Determinants of SPontaneous Extrapyramidal Symptoms in Elderly Psychiatric Inpatients Diagnosed With Alzheimer's Disease, Major Depressive Disorder, or Psychotic Disorders
Abstract
Extrapyramidal symptoms (EPS) occur more frequently in dementia of the Alzheimer's type (DAT) than in normal aging. Other late-life mental disorders, however, have also been associated with EPS. To examine whether EPS are increased in DAT patients relative to neuropsychiatric control subjects, the authors compared EPS in 127 neuroleptic-free elderly patients diagnosed with either DAT, major depressive disorder (MDD), or a psychotic disorder (SCHIZ/DELUS). They also examined whether depressive or psychotic symptoms were associated with EPS independently of diagnosis. Severity of parkinsonian rigidity was found to be independently associated with DAT. Rank order of rigidity was DAT>MDD> SCHIZ/DELUS. Bradykinesia, although not associated with diagnostic group, was positively correlated with withdrawn depression. These findings suggest that rigidity is associated with DAT independently of any concurrent psychotic or depressive process, whereas bradykinesia does not appear to be specific to DAT among late-life neuropsychiatric illnesses.
The neurologic findings that include muscular rigidity, bradykinesia, resting tremor, and flexion posture have historically been referred to as extrapyramidal symptoms (EPS) because of their frequent association with diseases involving the basal ganglia.1 Several reports, however, have documented the frequent occurrence of EPS in patients diagnosed with dementia of the Alzheimer's type (DAT)2–5 and in the related condition, senile dementia of the Lewy body type (SDLBT).6 DAT with EPS may represent a clinical subtype of DAT with a faster decline in self-care and cognition.7,8 Moreover, the presence of EPS at baseline may identify a subgroup of dementia patients with increased sensitivity to neuroleptic-induced parkinsonism.6,9
Notably, normal aging has also been associated with the development of spontaneous EPS,2,10,11 as have primary psychiatric disorders such as major depression and schizophrenia.12–16 It is therefore possible that the presence of EPS in some DAT patients is a consequence of age alone, associated with the development of concurrent depressive or psychotic symptoms that frequently complicate the course of DAT,17–19 or is merely a nonspecific concomitant of neuropsychiatric illness in late life. Funkenstein et al.20 have previously reported that the increased frequency of EPS in DAT patients persists even after controlling for the effects of age. To our knowledge, however, no prior study has examined whether clinical measures of EPS are increased in patients with DAT when compared to patients with other late-life neuropsychiatric syndromes, or whether the expression of clinical EPS in DAT patients is related to the presence of concurrent depressive or psychotic symptoms.
In this study, we set out to determine whether extrapyramidal symptoms differed among neuroleptic-free elderly patients with DAT, major depressive disorder (MDD), and schizophrenia or delusional disorder (SCHIZ/DELUS). Multivariate statistical techniques were used to control for the effects of age and the severity of depressive or psychotic symptoms.
METHODS
Clinical Evaluation
The study was conducted on the Geriatric Clinical Research Unit at Western Psychiatric Institute and Clinic over 26 months from 4/26/91 to 6/30/93. All patients over the age of 50 who were admitted to the unit for at least 72 hours during this period received a comprehensive multidisciplinary evaluation, including a psychiatric history and mental status examination, a social history, a medical history with physical and neurologic examination, laboratory testing, electroencephalogram, and neuroimaging (MRI, or CT scan when patients had contraindications to MRI or were unable to cooperate with the MRI procedures), as previously described.19,21,22 Axis I and Axis II diagnoses were determined for each patient according to DSM-III-R23 criteria, using all available information from the patient, family or caregivers, outpatient physicians, and the patient's medical records, in addition to the evaluations performed on the unit. The diagnostic criteria of DSM-III-R were applied in weekly consensus diagnostic conferences attended by at least three faculty psychiatrists, including the patient's attending psychiatrist, all of whom had specialized expertise in geriatric psychiatry. The psychiatric diagnosis corresponding to the symptoms that led to admission was designated the “primary diagnosis.”
Patients were also rated by a trained research clinician on a battery of psychometric scales including the Brief Psychiatric Rating Scale (BPRS),24 the Hamilton Rating Scale for Depression (Ham-D),25 and a standardized version of the Mini-Mental State Examination (MMSE).26 A second research clinician, who was blind to the patient's clinical history, then performed the comprehensive Geriatric Movement Disorders Assessment, which included ratings on the Simpson Extrapyramidal Side Effect Scale (SEPSE).27 The SEPSE provided ratings of 10 parkinsonian signs: gait, arm drop, shoulder rigidity, elbow rigidity, wrist rigidity, leg pendulousness, head drop, glabella tap, tremor, and salivation. Each of these items is scored from 0 to 4, with a score of 0 corresponding to “absent,” a score of 1 corresponding to “minimally present,” and scores of 2 to 4 reflecting increasing severity from mild to severe. To avoid inappropriate rating of gegenhalten, examiners were trained to disregard this “active” resistance and rate only the severity of rigidity that was lead-pipe or cogwheeling in nature. We have previously demonstrated the ability to train geriatric research clinicians to perform the Geriatric Movement Disorders Assessment reliably compared with an experienced geriatric psychiatrist.28 Throughout the study period, reliability was maintained by periodic retraining of the six research clinicians. On repeated reliability testing conducted prior to, during, and after the conclusion of the study interval, intraclass correlation coefficients for the SEPSE total score ranged from 0.70 to 0.89.
