Association of Cortical and Subcortical β-Amyloid With Standardized Measures of Depressive and Anxiety Symptoms in Adults Without Dementia
Abstract
Objective:
The purpose of this study was to test the hypothesis that subcortical β-amyloid (Aβ) deposition was associated with elevated scores on standardized measures of depressive and anxiety symptoms when compared with cortical (Aβ) deposition in persons without dementia.
Methods:
The authors performed a cross-sectional study, derived from the population-based Mayo Clinic Study of Aging, comprising participants aged ≥70 years (N=1,022; 55% males; 28% apolipoprotein E [APOE] ε4 carriers; without cognitive impairment, N=842; mild cognitive impairment; N=180). To assess Aβ deposition in cortical and subcortical (the amygdala, striatum, and thalamus) regions, participants underwent Pittsburgh Compound B positron emission tomography (PiB-PET) and completed the Beck Depression Inventory-II (BDI-II) and the Beck Anxiety Inventory (BAI). The investigators ran linear regression models to examine the association between PiB-PET standardized uptake value ratios (SUVRs) in the neocortex and subcortical regions and depressive and anxiety symptoms (BDI-II and BAI total scores). Models were adjusted for age, sex, education level, and APOE ε4 carrier status and stratified by cognitive status (without cognitive impairment, mild cognitive impairment).
Results:
Cortical PiB-PET SUVRs were associated with depressive symptoms (β=0.57 [SE=0.13], p<0.001) and anxiety symptoms (β=0.34 [SE=0.13], p=0.011). PiB-PET SUVRs in the amygdala were associated only with depressive symptoms (β=0.80 [SE=0.26], p=0.002). PiB-PET SUVRs in the striatum and thalamus were associated with depressive symptoms (striatum: β=0.69 [SE=0.18], p<0.001; thalamus: β=0.61 [SE=0.24], p=0.011) and anxiety symptoms (striatum: β=0.56 [SE=0.18], p=0.002; thalamus: β=0.65 [SE=0.24], p=0.008). In the mild cognitive impairment subsample, Aβ deposition, regardless of neuroanatomic location, was associated with depressive symptoms but not anxiety symptoms.
Conclusions:
Elevated amyloid deposition in cortical and subcortical brain regions was associated with higher depressive and anxiety symptoms, although these findings did not significantly differ by cortical versus subcortical Aβ deposition. This cross-sectional observation needs to be confirmed by a longitudinal study.
Neuropsychiatric symptoms are widespread among individuals with mild cognitive impairment and dementia (1–4), with anxiety and depression being two of the most common neuropsychiatric symptoms in old age (5). Neuropsychiatric symptoms are a risk factor for cognitive decline, even among persons without cognitive impairment, and are associated with an increased risk of dementia among persons with mild cognitive impairment (6–9).
Neuropsychiatric symptoms have also been associated with neuroimaging biomarker abnormalities in the context of brain aging. We, as well as other investigators, have reported associations between neuropsychiatric symptoms and abnormal β-amyloid (Aβ) deposition in persons without cognitive impairment (10–14), as well as in individuals with mild cognitive impairment and dementia (10–12, 15, 16). A regional progression pattern of amyloid deposition has been reported (17, 18); however, to date, it is unclear whether amyloid pathology in various brain regions is associated with emotional behavior. A recent study based on a convenience sample of older adults without cognitive impairment may have been the first to report that the association between anxiety symptoms and Aβ burden may vary by the neuroanatomic location of the amyloidosis (i.e., cortical versus subcortical Aβ deposition), with the striatum, thalamus, and amygdala included as subcortical structures in the analysis (19). Furthermore, given the well-established association between the amygdala and emotion (20–22), there may indeed be merit in investigating different subcortical structures, such as the amygdala, striatum, and thalamus, separately.
Therefore, the aim of the present study was to examine the association between cortical Aβ deposition and depressive and anxiety symptoms, and between Aβ deposition in the amygdala, striatum, and thalamus and depressive and anxiety symptoms. All analyses were conducted among community-dwelling older adults without dementia; we also carried out analyses stratified by cognitive status (i.e., individuals without cognitive impairment and persons with mild cognitive impairment). On the basis of the observation made by the Harvard Aging Brain Study (19), we hypothesized that amyloid deposition would be associated with elevated scores on standardized measures of depressive and anxiety symptoms and that this association would be particularly evident in subcortical structures, which may indicate more advanced amyloidosis beyond the cortex and may be regarded as a marker of pathologic progression.
