Factors Associated With New-Onset Depression After Stroke
Abstract
To better identify stroke survivors at risk for depression who may benefit from early prevention through targeted strategies in the acute-subacute poststroke period, we examined 118 Framingham Heart Study stroke survivors with longitudinal prestroke depression assessments. Among those who developed poststroke depression, most lacked a history of depressive symptoms 5 years prior to their stroke. Sex (female), advanced age, and prestroke factors (smoking and functional dependence) were associated with new-onset depression poststroke. These findings suggest fully characterizing and accounting for prestroke factors, including psychosocial and behavioral determinants, may inform the predictive modeling needed to determine whether targeted preventive trials early in stroke recovery will improve stroke outcomes.
Poststroke depression has been increasingly recognized as one of the most important consequences of stroke, as depression is common and often recurrent,1–3 associated with poor outcomes,4 costly,5,6 and difficult to treat given currently available treatments.7 The impact of poststroke depression is perhaps most pronounced as a barrier to recovery of functional status,8 independence,9 and long term survival after hospital discharge.10–12 Although antidepressants are now more frequently prescribed after stroke, there has been no clear decrease in the prevalence of poststroke depression.2,7 Because the effectiveness of antidepressants and other interventions treating and resolving poststroke depression appear limited, prevention has gained much more interest. Accumulating evidence suggests that long term reductions in depressive symptoms are best achieved through targeting individuals prior to the onset of their symptoms, rather than after the disorder emerges.13 However, to determine whether early treatments such as antidepressants can prevent stroke-related depression, it is critical to predict which patients are most likely to develop poststroke depression at the time of stroke. We undertook this study to better identify these patients of higher susceptibility.
In order to prevent cases of poststroke depression by identifying patients before the onset of depression, there is a need to understand new-onset depression poststroke and the prestroke risk factors that predict its development. New-onset depression poststroke is a distinct and important case of poststroke depression, as it may help clarify the influence of risk factors for depression unique to stroke. Using the combination of prestroke factors and cases of new-onset depression poststroke may help identify those cases where an early empiric antidepressant after stroke or a behavioral intervention may prevent the development of poststroke depression.14 Candidate risk and protective factors linked to poststroke depression have been proposed, though extant studies are limited in three primary ways. First, prior studies are typically confined to clinical and convenience samples where information about prestroke factors is often limited. Second, previous research has largely focused on poststroke predictors [for examples, see1,4] rather than on risk factors present prior to the stroke. Third, studies examining prestroke measures (including depressive symptoms) do so in a retrospective manner, which is less reliable than prospective ascertainment. Thus, to better identify stroke survivors at risk for depression who may benefit from a targeted prevention strategy in the acute to subacute poststroke period, we examined whether prestroke factors were associated with the risk for developing new-onset depression poststroke.
Methods
Study Sample
We analyzed data from respondents in the Framingham Heart Study (FHS), one of the longest running and most closely monitored observational community-based cohorts in the United States. (For additional details about FHS, see prior publications.15) We focused on a subset of 832 participants from the Original and Offspring cohorts who experienced a first stroke between 1981 and 2003 and had information specifically regarding depressive symptoms or antidepressant medication use within 5 years before and after their incident stroke (Figure 1). Prestroke risk factors were obtained from the most recent examination within the 5-year period preceding incident stroke. Written informed consent was obtained from all participants. The Institutional Review Board of Boston University Medical Center approved the consent form and original study design.
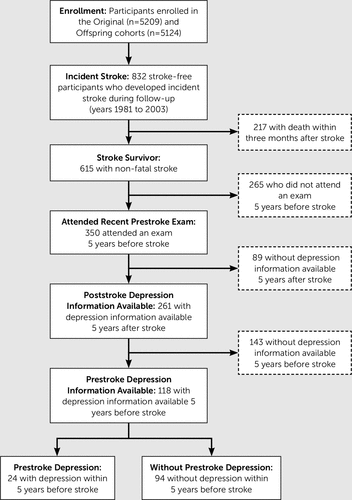
FIGURE 1. Flow of Participants Through Derivation of the Analytic Group.
Measures
Stroke assessment.