Neuroleptic histories for each patient were obtained by a research psychiatrist drawing on all available sources of information, including the patient, the patient's family, the treating physician, and the available medical records. Whether the patient had ever received any neuroleptic, including amoxapine, metoclopramide, or prochlorperazine, was recorded, and the interval since each patient last used neuroleptics was categorized as current use, use within the past 3 months, use 3–12 months prior to admission, use >1 year prior to admission, and never used. Similarly, each patient's lifetime duration of neuroleptic use was recorded, categorized as <3 months, 3–12 months, or >1 year. EPS might be masked by the current use of a tricyclic antidepressant or other anticholinergic/antiparkinsonian medication (TCA/antiparkinsonian)29 or induced by the use of a selective serotonin reuptake inhibitor (SSRI);30 we therefore also recorded whether patients were receiving these medications at the time of admission.
Subjects
During the study period, there were 643 consecutive admissions of patients at least 50 years of age. Thirty-six patients with an Axis III diagnosis of either Parkinson's disease or progressive supranuclear palsy were excluded from further analysis. We also excluded 17 patients for whom a neuroleptic history could not be obtained and 13 patients who could not comply with the Geriatric Movement Disorders Assessment. Repeat admissions accounted for another 120 of the cases, and these were not included in the data analysis. Of the remaining 457 patients, 262 were neuroleptic free for at least 3 months prior to admission. Dementia of the Alzheimer's type, major depressive disorder, and schizophrenia or delusional disorder were the primary psychiatric diagnoses for 146 of these patients. Nineteen patients, however, were later excluded because they were receiving neuroleptic medication or lithium in the interval between admission and completion of the Geriatric Movement Disorders Assessment (n=14) or failed to complete at least 3 of the 4 items comprising the Rigidity subscore of the SEPSE (n=5). Thus, this report comprises a total sample of 127 patients who had been neuroleptic free for at least 3 months and were able to complete the clinical evaluation described above during their first admission.
The data presented in this study were obtained as part of a clinical investigation of elderly patients admitted to the Geriatric Clinical Research Unit according to a protocol approved by the Biomedical Institutional Review Board of the University of Pittsburgh.
Analytic Procedures
Statistical analyses were conducted by using the SPSS for Windows 6.0 statistical software package.31 A three-step approach was taken. First, to aid in the definition of relevant subscales of our psychometric measures for this population, a principal components analysis of the BPRS, Ham-D, MMSE, and SEPSE was undertaken. The goal for the BPRS and Ham-D was to identify separate measures of psychosis and depression and to identify any possible contributions to these measures of motor symptoms (such as motoric slowing) that might represent underlying EPS. For the BPRS, this led to the identification of a psychosis subscale (BPRS-Psychosis) consisting of the items for suspiciousness, unusual thought content, and hallucinatory behavior. Two depression factors of the BPRS were identified. One, corresponding to a retarded subtype of depression, included the items for depressed mood, motor retardation, blunted affect, and emotional withdrawal. Because motor retardation and blunted affect might be better considered as manifestations of the EPS bradykinesia/hypokinesia, they were removed and treated as a dependent variable, Bradykinesia. The sum of the remaining two items, depressed mood and emotional withdrawal, formed the BPRS-Depressed-Withdrawn subscale. The other factor, corresponding to an anxious subtype of depression (BPRS-Depressed-Anxious) included depressed mood, somatic concern, anxiety, guilt feeling, and tension. Similarly, the Ham-D demonstrated two factors; the first included the items for depressed mood, psychomotor retardation, and anergia. As for the BPRS, the psychomotor retardation item was removed to form the Ham-D-Anergic subscale. The Ham-D-Anxious included the items for depressed mood, psychological anxiety, somatic anxiety, anergia, and hypochondriasis. For the MMSE, no subscales were identified.
Principal components analysis of the SEPSE identified a Rigidity factor with contributions from the wrist rigidity, elbow rigidity, shoulder rigidity, and leg pendulousness items. The items gait, arm drop, and head drop (a measure of neck rigidity), which might have been anticipated to contribute to this factor, did not, possibly because of frequent missing values (21%, 22%, and 27%, respectively) for these items, which require patients to stand and ambulate independently. Tremor made a small contribution to the rigidity factor and a moderate contribution to a separate factor with additional loadings for gait and arm drop. Because these latter items were frequently missing, however, the individual tremor item was identified as a separate subscore for further analyses (Tremor).
In the second step, the associations of the EPS Rigidity, Bradykinesia, and Tremor with diagnostic group was examined. Because the distribution of Rigidity and Bradykinesia were positively skewed, however, they were first transformed to their square roots. For the analysis of Tremor, Tremor was first categorized as “absent” (score of 0 on tremor item of SEPSE) or “present” (score≥1 on tremor item of SEPSE). In the third step, we assessed the independent contributions of diagnostic group and the other demographic and clinical variables (age, gender, race, total duration of neuroleptic use, interval since last neuroleptic use, SSRI use, TCA/antiparkinsonian use, MMSE, BPRS-Psychosis, BPRS-Depressed-Withdrawn, BPRS-Depressed-Anxious, Ham-D-Anergic, and Ham-D-Anxious) to the dependent variables (Rigidity, Bradykinesia, and Tremor). Linear regression analysis was used to examine the relationships with Rigidity and Bradykinesia. Stepwise elimination was chosen to eliminate covariant variables, allowing development of the most concise model. The assumption of normality of the transformed variables was confirmed by analysis of the residuals. For Tremor, multiple logistic regression was performed, using the likelihood ratio and backward elimination to again eliminate covariant variables in determining the most concise model.