Methods
Study Design and Sample
We conducted a cross-sectional study derived from the ongoing population-based Mayo Clinic Study of Aging in Olmsted County, Minnesota. We included participants without dementia (without cognitive impairment, N=842; with mild cognitive impairment, N=180) aged ≥70 years who underwent neurological evaluation, risk factor ascertainment, and neuropsychological testing, as well as Pittsburgh Compound B positron emission tomography (PiB-PET), to assess Aβ deposition in cortical and subcortical regions (i.e., the amygdala, striatum, and thalamus). Patient data were collected from January 2006 through October 2018.
Standard Protocol Approvals, Registrations, and Patient Consent
The Mayo Clinic Study of Aging was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center. All participants provided written informed consent.
Neurocognitive Evaluation
Participants underwent face-to-face evaluation, including a neurological examination, a study coordinator visit, and neuropsychological testing. Details on the face-to-face evaluation are described elsewhere (23). Briefly, the neurological evaluation comprised a neurological history review, administration of the Short Test of Mental Status (24), and a neurological examination. The study coordinator visit included assessment with the Clinical Dementia Rating Scale (25). Neuropsychological testing was administered by a psychometrist who was supervised by a board-certified neuropsychologist in order to assess performance in the following four cognitive domains: memory (delayed recall trials from the Auditory Verbal Learning Test [26]; the Wechsler Memory Scale-Revised [27] logical memory and visual reproduction subtests); language (the Boston Naming Test [28], category fluency subtests [29]); visuospatial skills (the Wechsler Adult Intelligence Scale-Revised [30], picture completion and block design subtests); and attention/executive function (Trail-Making Test, Part B [31]; the Wechsler Adult Intelligence Scale-Revised [30], digit symbol substitution subtest). An expert consensus panel consisting of physicians, study coordinators, and neuropsychologists reviewed the results for each participant and determined whether a participant was without cognitive impairment (on the basis of normative data developed in this community [32]) or had mild cognitive impairment based on published criteria (33, 34).
PiB-PET Acquisition
We performed amyloid PET imaging using the Pittsburgh Compound B tracer (11[C]-PiB). Details on PiB-PET imaging in the Mayo Clinic Study of Aging are described elsewhere (35, 36). Briefly, PiB scans, consisting of four 5-minute dynamic frames, were acquired from 40 to 60 minutes after intravenous injection with 292–728 MBq of 11[C]-PiB. Images were analyzed using an in-house fully automated image-processing pipeline in which image voxel values were extracted from automatically labeled regions of interest propagated from regions defined on each participant’s own MRI. A global amyloid PET standardized uptake value ratio (SUVR) was measured in cortical (i.e., prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus) and subcortical (i.e., the amygdala, striatum, and thalamus) regions and normalized to the cerebellar gray matter.
Assessment of Neuropsychiatric Symptoms
Neuropsychiatric symptoms were assessed using the Beck Depression Inventory (BDI-II) (37) and the Beck Anxiety Inventory (BAI) (38). The BDI-II measures common depressive symptoms, such as feelings of guilt or loss of interest, over the past 2 weeks. The BAI measures common anxiety symptoms, such as nervousness or fear of losing control, over the past week. Both inventories are validated and comprise 21 items. The severity of each item is rated on a Likert-type scale ranging from 0 to 3, with a total score ranging from 0 to 63. A higher score indicates higher severity of depressive and anxiety symptoms, respectively.
