regression analysis, the high-effort condition of these same four indices was again most strongly associated with depression severity, whereas the indices from the low-effort condition no longer were retained as significant predictors.
It is important to note that study patients on average were severely depressed and were psychiatric inpatients taking a variety of different psychotropic medications. Therefore, these results may not generalize to less severely depressed patients. Nevertheless, the present findings reinforce the role of attentional effort in the effects observed through this study.
Factors probably associated with the underlying neurophysiological mechanisms of major affective illness appear to affect attention by diminishing the depressed person's ability to summon adequate attentional resources in the face of effortful task demands. This difficulty may in turn reflect the motivational and energetic deficits that are central to affective disorders.69 The results of this study may have eventual clinical application if attentional probes can be developed that are graded on the basis of effortful task demand to determine the degree of functional impairment associated with the neurophysiological disturbance accompanying major affective illness. Additional studies are needed to establish optimal parameters for such tasks.
 |
 |
 |
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994, pp 317-392Google Scholar
2 Friedman A: Minimal effects of severe depression on cognitive functioning. J Abnorm Psychol 1964; 69:237-243Crossref, Medline, Google Scholar
3 Austin MP, Ross M, Murray C, et al: Cognitive function in major depression. J Affect Disord 1992; 25:21-30Crossref, Medline, Google Scholar
4 Blackburn IM, Roxborough HM, Muir WJ, et al: Perceptual and physiological dysfunction in depression. Psychol Med 1990; 20:95-103Crossref, Medline, Google Scholar
5 Blackburn I: Mental and psychomotor speed in depression and mania. Br J Psychiatry 1975; 126:329-335Crossref, Medline, Google Scholar
6 Bulbena A, Berrios GE: Cognitive function in the affective disorders: a prospective study. Psychopathology 1993; 26:6-12Crossref, Medline, Google Scholar
7 Burgess JW: Neurocognition in acute and chronic depression: personality disorder, major depression, and schizophrenia. Biol Psychiatry 1991; 30:305-309Crossref, Medline, Google Scholar
8 Donnelly E, Murphy D, Goodwin F, et al: Intellectual functioning in affective disorder. Br J Psychiatry 1982; 140:633-636Crossref, Medline, Google Scholar
9 Golinkoff M, Sweeney JA: Cognitive impairments in depression. J Affect Disord 1989; 17:105-112Crossref, Medline, Google Scholar
10 Ilsley JE, Moffoot AP, O'Carroll RE: An analysis of memory dysfunction in major depression. J Affect Disord 1995; 35:1-9Crossref, Medline, Google Scholar
11 Kuzis G, Sabe L, Tiberti C, et al: Cognitive functions in major depression and Parkinson disease. Arch Neurol 1997; 54:982-986Crossref, Medline, Google Scholar
12 Sackeim HA, Steif BL: Neuropsychology of depression, in Depression and Mania, edited by Georgotas A, Cancro R. New York, Elsevier, 1988Google Scholar
13 Sweeny JA, Wetzler S, Stokes P, et al: Cognitive functioning in depression. J Clin Psychol 1989; 45:836-842Medline, Google Scholar
14 Willner P: Cognitive functioning in depression: a review of theory and research. Psychol Med 1984; 14:807-823Crossref, Medline, Google Scholar
15 Zakzanis KK, Leach L, Kaplan E: On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 1998; 11:111-119Medline, Google Scholar
16 Rush AJ, Weissenburger J, Vinson DB, et al: Neuropsychological dysfunctions in unipolar nonpsychotic major depressions. J Affect Disord 1983; 5:281-287Crossref, Medline, Google Scholar
17 Flor-Henry P: Laterality shifts of cerebral dominance, sinistrality, and psychosis, in Hemisphere Asymmetries of Function in Psychopathology, edited by Gruzelier J, Flor-Henry P. New York, Elsevier, 1979, pp 3-20Google Scholar
18 Taylor MA, Greenspan B, Abrams R: Lateralized neuropsychological dysfunction in affective disorder and schizophrenia. Am J Psychiatry 1979; 136:1031-1034Google Scholar