RESULTS
Demographic and Clinical Characteristics
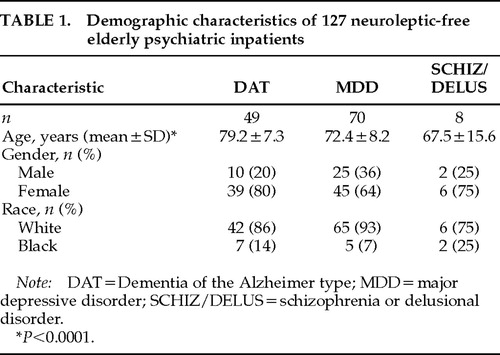
The diagnostic and demographic characteristics of the subjects are presented in Table 1. The majority of patients were diagnosed with MDD (55%), with 39% receiving a diagnosis of DAT and 6% a diagnosis of SCHIZ/DELUS. The mean age (±SD) of patients was 74.7±9.2 (range 51–98) and differed significantly between diagnostic groups (F=12.5, df=2, P<0.0001). In contrast, the groups did not differ in gender or racial distribution (χ2=3.3, df=2, P=0.2 and χ2=3.2, df=2, P=0.2, respectively).
The majority of patients in the DAT and MDD groups (76% and 67%, respectively) and 38% of the patients in the SCHIZ/DELUS group were never treated with neuroleptics (Figure 1A). The distribution of lifetime duration of neuroleptic treatment, however, did not differ among the groups (exact multinomial probability: P=0.4). By design, no patient had received neuroleptic treatment within 3 months of admission, and only a small minority in each diagnostic group had received neuroleptic treatment within the past year (Figure 1B): 10% of both the DAT and MDD patients, and 12% of the SCHIZ/DELUS patients. Differences among the groups in the distribution of interval since last neuroleptic use were also nonsignificant (exact multinomial probability: P=0.2). In contrast, 33% of MDD patients versus 14% of DAT and 12% of SCHIZ/DELUS patients were receiving a TCA/antiparkinsonian drug at the time of admission (χ2=6.0; df=2, P=0.05). A similar relationship was observed for current use of an SSRI (14%, 2%, and 0%, respectively; exact multinomial probability: P=0.05).
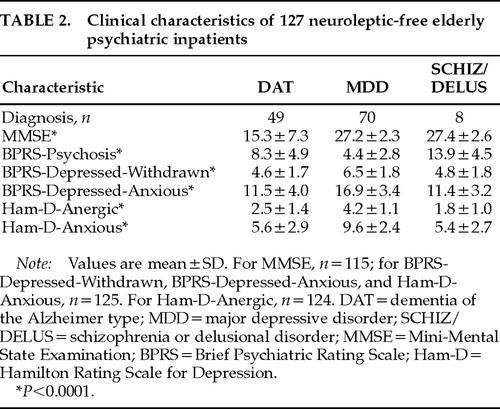
Mean scores for the diagnostic groups on the MMSE and the identified subscales of the BPRS and Ham-D are presented in Table 2. The differences among the groups for each of these variables were highly significant (all P<0.0001) and in the expected directions. Thus, DAT patients had lower mean MMSE scores than MDD and SCHIZ/DELUS patients. Mean BPRS-Psychosis scores were highest in the SCHIZ/DELUS group. Finally, the MDD group scored highest on all four measures of depression (BPRS-Depressed-Withdrawn, BPRS-Depressed-Anxious, Ham-D-Anergic, and Ham-D-Anxious).
Association of EPS with Diagnosis
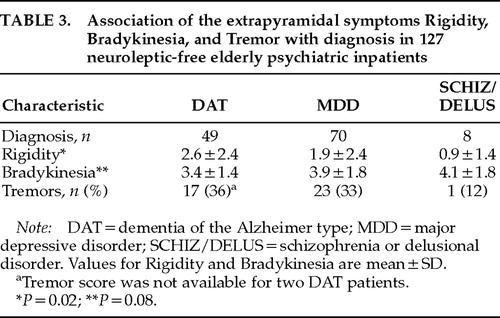
The association of Rigidity, Bradykinesia, and Tremor with diagnostic group was next examined (Table 3). Mean Rigidity scores were highest in DAT patients, followed by MDD patients and SCHIZ/DELUS patients, a significant difference (F=3.8, df=2, P=0.02). Bradykinesia demonstrated a trend toward a significant association with diagnostic group, but in the opposite rank order (SCHIZ/DELUS>MDD>DAT; test of linearity: F=3.2, df=1, P=0.08). In contrast, there was no significant association between Tremor and diagnosis. Because an association between EPS and diagnostic group might be mediated (or obscured) by the association of EPS with the other sociodemographic and clinical variables identified above (age, gender, race, diagnostic group, total duration of neuroleptic use, interval since last neuroleptic use, SSRI use, TCA/antiparkinsonian use, MMSE, BPRS-Psychosis, BPRS-Depressed-Withdrawn, BPRS-Depressed-Anxious, Ham-D-Anergic, and Ham-D-Anxious), multiple regression analysis was then conducted to assess the independent contributions of these variables and diagnostic group to Rigidity, Bradykinesia, and Tremor. For Rigidity, only diagnostic group demonstrated a significant independent association (B [partial regression coefficient]=–0.36, t=–2.6, P=0.01). For bradykinesia, BPRS-Depressed-Withdrawn, BPRS-Depressed-Anxious, and gender all demonstrated significant independent associations (B=0.09, t=4.6, P<0.0001; B=–0.02, t=–2.2, P=0.03; and B=–0.17, t=–2.2, P=0.03, respectively). For Tremor, none of the measured variables demonstrated significant associations, although there was a trend toward a significant association for diagnostic group (LL [log likelihood ratio]=–68, df=2, P=0.07).