Full details regarding the FHS stroke surveillance protocol, including diagnosis, classification, and assessment of severity have been published previously.16 We examined three features of adjudicated incident strokes: type, severity, and laterality. Hemorrhagic strokes were a minority of incident strokes, including subarachnoid hemorrhage. Ischemic or hemorrhagic stroke severity was classified by the neurological deficits found on examination during the acute presentation of stroke and was further classified into four categories: none (no deficit), mild (deficit present in visual, motor, sensory, or language domains, but without functional impairment), moderate (deficit requiring assistance in one of the domains mentioned above), and severe (deficit requiring assistance in at least two domains). Stroke laterality was classified as either right or left cerebral hemispheric involvement. Additional information regarding stroke size and neuroanatomic location were not available in this cohort. All participants analyzed survived at least 3 months after stroke for a first poststroke visit.
Poststroke depression.
Consistent with prior studies,11,17–19 we defined depression using two data sources. The first was using the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item screening tool capturing levels of depressive symptoms.20 Participants were asked to report how often in the past week they experienced each symptom; response options ranged from 0, “rarely or none of the time (less than 1 day),” to 3, “most or all of the time (5–7 days).” Total CES-D scores ranged from 0 to 60, with higher scores reflecting greater depressive symptoms. Consistent with CES-D guidelines20 and following other studies,21 we used total scores greater than or equal to 16 to indicate high depressive symptomatology. Although not a diagnostic tool, this cutoff has been shown in prior studies of stroke patients to distinguish those with and without a DSM-IV diagnosis of major depressive disorder (sensitivity=.75; specificity=.88).21
The second data source was initiation of antidepressant medication. Participants were asked to bring all current medications in their original pill bottles to follow-up clinic visits where staff would record all label information, including names of medications used regularly (more than 2 weeks), dose, and duration of use (used by FHS to determine initiation of “treatment for depression”). Medications classified as antidepressants were selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclics, and modified cyclic agents. These antidepressants are often prescribed to treat depression, though they are also used to treat other psychiatric disorders that can co-occur with depression (e.g. generalized anxiety) and can be used to treat other conditions such as migraine.
Using these two data sources, we defined prestroke depression as the presence of either high CES-D scores or antidepressant medication use in the 5-year period before incident stroke and poststroke depression as the presence of either high CES-D scores or antidepressant medication use in the 5-year period after incident stroke. Those without prestroke depression who went on to develop poststroke depression were defined as new-onset depression cases. By defining “depression” cases based on both the CES-D and antidepressant medication use, we were able to minimize the proportion of potential false negative cases and obtain a reasonable proxy for experiences of depression.
Although first onset of depression can occur throughout the life course with higher risk in early adulthood, our case definition using these measures serves as the best available proxy for a diagnosis of depression in absence of the diagnostic gold standard (i.e. a psychiatrist-administered semistructured clinical interview using the criteria set forth by DSM-IV). Since depression can be recurrent, measuring depressive symptoms within the 5 years prior to stroke is likely a large enough time period to capture those with more chronic depression.
Exposures.
We examined candidate risk factors in four domains, selected based on prior associations with poststroke depression,4 and assessed at the most recent examination within 5 years prior to stroke onset. Demographic factors: sex, age (continuous), and education (college degree). Health behavior: smoking status (smoked cigarettes regularly in last year? 0=no; 1=yes).4,22Clinical factors, assessed through physical exams, medical records, and diagnostic studies, were stroke laterality and severity in addition to prestroke prevalent cardiovascular disease, systolic blood pressure, diabetes, body mass index (BMI), and total serum cholesterol.4Functional capacity: dependence in bathing, transferring,23 and walking a half mile24 (dichotomized as either dependent [0=no, unable to do independently; 2=yes, but with human assistance] or independent [1=yes, able to do independently]).4
Statistical analysis.