Statistical Analysis
We compared baseline characteristics between participants without cognitive impairment and those with mild cognitive impairment using Kruskal-Wallis tests (for continuous outcomes, such as age, reported as the mean and standard deviation) and chi-square tests (for categorical outcomes, such as male sex, reported as the number and percentage). To address our research question, we conducted linear regression analyses to examine the association between cortical PiB-PET SUVRs as well as the amygdala, striatum, and thalamus PiB-PET SUVRs (continuous measures; these were the presumed independent variables in our analyses) and depressive and anxiety symptoms (BDI-II and BAI total scores; these were the presumed dependent variables in our analyses). All models were adjusted for age, sex, education level, and apolipoprotein E (APOE) ε4 carrier status. To best meet the assumptions of the linear regression model, we log transformed the PiB-PET variables; for BDI-II and BAI scores, we added 1 and then log transformed the variables. Owing to the fact that a log of 0 is undefined, and (as expected in this population-based sample) several BDI-II and BAI scores were 0 in our sample, we needed to add 1 to each of these before conducting the log transformation. Analyses were conducted for the whole sample and separately for the two subsamples (without cognitive impairment and with mild cognitive impairment). To test for interaction between APOE ε4 with cortical and subcortical PiB-PET, we reran our linear regression models including interaction terms (e.g., APOE*cortical PiB-PET). Because there were no significant interactions, we did not run models separately for those with and without an APOE ε4 allele. Because we aimed to examine the associations between cortical and subcortical Aβ and standardized measures of depressive and anxiety symptoms in order to generate hypotheses for future longitudinal research, we did not adjust for multiple comparison to avoid increasing type II error. Visual displays of data are presented in Figure 1. Analyses were conducted using the two-tailed alpha level of 0.05 and performed with SAS, version 9.4 (SAS Institute, Cary, N.C.) and R (R Foundation for Statistical Computing, Vienna).
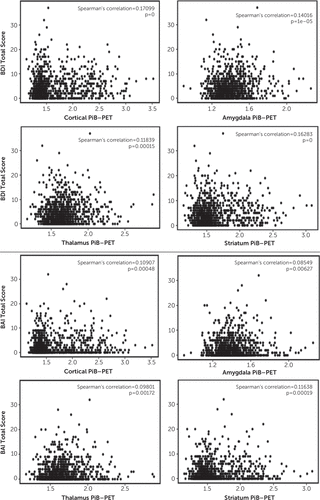
FIGURE 1. Scatterplots of the associations between cortical and subcortical β-amyloid deposition and depressive and anxiety symptoms in patients without cognitive impairment and with mild cognitive impairmenta
a BAI=Beck Anxiety Inventory, BDI=Beck Depression Inventory-II, PiB-PET=Pittsburgh Compound B positron emission tomography.
Results
The study sample comprised 842 individuals without cognitive impairment and 180 persons with mild cognitive impairment; 563 persons (55%) were males, and 286 (28%) were APOE ε4 carriers. The mean age was 78.8 (SD=5.6) years, and the mean years of education was 14.4 (SD=2.8). Persons with mild cognitive impairment had significantly higher BDI-II and BAI total scores than persons without cognitive impairment. The frequency of persons with clinically relevant depression (BDI-II score ≥13) and anxiety (BAI score ≥10) was also higher in the subsample with mild cognitive impairment versus without cognitive impairment. Finally, persons with mild cognitive impairment had significantly higher PiB-PET SUVRs for both the cortical and subcortical regions (Table 1).
| No cognitive impairment (N=842) | Mild cognitive impairment (N=180) | Total (N=1,022) | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | p |
| Male | 453 | 53.8 | 110 | 61.1 | 563 | 55.1 | 0.074b |
| Age (years) (mean±SD) | 78.3 | 5.4 | 81.1 | 5.7 | 78.8 | 5.6 | <0.001c |
| Education (years) (mean±SD) | 14.6 | 2.8 | 13.7d | 3.1 | 14.4d | 2.8 | <0.001c |
| APOE ε4 carrier | 218e | 26.0 | 68 | 37.8 | 286e | 28.1 | 0.001b |
| BDI-II | |||||||
| Total score (mean±SD) | 4.3e | 4.5 | 6.3 | 5.9 | 4.7e | 4.9 | <0.001c |
| Score ≥13 | 51e | 6.1 | 25 | 13.9 | 76e | 7.5 | <0.001b |
| BAI | |||||||
| Total score (mean±SD) | 2.4d | 3.7 | 4.2 | 5.0 | 2.7d | 4.0 | <0.001c |
| Score ≥10 | 40d | 4.8 | 20 | 11.1 | 60d | 5.9 | 0.001b |
| PiB-PET, SUVR (mean±SD) | |||||||
| Cortical | 1.60 | 0.38 | 1.87 | 0.53 | 1.64 | 0.43 | <0.001c |
| Amygdala | 1.39 | 0.15 | 1.47 | 0.21 | 1.40 | 0.16 | <0.001c |
| Striatum | 1.58 | 0.27 | 1.78 | 0.38 | 1.62 | 0.30 | <0.001c |
| Thalamus | 1.64 | 0.20 | 1.77 | 0.27 | 1.66 | 0.22 | <0.001c |
TABLE 1. Demographic and clinical characteristics of participants without cognitive impairment and with mild cognitive impairmenta
Associations of Cortical PiB-PET SUVRs With BDI-II and BAI Total Scores
In the overall sample, the cortical PiB-PET SUVR was associated with the BDI-II total score (β=0.57 [SE=0.13], p<0.001) and the BAI total score (β=0.34 [SE=0.13], p=0.011) (Table 2, Figure 1). In subsample analyses, cortical PiB-PET was only associated with the BDI-II total score (without cognitive impairment: β=0.38 [SE=0.15], p=0.013; mild cognitive impairment: β=0.86 [SE=0.27], p=0.002; Table 2).