To rule out the possibility that the results of the regression analyses might reflect the influence of outliers in the small SCHIZ/DELUS group, all three analyses were repeated excluding the SCHIZ/DELUS patients. For Rigidity, diagnostic group continued to demonstrate an independent statistical association, although now a second independent association, with BPRS-Depressed-Withdrawn, was also observed (B=–0.50, t=–2.6, P=0.01, and B=0.10, t=2.1, P=0.04, respectively). For Bradykinesia, the independent associations of BPRS-Depressed-Withdrawn and gender persisted (B=0.07, t=3.6, P=0.0005; and B=–0.22, t=–2.7, P=0.008, respectively), and an independent association of age was also seen (B=0.01, t=2.3, P=0.02). The previously observed association between Bradykinesia and BPRS-Depressed-Anxious was no longer observed. Finally, for Tremor, no association with any of the independent variables was found when SCHIZ/DELUS patients were excluded.
DISCUSSION
We compared the severity of three aspects of EPS—parkinsonian rigidity, bradykinesia, and tremor—in neuroleptic-free elderly psychiatric inpatients diagnosed with either dementia of the Alzheimer's type, major depressive disorder, or a psychotic disorder (schizophrenia or delusional disorder). Parkinsonian rigidity, but not bradykinesia or tremor, was independently associated with diagnostic group, with a rank order of severity of rigidity of DAT>MDD>SCHIZ/DELUS. Although not associated with diagnostic group, bradykinesia was positively associated with a measure of withdrawn depression and with male gender. No significant association was found between the presence of tremor and any of the identified variables.
We found significantly elevated ratings of parkinsonian rigidity in our DAT patients in comparison to the MDD and SCHIZ/DELUS groups, but bradykinesia and tremor did not differ among groups. Because we did not include normal elderly control subjects in the current study, we cannot conclude whether the degree of rigidity, bradykinesia, and tremor observed in our patients was abnormal for individuals of this age. Abnormalities of bradykinesia, however, have been reported in all three diagnostic groups relative to normal control subjects,12,13,15,20,32 with abnormalities in parkinsonian rigidity and tremor reported for DAT and SCHIZ/DELUS patients.2,15,20,32 The finding of a difference in Rigidity scores between our patients diagnosed with DAT and elderly psychiatric control subjects therefore extends these prior reports by suggesting that parkinsonian rigidity is specifically elevated in DAT and is not merely a nonspecific concomitant of aging or of late-life mental disorders. In contrast, these findings suggest that the reported increases of bradykinesia and tremor in DAT patients are not unique to this diagnosis, but may be mediated through mechanisms common among late-life neuropsychiatric illnesses.
We are aware of only one prior report that examined differences in spontaneous EPS between DAT and psychiatric patient groups; however, instrumental rather than clinical measures of rigidity, movement time (a measure of bradykinesia), and tremor were used. Caligiuri et al.15 examined 13 elderly patients diagnosed with DAT and 13 diagnosed with schizophrenia. They found that only on the measure of movement time did the DAT patients show significantly more EPS than the patients with schizophrenia. Although the discrepancy between the findings of Caligiuri et al. and those of the present study, which found an independent association between psychiatric diagnosis and rigidity, but not bradykinesia, are not readily resolvable, they may be due to the different methodology of assessing EPS. Instrumental techniques for the measurement of EPS appear to be sensitive to subclinical abnormalities.16,32 Thus, it is possible that instrumental measures might identify subtle between-group differences not appreciable on clinical evaluation (in bradykinesia, for example). Similarly, if, as a consequence of increased sensitivity, instrumental measures have a lower “ceiling” than clinical EPS measurement, the use of instrumental techniques might not detect clinically observed differences between patient groups (in rigidity, for example). It is also possible that the discrepancy between the current findings and those of Caligiuri et al. might also result from the lack of generalizability attendant on the small number of SCHIZ/DELUS patients in both studies and the small number of DAT patients in the latter report. Because our finding of an association between diagnostic group and increased rigidity persisted when we limited our analysis to the larger DAT and MDD groups, however, the finding seems unlikely to have resulted from this form of a type I error.
We also examined whether the presence of EPS in our elderly psychiatric patients was due to an association with the severity of depressive or psychotic symptoms, rather than to an association with a diagnosis of a depressive or psychotic disorder per se. Thus, bradykinesia, although not demonstrating a specific association with a diagnosis of depression, was correlated with measures of depression across diagnostic groups. The severity of bradykinesia was positively correlated with the BPRS measure of withdrawn depression and, correspondingly, was negatively correlated with the BPRS measure of anxious depression. Although similar symptoms rated on the Ham-D (Ham-D-Anergic and Ham-D-Anxious) were not independently associated with bradykinesia, this is explained in part by the substantial covariance of the corresponding Ham-D and BPRS subscales (r=0.6 and r=0.8, respectively, both P<0.001). We identified one prior study, limited to patients diagnosed with MDD, that reported on the relationship between EPS and severity of depressive symptoms. Rogers et al.13 found no correlation between total Ham-D score and an instrumental measure of movement time, nor did change in total Ham-D score after treatment correlate with change in movement time. Subscales of the Ham-D were not examined, however, which leaves the possibility that any associations were obscured by the opposing contributions of anxious and anergic factors. Interestingly, measures of clinical EPS in their MDD patients did improve significantly with treatment of depression, although the authors did not report which specific EPS were improved or their correlations with the reduction in depressive symptoms. Taken with the current findings, this linkage is consistent with a common mechanism contributing to the expression of certain depressive symptoms and bradykinesia.