We conducted univariate analyses to describe the distribution of all study variables in this sample. We then ran bivariate analyses to examine the differences between those with and without prestroke depression using either Wilcoxon Rank Sum (two groups) or Kruskal-Wallis (N groups) tests for nonparametric variables, and t-test (two groups) or one-way analysis of variance (N groups) for normally distributed variables. Fisher’s exact test or the chi-square test was used for categorical variables. Given the number of bivariate comparisons conducted, statistical significance for bivariate analyses was set to a two-sided α≤.003 after Bonferroni correction. We then used multiple logistic regression to examine the associations between each predictor and new-onset depression poststroke. Models were adjusted for age, sex, and time from prestroke exam to stroke in order to adequately maximize stability of each model for the available sample size. Statistical significance for regression analysis was set at a two-sided α≤.05. All analyses were conducted in SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Among the participants for whom prestroke assessment of depressive symptoms was available within 5 years before and after stroke, 94/118 (80%) did not have recent history of depressive symptoms before stroke. The mean (SD) times from prestroke CES-D assessment to stroke and from stroke to poststroke CES-D were 2.1 (1.4) years and 1.9 (1.3) years, respectively. The mean (SD) times from prestroke medication inventory to stroke and from stroke to poststroke medication inventory were 1.9 (1.5) years and 2.2 (1.4) years, respectively. There were no significant differences between participants who did not have depression information available within 5 years after stroke compared with those that did. Poststroke depression of any type (i.e. including those with previous history of depression) was present in 49/118 (42%) where 11 were on antidepressant medication and had CES-D≥16, 28 were not on antidepressant medication but had a CES-D≥16, and 10 were on antidepressant medication and had CES-D<16. Of these cases, a majority (34/49, 69%) did not have recent history of depressive symptoms 5 years prior to stroke and thus were classified as having new-onset depression poststroke. Among the 34 participants with new-onset poststroke depression, nine were on antidepressant medication and had CES-D≥16, 18 were not on antidepressant medication but had CES-D≥16, and seven were on antidepressant medication and had CES-D<16). At-risk participants in the primary analytic sample who developed new-onset depression after stroke (34/94, 36%), compared with those who did not, were more likely to be older, women, current smokers, and dependent in activities of daily living (Table 1).
| Characteristic | Total Cohort | Absence of Poststroke Depression | Poststroke Depression | p value |
|---|---|---|---|---|
| Total no. | 94 | 60 | 34 | |
| Age at time of stroke, mean±SD, years | 78.1±9.4 | 76.1±9.4 | 81.6±8.4 | .005 |
| Women | 48 (51) | 24 (40) | 24 (71) | .004 |
| College degree | 25 (27) | 18 (31) | 7 (21) | NS |
| Current smoker | 8 (9) | 2 (3) | 6 (18) | .024 |
| BMI, median [Q1, Q3], kg/m2 | 26.7 [24.2, 30.3] | 26.4 [24.1,30.1] | 27.7 [24.9,32.1] | NS |
| Cardiovascular disease | 29 (31) | 17 (28) | 12 (35) | NS |
| Diabetes mellitus | 19 (25) | 15 (27) | 4 (18) | NS |
| SBP, mean±SD, mmHg | 144.1±2.2 | 142.5±2.2 | 146.9±2.3 | NS |
| Total cholesterol, mean±SD, mg/dL | 196.7±4.2 | 193.7±4.1 | 202.8±4.4 | NS |
| Dependent bathing | 13 (14) | 3 (5) | 10 (29) | .002 |
| Dependent transferring | 12 (13) | 4 (7) | 8 (24) | .026 |
| Dependent ambulation for half mile | 23 (25) | 10 (17) | 13 (39) | .017 |
| Stroke severity | NS | |||
| No deficit | 8 (8) | 7 (12) | 1 (3) | |
| Mild | 58 (62) | 38 (63) | 20 (59) | |
| Moderate | 18 (19) | 11 (18) | 7 (21) | |
| Severe | 10 (11) | 4 (7) | 6 (18) | |
| Stroke type | ||||
| Ischemic | 86 (91) | 53 (88) | 33 (97) | NS |
| Stroke laterality | NS | |||
| Left | 43 (49) | 27 (49) | 16 (48) | |
| Right | 45 (51) | 28 (51) | 17 (52) |
TABLE 1. Baseline Characteristics of 94 Respondents With Incident Stroke, Depressive Information Available 5 Years Before and After Stroke, and Absence of Prestroke Depressiona
The odds of developing new-onset depression poststroke was 1.08 greater for each 1-year difference in age at the time of stroke (95% CI, 1.02–1.15; df=1; p=.009) (Table 2). Controlling for age only, women had a 3.30-fold increase in the odds of experiencing new-onset depression poststroke compared with men (95% CI, 1.30–8.37; df=2; p=.012). After controlling for age, sex, and time from prestroke assessment to date of incident stroke, we found that smokers had 8.87 times the odds of developing new-onset depression poststroke compared with nonsmokers (95% CI, 1.19–66.11; df=3; p=.033) and dependent bathing prior to stroke onset had an OR of 4.65 (95% CI, 1.00–21.69; df=3; p=.050).