19 Taylor MA, Redfield J, Abrams R: Neuropsychological dysfunction in schizophrenia and affective disease. Biol Psychiatry 1981; 16:467-478Medline, Google Scholar
20 Merrin E: Motor and sighting dominance in schizophrenia and affective disorder: evidence for right hand grip strength prominence in schizophrenia and bipolar illness. Br J Psychiatry 1984; 146:539-544Crossref, Google Scholar
21 Amsterdam JD, Mozley PD: Temporal lobe asymmetry with iofetamine (IMP) SPECT imaging in patients with major depression. J Affect Disord 1992; 24:43-53Crossref, Medline, Google Scholar
22 Adler G, Adler J, Schneck M, et al: Influence of stimulation parameters on auditory stimulus processing in schizophrenia and major depression: an auditory evoked potential study. Acta Psychiatr Scand 1990; 81:453-458Crossref, Medline, Google Scholar
23 Backman L, Forsell Y: Episodic memory functioning in a community-based sample of old adults with major depression: utilization of cognitive support. J Abnorm Psychol 1994; 103:361-370Crossref, Medline, Google Scholar
24 Calev A, Korin Y, Shapira B, et al: Verbal and non-verbal recall by depressed and euthymic affective patients. Psychol Med 1986; 16:789-94Crossref, Medline, Google Scholar
25 Hart RP, Kwentus JA, Hamer RM, et al: Selective reminding procedure in depression and dementia. Psychol Aging 1987; 2:111-115Crossref, Medline, Google Scholar
26 Nunn JD, Mathews A, Trower P: Selective processing of concern-related information in depression. Br J Clin Psychol 1997; 36:489-503Crossref, Medline, Google Scholar
27 Sabbe B, Hulstijn W, Van Hoof J, et al: Fine motor retardation and depression. J Psychiatr Res 1996; 30:295-306Crossref, Medline, Google Scholar
28 Stip E, Lecours AR, Chertkow H, et al: Influence of affective words on lexical decision task in major depression. J Psychiatry Neurosci 1994; 19:202-207Medline, Google Scholar
29 Williams JM, Scott J: Autobiographical memory in depression. Psychol Med 1988; 18:689-695Crossref, Medline, Google Scholar
30 Malonee J, Helmsley D: Lowered responsiveness and auditory signal detectability during depression. Psychol Med 1977; 7:717-722Crossref, Medline, Google Scholar
31 Cooley EL, Nowicki S Jr: Discrimination of facial expressions of emotion by depressed subjects. Genet Soc Gen Psychol Monogr 1989; 115:449-465Medline, Google Scholar
32 Martin DJ, Oren Z, Boone K: Major depressives' and dysthymics' performance on the Wisconsin Card Sorting Test. J Clin Psychol 1991; 47:684-690Crossref, Medline, Google Scholar
33 Benoit G, Fortin L, Lemelin S, et al: Selective attention in major depression: clinical retardation and cognitive inhibition. Can J Psychol 1992; 46:41-52Crossref, Medline, Google Scholar
34 El Massioui F, Lesevre M: Attention impairment and psychomotor retardation in depressed patients: an event related potential study. Electroencephalogr Clin Neurophysiol 1988; 70:46-55Crossref, Medline, Google Scholar
35 Hart RP, Wade JB, Calabrese VP, et al: Vigilance performance in Parkinson's disease and depression. J Clin Exp Neuropsychol 1998; 20:111-117Crossref, Medline, Google Scholar
36 Knott, Lapierre Y, Griffiths, et al: Event related potentials and selective attention in major depressive illness. J Affect Disord 1991; 23:43-48Crossref, Medline, Google Scholar
37 Lemelin S, Baruch P: Clinical psychomotor retardation and attention in depression. J Psychiatr Res 1998; 32:81-8Crossref, Medline, Google Scholar
38 Nelson EB, Sax KW, Strakowski SM: Attentional performance in patients with psychotic and nonpsychotic major depression and schizophrenia. Am J Psychiatry 1998; 155:137-139Crossref, Medline, Google Scholar
39 O'Carroll RE, Rogers A, Lawrie SM, et al: Laterality of visuo-spatial attention in acute and chronic schizophrenia, major depression and in healthy controls. Psychol Med 1995; 25:1091-1095Google Scholar
40 Roy-Byrne PP, Weingartner H, Bierer LM, et al: Effortful and automatic cognitive processes in depression. Arch Gen Psychiatry 1986; 43:265-267Crossref, Medline, Google Scholar
41 Suffin SC, Emory WH: Neurometric subgroups in attentional and affective disorders and their association with pharmacotherapeutic outcome. Clin Electroencephalogr 1995; 26:76-83Crossref, Medline, Google Scholar