We also found an independent association between increased bradykinesia and male gender. Mean bradykinesia ratings in our male patients were 4.3±1.6, versus 3.5±1.6 in our female patients. Gender differences in severity of EPS have been previously reported in DAT,33,34 with greater frequency of degenerative changes of the substantia nigra in male patients at postmortem examination.35 Gender differences in the prevalence of idiopathic Parkinson's disease have also been reported.36 Although several lines of preclinical investigation indicate that estrogens modulate dopaminergic neurotransmission,37–40 this is unlikely to explain the observed difference in our elderly patients, since the females were postmenopausal and only infrequently received estrogen replacement therapy (unpublished data). Further exploration of the role of gonadal steroids in modulating dopaminergic neurotransmission and motor function in elderly populations, however, may be warranted.
Unlike our finding of an association between bradykinesia and withdrawn depression, we found no association between depression and either rigidity or tremor, nor between any EPS and measures of psychosis. This stands in contrast to reports of an association of EPS with psychotic symptoms in studies limited to DAT patients.4,17 To examine this issue in our sample, we re-analyzed the association of psychotic symptoms with Rigidity, Tremor, and Bradykinesia in our DAT patients only; however, no significant relationships were found. In contrast, the observed correlations between bradykinesia and symptoms of depressive withdrawal remained significant within the DAT group (r=0.3, P=0.02). An important difference between the present study and the earlier reports of Gilley et al.17 and Mayeux et al.4 was the current use of patients presenting for acute psychiatric hospitalization for treatment of psychosis, depression, or other behavioral syndromes.19 Thus, when we confined our analysis to DAT patients, we were contrasting our DAT patients with psychotic symptoms to our DAT patients without psychosis but with prominent symptoms of depression, irritability, or aggression. This may have limited our power to detect an association of EPS and psychosis, since agitation and irritability have also been associated with EPS.4,41
Although the specific mechanism underlying the association of EPS with DAT is not known, there is evidence to suggest that degradation of nigrostriatal dopamine pathways is in part responsible. Losses of 30% to 50% of dopaminergic neurons in the substantia nigra, with a 50% reduction of dopamine concentration in the striatum, have been reported in normal aging (for a recent review, see Palmer and DeKosky42). Reductions in dopaminergic function beyond that seen with age alone have also been reported in DAT.43–45 This pattern of degeneration of dopaminergic function parallels the clinical occurrence of EPS, which are more frequent in DAT patients than in normal elderly subjects, and more frequent in normal elderly than in normal young subjects.10,20 A similar association between EPS and decreases in dopaminergic function has been reported within groups of patients diagnosed with DAT. Thus, Kaye et al.47 reported cerebrospinal fluid homovanillic acid (HVA) concentrations in 10 DAT patients with EPS to be 40% of the corresponding values in 27 DAT patients without EPS. A decrease in the CSF concentration of biopterin (an enzyme cofactor for the rate-limiting step of dopamine synthesis) was also found. These differences remained significant after controlling for the effects of age and severity of cognitive impairment. Similarly, we found lower concentrations of plasma HVA to be correlated with increasing severity of parkinsonian rigidity in DAT patients.48
Findings from postmortem studies both support and extend the in vivo data. Antemortem EPS in DAT patients are associated with postmortem evidence of reduction in dopamine concentration, HVA concentration, and number of dopaminergic neurons.34,49,50 Antemortem EPS in DAT patients are also associated with postmortem evidence of cortical and subcortical Lewy bodies.51,52 In the absence of a longitudinal design with neuropathologic confirmation of diagnosis, we cannot establish the frequency of SDLBT among our patients. Nor did we attempt to apply proposed diagnostic criteria for SDLBT6 in an antemortem effort to identify the SDBLT patients among our DAT group. Current estimates of the frequency of cortical and subcortical Lewy bodies in patients receiving an antemortem diagnosis of DAT, however, range from 20% to 30% (for a recent review, see Ellis et al.53), making it likely that a similar proportion of our DAT patients, and a larger percentage of our DAT patients with prominent EPS, would demonstrate Lewy bodies at postmortem exam. Whether Lewy body pathology contributes directly to the risk for EPS or is merely a marker for the associated degeneration of dopamine systems remains to be determined.
Determination of whether the differences in severity of EPS among DAT, MDD, and SCHIZ/DELUS patients observed in the present study also reflect progressive impairments in dopaminergic function requires direct comparison of neurochemical and neuropathological markers of dopaminergic function among these groups. Although a substantial number of studies have examined dopaminergic measures in schizophrenic patients (for review, see Csernansky and Newcomer,54 Kahn and Davis,55 or Friedhoff and Silva56), evaluation of the relationship of these measures to EPS in unmedicated patients or comparisons with MDD and DAT groups are infrequent. A number of studies, however, have examined dopamine receptor density in either schizophrenic or DAT patients in comparison to age-matched normal control subjects. Dopamine receptor densities appear to be elevated in untreated schizophrenic patients,57 whereas reduced receptor densities are reported in DAT patients44—findings consistent with the differences in EPS observed in our patients.