| Individual Models | |||
|---|---|---|---|
| 34/94 (Cases/N) | p value | ||
| Odds Ratio (95% CI) | |||
| Demographic factors | |||
| Age at stroke | 1.08 | (1.02–1.15) | .009 |
| Womena | 3.30 | (1.30–8.37) | .012 |
| College degreeb | 1.17 | (.37–3.73) | NS |
| Prestroke risk factorsc | |||
| Cardiovascular disease | 1.74 | (.58–5.26) | NS |
| Systolic blood pressure | 1.10 | (.87–1.39) | NS |
| Total cholesterol | 1.10 | (.95–1.27) | NS |
| Diabetes mellitus | .51 | (.14–1.88) | NS |
| Current smoking | 8.87 | (1.19–66.11) | .033 |
| Body mass index | 1.08 | (.96–1.20) | NS |
| Dependent bathing | 4.65 | (1.00–21.69) | .050 |
| Dependent transferring | 2.30 | (.55–9.66) | NS |
| Dependent ambulation for half mile | 1.40 | (.44–4.45) | NS |
| Stroke severity | 1.35 | (.73–2.47) | NS |
| Stroke location (left versus right) | .83 | (.32–2.18) | NS |
TABLE 2. Association Between Prestroke Risk Factors and 5-Year New-Onset Depression Poststroke
In post hoc sensitivity analyses, we defined new-onset poststroke depression cases solely by CES-D≥16 (i.e. the seven participants that were on antidepressant medication and had CES-D<16 were no longer classified as cases). Advanced age (OR 1.15; 95% CI, 1.06–1.24; df=3; p=.001) and prestroke smoking (OR 13.95; 95% CI, 1.60–121.39; df=3; p=.017) remained associated. Odds of developing new-onset depression poststroke was 1.15 greater for each one-point difference in BMI (95% CI, 1.01–1.31; df=3; p=.041) (Table 3). We also examined the association between sex and new-onset poststroke depression in one model additionally adjusted for age and smoking and a second model additionally adjusted for age and dependent bathing. Adjustment for smoking had a minor impact on effect with a change in OR from 3.30 (95% CI, 1.30–8.37; df=4; p=.012) to 2.99 (95% CI, 1.10–8.05; df=4; p=.032). Adjustment for dependent bathing, reduced the OR to 2.36 (95% CI, 0.84–6.65; p=.104). The overall effect of dependent bathing in women (N=48) was a 9.82-fold increase in the odds of developing new-onset poststroke depression (95% CI, 1.24–77.51; df=4; p=.030).
| Individual Models | |||
|---|---|---|---|
| 27/94 (Cases/N) | p value | ||
| Odds Ratio (95% CI) | |||
| Demographic factors | |||
| Age at stroke | 1.15 | (1.06–1.24) | .001 |
| Womena | 2.61 | (.94–7.27) | NS |
| College degreeb | 1.22 | (.33–4.47) | NS |
| Prestroke risk factorsc | |||
| Cardiovascular disease | 1.27 | (.42–3.89) | NS |
| Systolic blood pressure | 1.10 | (.86–1.41) | NS |
| Total cholesterol | 1.09 | (.93–1.27) | NS |
| Diabetes mellitus | 1.01 | (.26–3.92) | NS |
| Current smoking | 13.95 | (1.60–121.39) | .017 |
| Body mass index | 1.15 | (1.01–1.31) | .041 |
| Dependent bathing | 3.63 | (.81–16.22) | NS |
| Dependent transferring | 3.25 | (.79–13.41) | NS |
| Dependent ambulation for half mile | 1.82 | (.95–3.59) | NS |
| Stroke severity | 1.84 | (.95–3.59) | NS |
| Stroke location (left versus right) | .91 | (.32–2.60) | NS |
TABLE 3. Association Between Prestroke Risk Factors and 5-Year New-Onset Depression Poststroke Defined by Center for Epidemiologic Studies Depression Scale
Discussion
Our study demonstrated that among those who developed poststroke depression, the majority did not have depression 5 years prior to their stroke (34/49, 69%); however, the risk of poststroke depression is still higher in those with prestroke depression (15/24, 63%) than in those with no prestroke depression (34/94, 36%). We found that women, older adults, smokers, and those with some dependence in activities of daily living at the time of stroke were more likely to develop a first episode of depression poststroke. The results of post hoc sensitivity analyses add confidence to identified associations with advanced age and smoking and suggest that women may be more likely to become depressed poststroke if they have functional impairment before the stroke.