42 Thomas P, Goudemand M, Rousseaux M: Attentional resources in major depression. Eur Arch Psychiatry Clin Neurosci 1999; 249:79-85Crossref, Medline, Google Scholar
43 Weingartner H, Cohen RM, Murphy DL, et al: Cognitive processes in depression. Arch Gen Psychiatry; 38:42-47Google Scholar
44 Cohen RA: Neuropsychology of Attention. New York, Plenum, 1994Google Scholar
45 Kahneman D: Attention and Effort. Englewood Cliffs, NJ, Prentice Hall, 1973Google Scholar
46 Tancer ME, Brown TM, Evans DL, et al: Impaired effortful cognition in depression. Psychiatry Res 1990; 31:161-118Crossref, Medline, Google Scholar
47 Gipson WT: Fatigue and depression in the patient in the intensive care unit. Prim Care 1991; 18:359-367Medline, Google Scholar
48 Ellis HC: Focused attention and depressive deficits in memory. J Exp Psychol 1991; 120:310-312Crossref, Google Scholar
49 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd edition. Washington, DC, American Psychiatric Association, 1980Google Scholar
50 Zimmerman M, Coryell W: The Inventory to Diagnose Depression (IDD): a self-report scale to diagnose major depressive disorder. J Consult Clin Psychol 1987; 55:55-59Crossref, Medline, Google Scholar
51 Shipley WC: Institute of Living Scale. Los Angeles, Western Psychological Services, 1946Google Scholar
52 Posner MI, Boies SJ: Components of attention. Psychol Rev 1971; 78:391-408Crossref, Google Scholar
53 Thorne DR, Genser SG, Sing HC, et al: Walter Reed Performance Assessment Battery. Neurobehavioral Toxicology and Teratology 1985; 7:415-418Medline, Google Scholar
54 Army Individual Test Battery: Manual for Directions and Scoring. Washington, DC, War Department, Adjutant General's Office, 1944Google Scholar
55 Spreen O, Benton AL: Neurosensory Center Comprehensive Examination for Aphasia (NCCEA), experimental edition. Iowa City, IA, University of Iowa Department of Neurology, 1965Google Scholar
56 Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Interpretation. Tucson, AZ, Neuropsychology Press, 1985Google Scholar
57 Smith A: Symbol Digit Modalities Test Manual. Los Angeles, Western Psychological Services, 1982Google Scholar
58 Stroop JR: The Manual for the Stroop Color and Word Test. Wood Dale, IL, Stoelting, 1978Google Scholar
59 Craik FIM, Lockhart RS: Levels of Processing: a framework for memory research. Journal of Verbal Learning and Verbal Behavior 1972; 11:671-684Crossref, Google Scholar
60 Thorndike EL, Lorge I: The Teacher's Word Book of 30,000 Words. New York, Teacher's College/Columbia University Bureau of Publications, 1944Google Scholar
61 Cegalis JA, Bowlin J: VIGIL: User's Manual and Reference Guide. Nashua, NH, For Thought, Ltd, 1991Google Scholar
62 Hasher L, Zacks RT: Automatic and effortful processing in memory. J Exp Psychol 1979; 108:356-388Crossref, Google Scholar
63 Shiffrin RM, Schneider W: Automatic and controlled processing revisited. Psychol Rev 1984; 91:269-276Crossref, Medline, Google Scholar
64 Buchsbaum MS, DeLisi LE, Holcomb HH, et al: Anteroposterior gradients in cerebral glucose use in schizophrenia and affective disorders. Arch Gen Psychiatry 1984; 41:1159-1166Google Scholar
65 Bruder GE, Stewart JW, Towey JP, et al: Abnormal cerebral laterality in bipolar depression: convergence of behavioral and brain event-related potential findings. Biol Psychiatry 1992; 32:33-47Crossref, Medline, Google Scholar
66 Cohen RM, Semple WE, Gross M, et al: Evidence for common alterations in cerebral glucose metabolism in major affective disorders and schizophrenia. Neuropsychopharmacology 1989; 2:241-254Crossref, Medline, Google Scholar
67 Rief W, Hermanutz M: Responses to activation and rest in patients with panic disorder and major depression. Br J Clin Psychol 1996; 35:605-616Crossref, Medline, Google Scholar
68 Fiske A, Schneider W: Memory as a function of attention, level of processing, and automatization. J Exp Psychol Learn Mem Cogn 1984; 10:181-197Crossref, Medline, Google Scholar
69 Richards PM, Ruff RM: Motivational effects on neuropsychological functioning: comparison of depressed and non-depressed individuals. J Clin Consult Psychol 1989; 57:396-402Crossref, Medline, Google Scholar