Two small studies have directly compared in vivo dopaminergic markers in DAT and MDD subjects. We found the prolactin response to neuroleptic challenge to be significantly more variable in 7 DAT patients when compared with 4 patients diagnosed with MDD with psychotic features.58 Wolfe et al.59 examined cerebrospinal fluid HVA in 9 patients with DAT, 4 patients with MDD, and 8 patients with idiopathic Parkinson's disease. Mean HVA concentrations were highest in the DAT group, followed in descending order by Parkinson's disease and MDD patients. Interpretation of these reports is limited, however, by the small number of patients studied. This is especially true since HVA is sensitive to both psychomotor retardation (associated with decreased HVA) and psychomotor agitation (associated with increased HVA) in patients with MDD.60
Interestingly, reduced dopaminergic function may also underlie the association between bradykinesia and depressive withdrawal in our sample. Several lines of evidence suggest that impaired dopaminergic neurotransmission may lead to the onset of depressive symptoms and psychomotor slowing.61 In fact, in the report by Wolfe et al.,59 when patients from all diagnostic groups were combined, those with low HVA concentrations demonstrated significantly more EPS, mental slowing, and depression than those with high HVA concentrations.
Several important limitations of the current study need to be addressed. Because of the cross-sectional nature of our study design, it is possible that some percentage of the observed EPS in our patients were actually the early manifestations of another neurologic illness that would become manifest if follow-up evaluations were conducted (for instance, subclinical Parkinson's disease in a patient with MDD). Conversely, it is possible that in some patients EPS occurred secondary to prior neuroleptic use and would dissipate on follow-up examination. Anecdotal reports have suggested that this form of persistent drug-induced parkinsonism may be common in the elderly.62 Our data (the first to address this issue of which we are aware) would argue against the occurrence of this syndrome. We found no association between EPS and either lifetime duration of neuroleptic use or interval since last neuroleptic use in our patients. Thus, if persistent drug-induced parkinsonism does occur, it would not appear to contribute substantially to the variance in EPS rates in the current study.
An unavoidable consequence of limiting our study to patients who were neuroleptic free for at least 3 months prior to admission was that the resultant SCHIZ/DELUS patients were limited in number and had neuroleptic treatment histories that were atypical for patients with these diagnoses. As discussed earlier, generalization from this group must be viewed with caution. It does not appear, however, that inclusion of this group in the analysis unduly influenced the results of the analyses conducted. Our principal findings—the independent association of rigidity with diagnostic group and the associations of bradykinesia with depressive withdrawal and gender—persisted when we repeated the multivariate analyses excluding the SCHIZ/DELUS patients. The magnitude of the significant associations between diagnostic group, depressive withdrawal, and EPS in our patients, however, should not be overstated. Diagnostic group accounts for only 6% of the variance in rigidity scores, while depressive withdrawal explains only 14% of the variance in bradykinesia. Similarly, none of the identified variables was significantly associated with the presence of tremor in our patients. The substantial unexplained variability in the determinants of EPS in elderly neuropsychiatric patients may not be surprising given the clinical and neurobiologic heterogeneity of these disorders. Further clarification of the determinants of EPS across subgroups of elderly neuropsychiatric patients will require investigative strategies that assess concurrently the effects of diagnosis, depressive slowing, and in vivo measures of dopaminergic activity and/or dopamine receptors.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of the clinical and research staff of the Geriatric Clinical Research Unit at Western Psychiatric Institute and Clinic and thank Sharon Hutsko for her assistance in the preparation of the manuscript. Additional statistical consultation was graciously provided by Kim Hung Lo, M.S. This work was supported in part by Grants MH30915 and MH49786 from the National Institute of Mental Health. Dr. Sweet was the recipient of Scientist Development Award for Clinicians MH01153, and Dr. Zubenko was the recipient of Research Career Development Award MH00540. These findings were presented in part at the 9th annual meeting of the American Association for Geriatric Psychiatry, Tucson, AZ, February 16, 1996.
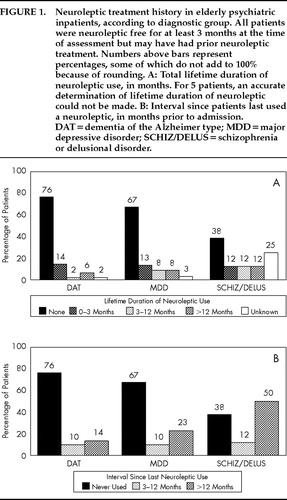
FIGURE 1. Neuroleptic treatment history in elderly psychiatric inpatients, according to diagnostic group. All patients were neuroleptic free for at least 3 months at the time of assessment but may have had prior neuroleptic treatment. Numbers above bars represent percentages, some of which do not add to 100% because of rounding. A: Total lifetime duration of neuroleptic use, in months. For 5 patients, an accurate determination of lifetime duration of neuroleptic could not be made. B: Interval since patients last used a neuroleptic, in months prior to admission. DAT=dementia of the Alzheimer type; MDD=major depressive disorder; SCHIZ/DELUS=schizophrenia or delusional disorder.