Prior studies in mostly hospital- and rehabilitation-based registries have identified advanced age3,4 at the time of stroke as associated with poststroke depression. Consistent with the established association between women and risk of depression, our findings suggest women are at higher odds of new-onset depression poststroke. This finding, however, is at odds with prior studies of poststroke depression, which have shown either no differences in risk by sex, or higher risk among men.25,26 Moreover, the greater odds of new-onset depression poststroke among smokers may be explained by the proposed pathophysiologic models for poststroke depression from previous associations implicating chronic inflammatory cytokines and poststroke neuroinflammatory response27 as well as impaired endothelial function28 and changes in vascular perfusion.22 This finding differs from one previous study that did not detect an association with smoking status29 though may be supported by the association with obesity in post hoc sensitivity analysis. The absence of associations with cardiovascular disease, hypertension, and diabetes mellitus may be related to either small effect sizes or a more direct pathophysiologic role of white matter microstructural damage, which has been more specifically associated with apathy as a contributing factor in the development of poststroke depression.30 The association with poststroke impairments in activities of daily living has been demonstrated,1 though our results indicate that prestroke functional status may also be an important factor to consider as it implies a potential relationship between greater cumulative years of functional disability and new-onset depression poststroke.
In our prospective study of participants followed at least 5 years prior to their stroke, the occurrence of poststroke depression and new-onset depression poststroke (42% and 29%, respectively) parallels previous poststroke depression estimates of about 33% in the first 3 months after stroke and suggests that the actual incidence of new-onset depression poststroke is greater than 30%.1,2,7 Clinical and convenience samples have reported more disparate incidence and prevalence estimates for poststroke depression. However, while the burden of poststroke depression has wide variation in its estimates, precise estimates of new-onset depression poststroke are even more challenging to discern.6,31 We suspect wider estimates from other studies may reflect selection bias (by including only the most disabled or least disabled stroke survivors), particularly in rehabilitation-based studies.32 This is likely due to inherent clinical challenges in detecting poststroke depression, known classification challenges in measuring psychiatric symptoms using retrospective reports, and the ability to distinguish new-onset depression poststroke from relapse of longstanding major depressive disorder.5 Our study overcomes this limitation by using a prospective cohort with minimal loss to follow-up, which also made it possible to uncover that 69% of poststroke depression occurred in those without a recent history of depressive symptoms before stroke.
Strengths of this study include its longitudinal design, large incident stroke sample, and the close surveillance of clinical end points with minimal loss to follow-up. Results, however, need to be interpreted in the context of limitations. First, we were unable to account for the potential effects of additional factors, such as family history of depression or stroke heterogeneity in size, etiology, and neuroanatomical involvement. Second, generalizability of results may be limited, given that these analyses were conducted in a sample of largely European ancestry. Third, an even larger study of stroke survivors who were free from stroke and depression at baseline may generate more precise effect estimates, especially when there is potential for nondifferential misclassification from a tendency to underdiagnose poststroke depression.5 The FHS mitigates some of these effects with its frequency of assessments.
Conclusions
Poststroke depression is common, particularly among those without a recent history of depressive symptoms prior to the stroke. Our results demonstrate that prestroke characteristics are associated with increased risk for a new episode of depression following stroke. Given that poststroke depression is linked with substantial morbidity and is resistant to treatment once it develops, these factors may help identify patients early in the course of stroke recovery who are at risk for depression as a crucial first step to design and test strategies to prevent poststroke depression.