| N | Dependent variable | Independent variable | β | SE | p |
|---|---|---|---|---|---|
| Whole sample | |||||
| 1,015 | BDI-II score | Cortical PiB-PET | 0.57 | 0.13 | <0.001 |
| 1,015 | BDI-II score | Amygdala PiB-PET | 0.80 | 0.26 | 0.002 |
| 1,015 | BDI-II score | Striatum PiB-PET | 0.69 | 0.18 | <0.001 |
| 1,015 | BDI-II score | Thalamus PiB-PET | 0.61 | 0.24 | 0.011 |
| 1,017 | BAI score | Cortical PiB-PET | 0.34 | 0.13 | 0.011 |
| 1,017 | BAI score | Amygdala PiB-PET | 0.48 | 0.26 | 0.063 |
| 1,017 | BAI score | Striatum PiB-PET | 0.56 | 0.18 | 0.002 |
| 1,017 | BAI score | Thalamus PiB-PET | 0.65 | 0.24 | 0.008 |
| Cognitively unimpaired subsample | |||||
| 836 | BDI-II score | Cortical PiB-PET | 0.38 | 0.15 | 0.013 |
| 836 | BDI-II score | Amygdala PiB-PET | 0.48 | 0.30 | 0.111 |
| 836 | BDI-II score | Striatum PiB-PET | 0.43 | 0.21 | 0.039 |
| 836 | BDI-II score | Thalamus PiB-PET | 0.26 | 0.28 | 0.355 |
| 838 | BAI score | Cortical PiB-PET | 0.23 | 0.15 | 0.124 |
| 838 | BAI score | Amygdala PiB-PET | 0.24 | 0.30 | 0.425 |
| 838 | BAI score | Striatum PiB-PET | 0.39 | 0.20 | 0.058 |
| 838 | BAI score | Thalamus PiB-PET | 0.48 | 0.28 | 0.086 |
| Mild cognitive impairment subsample | |||||
| 179 | BDI-II score | Cortical PiB-PET | 0.86 | 0.27 | 0.002 |
| 179 | BDI-II score | Amygdala PiB-PET | 1.19 | 0.49 | 0.016 |
| 179 | BDI-II score | Striatum PiB-PET | 1.09 | 0.36 | 0.003 |
| 179 | BDI-II score | Thalamus PiB-PET | 1.17 | 0.49 | 0.017 |
| 179 | BAI score | Cortical PiB-PET | 0.30 | 0.30 | 0.325 |
| 179 | BAI score | Amygdala PiB-PET | 0.52 | 0.53 | 0.336 |
| 179 | BAI score | Striatum PiB-PET | 0.58 | 0.39 | 0.146 |
| 179 | BAI score | Thalamus PiB-PET | 0.52 | 0.53 | 0.329 |
TABLE 2. Associations between cortical and subcortical β-amyloid deposition and depressive and anxiety symptoms in participants without cognitive impairment and with mild cognitive impairmenta
Associations of Subcortical PiB-PET SUVRs With BDI-II and BAI Total Scores
In the overall sample, the PiB-PET SUVR in the amygdala was associated with the BDI-II total score (β=0.80 [SE=0.26], p=0.002), the PiB-PET SUVR in the striatum was associated with the BDI-II (β=0.69 [SE=0.18], p<0.001) and BAI total scores (β=0.56 [SE=0.18], p=0.002), and the PiB-PET SUVR in the thalamus was associated with the BDI-II (β=0.61 [SE=0.24], p=0.011) and BAI total scores (β=0.65 [0.24], p=0.008) (Figure 1). Among participants without cognitive impairment, there was only one significant association, which was between the striatum PiB-PET and the BDI-II total score (β=0.43 [SE=0.21], p=0.039). Among participants with mild cognitive impairment, the PiB-PET SUVR in each subcortical region was significantly associated with the BDI-II total score (amygdala: β=1.19 [SE=0.49], p=0.016; striatum: β=1.09 [SE=0.36], p=0.003; thalamus: β=1.17 [SE=0.49], p=0.017) (Table 2).