 |
 |
 |
1. Adams RD, Victor M: Principles of Neurology. New York, McGraw-Hill, 1989Google Scholar
2. Koller WC, Wilson RS, Glatt SL, et al: Motor signs are infrequent in dementia of the Alzheimer's type. Ann Neurol 1984; 16:514–516Crossref, Medline, Google Scholar
3. Richards M, Marder K, Bell K, et al: Interrater reliability of extrapyramidal signs in a group assessed for dementia. Arch Neurol 1991; 48:1147–1149Crossref, Medline, Google Scholar
4. Mayeux R, Stern Y, Spanton S: Heterogeneity in dementia of the Alzheimer's type: evidence of subgroups. Neurology 1985; 35:453–461Crossref, Medline, Google Scholar
5. Mölsä PK, Marttila RJ, Rinne UK: Extrapyramidal signs in Alzheimer's disease. Neurology 1984; 34:1114–1116Crossref, Medline, Google Scholar
6. McKeith I, Fairbairn A, Perry R, et al: Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992; 305:673–678Crossref, Medline, Google Scholar
7. Stern Y, Mayeux R, Sano M, et al: Predictors of disease course in patients with probable Alzheimer's disease. Neurology 1987; 37:1649–1653Crossref, Medline, Google Scholar
8. Stern Y, Hesdorffer D, Sano M, et al: Measurement and prediction of functional capacity in Alzheimer's disease. Neurology 1990; 40:8–14Crossref, Medline, Google Scholar
9. Ganzini L, Heintz R, Hoffman WF, et al: Acute extrapyramidal syndromes in neuroleptic-treated elders: a pilot study. J Geriatr Psychiatry Neurol 1991; 4:222–225Crossref, Medline, Google Scholar
10. Critchley M: The neurology of old age. Lancet 1931; 1:1221–1230Crossref, Google Scholar
11. Richards M, Stern Y, Mayeux R: Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology 1993; 43:2184–2188Crossref, Medline, Google Scholar
12. Fleminger S: Depressive motor retardation. Int J Geriatr Psychiatry 1991; 6:459–468Crossref, Google Scholar
13. Rogers D, Lees AJ, Smith E, et al: Bradyphrenia in Parkinson's disease and psychomotor retardation in depressive illness: an experimental study. Brain 1987; 110:761–776Crossref, Medline, Google Scholar
14. Hart RP, Kwentus JA: Psychomotor slowing and subcortical-type dysfunction in depression. J Neurol Neurosurg Psychiatry 1987; 50:1263–1266Crossref, Medline, Google Scholar
15. Caligiuri MP, Lohr JB, Panton D, et al: Extrapyramidal motor abnormalities associated with late-life psychosis. Schizophr Bull 1993; 19:747–754Crossref, Medline, Google Scholar
16. Caligiuri MP, Lohr JB, Jeste DV: Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry 1993; 150:1343–1348Crossref, Medline, Google Scholar
17. Gilley DW, Whalen ME, Wilson RS, et al: Hallucinations and associated factors in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 1991; 3:371–376Link, Google Scholar
18. Tariot PN, Mack JL, Patterson MB, et al: The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer's Disease. Am J Psychiatry 1995; 152:1349–1357Crossref, Medline, Google Scholar
19. Zubenko GS, Rosen J, Sweet RA, et al: Impact of psychiatric hospitalization on behavioral complications of Alzheimer's disease. Am J Psychiatry 1992; 149:1484–1491Crossref, Medline, Google Scholar
20. Funkenstein HH, Albert MS, Cook NR, et al: Extrapyramidal signs and other neurologic findings in clinically diagnosed Alzheimer's disease: a community-based study. Arch Neurol 1993; 50:51–56Crossref, Medline, Google Scholar
21. Mulsant BH, Sweet RA, Rifai AH, et al: The use of the Hamilton Rating Scale for Depression in elderly patients with cognitive impairment and physical illness. Am J Geriatr Psychiatry 1994; 2:220–229Crossref, Medline, Google Scholar
22. Sweet RA, Mulsant BH, Gupta B, et al: Duration of neuroleptic treatment and prevalence of tardive dyskinesia in late life. Arch Gen Psychiatry 1995; 52:478–486Crossref, Medline, Google Scholar
23. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised. Washington, DC, American Psychiatric Association, 1987Google Scholar
24. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Res 1962; 10:799–812Google Scholar
25. Hamilton M: Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology 1967; 6:278–296Crossref, Medline, Google Scholar
26. Molloy DW, Alemayehu E, Roberts R: Reliability of a standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am J Psychiatry 1991; 148:102–105Crossref, Medline, Google Scholar
27. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
28. Sweet RA, DeSensi EG, Zubenko GS: Reliability and applicability of movement disorder rating scales in the elderly. J Neuropsychiatry Clin Neurosci 1993; 5:56–60Link, Google Scholar
29. Tune L, Coyle JT: Acute extrapyramidal side effects: serum levels of neuroleptics and anticholinergics. Psychopharmacology 1981; 75:9–15Crossref, Medline, Google Scholar
30. Shihabuddin L, Rapport D: Sertraline and extrapyramidal side effects (letter). Am J Psychiatry 1994; 151:288Medline, Google Scholar
31. SPSS Inc.: SPSS for Windows Release 6.0. Chicago, SPSS Inc., 1993Google Scholar
32. Kischka U, Mandir AS, Ghika J, et al: Electrophysiologic detection of extrapyramidal motor signs in Alzheimer's disease. Neurology 1993; 43:500–505Crossref, Medline, Google Scholar
33. Girling DM, Berrios GE: Extrapyramidal signs, primitive reflexes and frontal lobe function in senile dementia of the Alzheimer type. Br J Psychiatry 1990; 157:888–893Crossref, Medline, Google Scholar