1 : Natural history, predictors, and associations of depression 5 years after stroke: the South London Stroke Register. Stroke 2011; 42:1907–1911Crossref, Medline, Google Scholar
2 : Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 2014; 9:1017–1025Crossref, Medline, Google Scholar
3 : The natural history of depression up to 15 years after stroke: the South London Stroke Register. Stroke 2013; 44:1105–1110Crossref, Medline, Google Scholar
4 : Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke 2014; 9:1026–1036Crossref, Medline, Google Scholar
5 : Poststroke depression: a review. Can J Psychiatry 2010; 55:341–349Crossref, Medline, Google Scholar
6 : Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002; 52:253–264Crossref, Medline, Google Scholar
7 : Frequency of depression after stroke: a systematic review of observational studies. Stroke 2005; 36:1330–1340Crossref, Medline, Google Scholar
8 : Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 2011; 10:123–130Crossref, Medline, Google Scholar
9 Chollet F, Acket B, Raposo N, Albucher JF, Loubinoux I, Pariente J. Use of antidepressant medications to improve outcomes after stroke. Curr Neurol Neurosci Rep. 2013;13:318–324. Available at doi: 10.1007/s11910-012-0318-z.Google Scholar
10 : Prestroke factors associated with poststroke mortality and recovery in older women in the Women’s Health Initiative. J Am Geriatr Soc 2013; 61:1324–1330Crossref, Medline, Google Scholar
11 : Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med 2009; 169:2128–2139Crossref, Medline, Google Scholar
12 : Depression and cardiovascular sequelae in postmenopausal women. Arch Intern Med 2004; 164:289–298Crossref, Medline, Google Scholar
13 : Preventing depression: a global priority. JAMA 2012; 307:1033–1034Crossref, Medline, Google Scholar
14 : Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 1994; 25:1099–1104Crossref, Medline, Google Scholar
15 : Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 1951; 41:279–281Crossref, Medline, Google Scholar
16 : Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 2006; 296:2939–2946Crossref, Medline, Google Scholar
17 : Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (Systolic Hypertension in the Elderly). Arch Intern Med 1996; 156:553–561Crossref, Medline, Google Scholar
18 : Vitamin D supplementation and depression in the women’s health initiative calcium and vitamin D trial. Am J Epidemiol 2012; 176:1–13Crossref, Medline, Google Scholar
19 : Omega-3 fatty acid biomarkers and subsequent depressive symptoms. Int J Geriatr Psychiatry 2014; 29:747–757Crossref, Medline, Google Scholar
20 : The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401Crossref, Google Scholar
21 : Screening for poststroke major depression: a meta-analysis of diagnostic validity studies. J Neurol Neurosurg Psychiatry 2014; 85:198–206Crossref, Medline, Google Scholar
22 : “Vascular depression” hypothesis. Arch Gen Psychiatry 1997; 54:915–922Crossref, Medline, Google Scholar
23 : Studies of Illness in the Aged. The Index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963; 185:914–919Crossref, Medline, Google Scholar
24 : A Guttman health scale for the aged. J Gerontol 1966; 21:556–559Crossref, Medline, Google Scholar
25 : Poststroke depression: an 18-month follow-up. Stroke 2003; 34:138–143Crossref, Medline, Google Scholar
26 : Poststroke depression and functional outcome: a critical review of literature. Heart Lung 2009; 38:151–162Crossref, Medline, Google Scholar
27 : Inflammatory and neuroendocrine biomarkers of prognosis after ischemic stroke. Expert Rev Neurother 2011; 11:225–239Crossref, Medline, Google Scholar
28 : Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol 2010; 67:41–52Crossref, Medline, Google Scholar
29 : Predictors of depressive symptoms in patients with stroke: a three-month follow-up. Neurol Neurochir Pol 2010; 44:13–20Crossref, Medline, Google Scholar
30 : Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 2015; 138:3803–3815Crossref, Medline, Google Scholar
31 : What are the important factors in health-related quality of life for people with aphasia? A systematic review. Arch Phys Med Rehabil 2012; 93(Suppl):S86–S95Crossref, Medline, Google Scholar
32 : The Sunnybrook Stroke Study: a prospective study of depressive symptoms and functional outcome. Stroke 1998; 29:618–624Crossref, Medline, Google Scholar