Discussion
Here, we report a positive association between cortical Aβ deposition, as well as Aβ deposition in the amygdala, striatum, and thalamus, and depressive and anxiety symptoms in community-dwelling older adults without dementia (i.e., higher levels of SUVRs were associated with higher levels of depressive and anxiety symptoms). For example, each 1% increase in the cortical PiB-PET SUVR corresponded with approximately a 0.57% increase in the BDI-II total score and with a 0.34% increase in the BAI total score, on average. In subsample analyses, we observed that PiB-PET SUVRs in the neocortex, amygdala, striatum, and thalamus were associated with depressive symptoms among individuals with mild cognitive impairment. For individuals without cognitive impairment, such an association was observed only in the neocortex and striatum. However, the BAI score was not significantly related to the PiB-PET SUVR in the neocortex or subcortical tissues in either subgroup (without cognitive impairment and with mild cognitive impairment) but was significantly related to the PiB-PET SUVR in the neocortex, striatum, and thalamus in the study group as a whole. The coefficients were rather small, indicating that this association may be more related to the larger sample size and less related to a clinically relevant difference in the amyloid burden.
In a previous observation made by the Harvard Aging Study group, investigators showed that anxiety symptoms were significantly associated with PiB uptake in the striatum, thalamus, and amygdala but not in the neocortex among older adults without cognitive impairment (19). Thus, their results differ slightly from those of the present study, because we observed associations between both cortical and subcortical Aβ and depressive and anxiety symptoms. Several differences between our study and the study conducted by the Harvard group may account for the discrepancy in findings. For example, the convenience sample in the Harvard study was small and restricted to persons without cognitive impairment, whereas our sample included more than 1,000 community-dwelling nondemented individuals (i.e., without cognitive impairment as well as with mild cognitive impairment). Additionally, the primary outcome of interest in the Harvard study was anxiety as measured by the Hospital Anxiety and Depression Scale and after adjusting for Geriatric Depression Scale (GDS) scores, whereas our outcomes of interest were both depression and anxiety as measured by the BDI-II and BAI, respectively.
In addition, findings from other studies on the association between anxiety or depression and Aβ deposition have been inconsistent. For example, investigators from Sweden reported that anxiety symptoms but not depressive symptoms were associated with cerebral Aβ deposition in a small sample of 104 individuals without cognitive impairment and 53 persons with mild cognitive impairment and that low levels of anxiety were associated with Aβ, which may be a predictor of faster cognitive decline (11). In line with this, French researchers showed that anxiety symptoms were associated with higher frontal, cingulate, and global cerebral [18F]-florbetapir uptake in a large sample comprising persons without cognitive impairment and patients with mild cognitive impairment and Alzheimer’s disease; the associations remained significant for frontal and global uptake in the mild cognitive impairment subgroup (10). In a study using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database, the investigators did not observe an association between the frequency of anxiety or depressive symptoms and Aβ deposition but noted that persons with mild cognitive impairment and amyloid pathology had increased levels of anxiety (39). Researchers from the University of California Los Angeles reported a positive correlation between GDS scores and lateral temporal Aβ deposition in a sample of individuals without cognitive impairment and with mild cognitive impairment (12). In another study, investigators used the Australian Imaging, Biomarker and Lifestyle Study of Ageing to examine individuals without cognitive impairment and found that depression was not related to Aβ deposition at baseline, but having high Aβ deposition was associated with a significantly increased risk of new onset of depressive symptoms after 54 months (40). In a recent study using the construct of mild behavioral impairment, which is characterized by the onset of neuropsychiatric symptoms in older adults, investigators reported that a higher score on the Mild Behavioral Impairment Checklist was associated with global and striatal Aβ deposition in participants without cognitive impairment (41). Finally, our group reported weak but significant associations between BDI-II and BAI scores and elevated cortical amyloid deposition in community-dwelling participants without cognitive impairment in the Mayo Clinic Study of Aging (13), as well as higher odds of clinical depression and anxiety among participants with mild cognitive impairment with elevated Aβ deposition (14). This study builds on our previous research by additionally examining whether there is an association between Aβ deposition in the amygdala, striatum, and thalamus and behavioral symptoms among community-dwelling older adults without dementia.