34. Leverenz J, Sumi SM: Parkinson's disease in patients with Alzheimer's disease. Arch Neurol 1986; 43:662–664Crossref, Medline, Google Scholar
35. Herrmann M, Bartels C, Wallesch CW: Depression in acute and chronic aphasia: symptoms, pathoanatomical-clinical correlations and functional implications. J Neurol Neurosurg Psychiatry 1993; 56:672–678Crossref, Medline, Google Scholar
36. Mayeux R, Denaro J, Hemenegildo N, et al: A population-based investigation of Parkinson's disease with and without dementia. Arch Neurol 1992; 49:492–497Crossref, Medline, Google Scholar
37. Drago F, Scapagnini U: Hormonal modulation of central dopaminergic transmission. J Neural Transm 1986; 22(suppl):47–54Google Scholar
38. Raymond V, Beaulieu M, Labrie F: Potent antidopaminergic activity of estradiol at the pituitary level on prolactin release. Science 1978; 200:1173–1175Crossref, Medline, Google Scholar
39. Schaeffer JM, Hsueh AJW: 2-Hydroxyestradiol interaction with dopamine receptor binding in rat anterior pituitary. J Biol Chem 1979; 254:5606–5608Medline, Google Scholar
40. Koller WC, Weiner WJ, Klawans HL, et al: Influence of female sex hormones on neuroleptic-induced behavioral supersensitivity. Neuropharmacology 1980; 19:387–391Crossref, Medline, Google Scholar
41. Gilley DW, Wilson RS, Bennett DA, et al: Predictors of behavioral disturbance in Alzheimer's disease. J Gerontol 1991; 46:P362–P371Google Scholar
42. Palmer AM, DeKosky ST: Monamine neurons in aging and Alzheimer's disease. J Neural Transm 1993; 91:135–159Crossref, Medline, Google Scholar
43. Palmer AM, Bowen DM: 5-Hydroxyindoleacetic acid and homovanillic acid in the cerebrospinal fluid and caudate nucleus of histologically verified examples of Alzheimer's disease. Biochem Soc Trans 1995; 13:167–168Google Scholar
44. Reisine TD, Yamamura HI, Bird ED, et al: Pre- and postsynaptic neurochemical alterations in Alzheimer's disease. Brain Res 1978; 159:477–481Crossref, Medline, Google Scholar
45. Cross AJ, Crow TJ, Ferrier IN, et al: Striatal dopamine receptors in Alzheimer-type dementia. Neurosci Lett 1995; 52:1–6Crossref, Google Scholar
46. See reference 10 aboveGoogle Scholar
47. Kaye JA, May C, Daly E, et al: Cerebrospinal fluid monoamine markers are decreased in dementia of the Alzheimer type with extrapyramidal features. Neurology 1988; 38:554–557Crossref, Medline, Google Scholar
48. Sweet RA, Pollock BG, Mulsant BH, et al: Association of plasma homovanillate with behavioral symptoms in patients diagnosed with dementia: a preliminary report. Biol Psychiatry 1997; 42:1016–1023Crossref, Medline, Google Scholar
49. Perry EK, Marshall E, Perry RH, et al: Cholinergic and dopaminergic activities in senile dementia of Lewy body type. Alzheimer Dis Assoc Disord 1990; 4:87–95Crossref, Medline, Google Scholar
50. Langlais PJ, Thal L, Hansen L, et al: Neurotransmitters in basal ganglia and cortex of Alzheimer's disease with and without Lewy bodies. Neurology 1993; 43:1927–1934Crossref, Medline, Google Scholar
51. Hansen L, Salmon D, Galasko D, et al: The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology 1990; 40:1–8Crossref, Medline, Google Scholar
52. McKeith IG, Fairbairn AF, Bothwell RA, et al: An evaluation of the predictive validity and inter-rater reliability of clinical diagnostic criteria for senile dementia of Lewy body type. Neurology 1994; 44:872–877Crossref, Medline, Google Scholar
53. Ellis RJ, Caligiuri M, Galasko D, et al: Extrapyramidal motor signs in clinically diagnosed Alzheimer disease. Alzheimer Dis Assoc Disord 1996; 10:103–114Crossref, Medline, Google Scholar
54. Csernansky JG, Newcomer JW: Are there neurochemical indicators of risk for schizophrenia? Schizophr Bull 1994; 20:75–88Google Scholar
55. Kahn RS, Davis KL: New developments in dopamine and schizophrenia, in Psychopharmacology: The Fourth Generation of Progress, edited by Bloom FE, Kupfer DJ. New York, Raven, 1995, pp 1193–1203Google Scholar
56. Friedhoff AJ, Silva RR: The effects of neuroleptics on plasma homovanillic acid, in Psychopharmacology: The Fourth Generation of Progress, edited by Bloom FE, Kupfer DJ. New York, Raven, 1995, pp 1229–1233Google Scholar
57. Seeman P: Dopamine receptors in human brain diseases, in Dopamine Receptors: Receptor Biochemistry and Methodology, edited by Creese J, Fraser CM. New York, Alan R. Liss, 1987, pp 233–245Google Scholar
58. Sweet RA, Pollock BG, Mulsant BH, et al: Prolactin response to neuroleptic challenge in late-life psychosis. Psychopharmacol Bull 1995; 31:651–657Medline, Google Scholar
59. Wolfe N, Katz DI, Albert ML, et al: Neuropsychological profile linked to low dopamine: in Alzheimer's disease, major depression, and Parkinson's disease. J Neurol Neurosurg Psychiatry 1990; 53:915–917Crossref, Medline, Google Scholar
60. Wilner P: Dopaminergic mechanisms in depression and mania, in Psychopharmacology: The Fourth Generation of Progress, edited by Bloom FE, Kupfer DJ. New York, Raven, 1995, pp 921–931Google Scholar
61. Mann JJ, Kapur S: A dopaminergic hypothesis of major depression. Clin Neuropharmacol 1995; 18:S57–S65Google Scholar
62. Weiner WJ, Lang AE: Movement Disorders: A Comprehensive Survey. Mount Kisco, NY, Futura, 1989Google Scholar