Few studies have also examined the potential impact of a history of depression. For example, Donovan et al. (42) reported significantly higher Aβ burden among participants without cognitive impairment with current depression compared with those without a history of depression; they also found a correlation between higher Aβ deposition at baseline and a more pronounced increase in GDS scores. Furthermore, in a study using the ADNI database, investigators found that participants with amnestic mild cognitive impairment and a lifetime history of major depression had increased Aβ deposition in the bilateral frontal lobes compared with those without a lifetime history of depression (16). Similarly, lifetime history of major depression was associated with higher [18F]-florbetapir uptake in specific brain regions (43). In addition to the studies mentioned above, a systematic review of 19 studies found that 15 provided evidence of an association between depression and Aβ deposition in older adults (44). However, not all studies reported such associations (15, 45–47). Conflicting findings may be due in part to differences in methodology, such as smaller sample sizes or different sample compositions (e.g., combinations of participants without cognitive impairment and with mild cognitive impairment versus without cognitive impairment or with mild cognitive impairment only), varying degrees of depression severity between participants from different studies, or different tools used to assess depressive symptoms (e.g., GDS versus NPI-Q).
Results from our study show that, on average, both cortical and subcortical levels of amyloid deposition were associated with depressive and anxiety symptoms in the overall sample and that both cortical and subcortical amyloid deposition were associated with depressive but not anxiety symptoms in participants with mild cognitive impairment. Of note, subcortical Aβ deposition may indicate more advanced amyloidosis beyond the cortex and may be regarded as a marker of pathologic progression (19, 48, 49). Particularly, according to the five phases of amyloidosis as postulated by Thal et al. (48), Aβ deposition in neocortical regions reflects phase 1; phase 2 indicates Aβ deposition in allocortical regions, including the limbic system (e.g., the amygdala); and phase 3 indicates Aβ deposition in the striatum and diencephalic nuclei (e.g., the thalamus). As such, subcortical amyloid may merely be a marker of duration of the amyloid deposition process, and there may be no anatomic localization significance of the subcortical abundance of amyloid with regard to its potential association with neuropsychiatric symptoms. Furthermore, it is known that amyloid instigates the degenerative pathways that are in turn responsible for clinical deficits. Therefore, we postulate that amyloid deposition likely does not have a direct effect on depression or anxiety; rather, the degree of Alzheimer’s disease pathology as reflected by amyloid levels (i.e., lesser or greater Thal amyloid phase) may be associated with emotional behavior (50), even though our cross-sectional results reported here do not support a more pronounced association between Aβ deposition in the amygdala, striatum, or thalamus and behavioral symptoms compared with cortical Aβ deposition. Therefore, more research is needed to investigate the associations between amyloid deposition in different brain regions and neuropsychiatric symptoms.
The strengths of our study include its population-based design, large sample size, and rigorous statistical analysis that adjusted for APOE ε4 genotype status and traditional confounders, including age, which may be important with regard to the effect of age on striatal PiB uptake. In addition, older age and fewer years of education were significantly associated with higher BDI-II and BAI scores, and female sex was associated with higher scores on the BAI but not the BDI-II. APOE ε4 genotype status was not associated with anxiety and depression measures in our sample.
However, there are limitations to our study. As in any cross-sectional study, we were unable to assess causality. Additionally, unlike in clinical samples, the severity of depressive and anxiety symptoms was low in our community-dwelling sample of nondemented older adults. Therefore, we chose to use continuous BDI-II and BAI scores as presumed dependent variables rather than dichotomized outcomes (e.g., BDI-II scores <13 versus scores ≥13), even though we acknowledge the low scores and small variance on the BDI-II and BAI, which may reduce the significance of the association between the PET-PiB SUVRs and the dependent variables in our analysis. In addition, we did not include history of depression or anxiety disorder, as well as potential treatment. Finally, we did not adjust for vascular risk factors, because more research is needed to clarify whether Alzheimer’s disease and vascular pathology are associated (51) or whether amyloid and vascular pathologies are independent predictors of cognitive decline (52).
Conclusions
Elevated levels of amyloid deposition in both cortical and subcortical brain regions are associated with higher depressive and anxiety symptoms in older adults without dementia. In individuals with mild cognitive impairment, Aβ deposition in the neocortex, amygdala, striatum, or thalamus was associated with depressive but not anxiety symptoms. This cross-sectional observation merits further investigation and needs to be confirmed by a prospective cohort study.
1 : Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002; 288:1475–1483Crossref, Medline, Google Scholar
2 : Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement 2013; 9:602–608Crossref, Medline, Google Scholar
3 : A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. Am J Geriatr Psychiatry 2005; 13:460–468Crossref, Medline, Google Scholar
4 : Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65:1193–1198Crossref, Medline, Google Scholar
5 : Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord 2008; 25:115–126Crossref, Medline, Google Scholar
6 : Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis 2012; 31:411–420Crossref, Medline, Google Scholar
7 : Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 2014; 171:572–581Crossref, Medline, Google Scholar
8 : The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry 2013; 21:685–695Crossref, Medline, Google Scholar
9 : Neuropsychiatric symptoms, APOE ε4, and the risk of incident dementia: a population-based study. Neurology 2015; 84:935–943Crossref, Medline, Google Scholar
10 : Associations between neuropsychiatric symptoms and cerebral amyloid deposition in cognitively impaired elderly people. J Alzheimers Dis 2016; 49:387–398Crossref, Medline, Google Scholar
11 : Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol Aging 2020; 85:74–82Crossref, Medline, Google Scholar
12 : Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am J Geriatr Psychiatry 2009; 17:493–502Crossref, Medline, Google Scholar
13 : Depressive and anxiety symptoms and cortical amyloid deposition among cognitively normal elderly persons: the Mayo Clinic Study of Aging. Int Psychogeriatr 2018; 30:245–251Crossref, Medline, Google Scholar
14 : Cortical β-amyloid burden, neuropsychiatric symptoms, and cognitive status: the Mayo Clinic Study of Aging. Transl Psychiatry 2019; 9:123Crossref, Medline, Google Scholar
15 : Cortical amyloid β deposition and current depressive symptoms in Alzheimer disease and mild cognitive impairment. J Geriatr Psychiatry Neurol 2016; 29:149–159Crossref, Medline, Google Scholar
16 : Lifetime history of depression predicts increased amyloid-β accumulation in patients with mild cognitive impairment. J Alzheimers Dis 2016; 49:1189–1190Crossref, Medline, Google Scholar
17 : In vivo staging of regional amyloid deposition. Neurology 2017; 89:2031–2038Crossref, Medline, Google Scholar
18 : Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun 2017; 8:1214Crossref, Medline, Google Scholar
19 : Association of anxiety with subcortical amyloidosis in cognitively normal older adults. Mol Psychiatry (Epub ahead of print, August 16, 2018)
20 : The amygdala: functional organization and involvement in neurologic disorders. Neurology 2015; 84:313–324Crossref, Medline, Google Scholar
21 : The amygdala. Curr Biol 2007; 17:R868–R874Crossref, Medline, Google Scholar
22 : Emotion circuits in the brain. Annu Rev Neurosci 2000; 23:155–184Crossref, Medline, Google Scholar
23 : The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69Crossref, Medline, Google Scholar
24 : The Short Test of Mental Status: correlations with standardized psychometric testing. Arch Neurol 1991; 48:725–728Crossref, Medline, Google Scholar
25 : The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414Crossref, Medline, Google Scholar
26 : L’examen clinique en psychologie. Paris, Presses Universitaires de France, 1964Google Scholar
27 : Wechsler Memory Scale-Revised. New York, Psychological Corporation, 1987Google Scholar
28 : Boston Naming Test. Philadelphia, Lippincott Williams & Wilkins, 2001Google Scholar
29 : Mayo’s older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol 1998; 20:194–200Crossref, Medline, Google Scholar
30 : Wechsler Adult Intelligence Scale-Revised. New York, Psychological Corporation, 1981Google Scholar
31 : Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
32 : Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R, and AVLT norms for ages 56 through 97. Clin Neuropsychol 1992; 6:83–104Crossref, Google Scholar
33 : Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256:183–194Crossref, Medline, Google Scholar
34 : Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256:240–246Crossref, Medline, Google Scholar
35 : Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 2017; 13:205–216Crossref, Medline, Google Scholar
36 : Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med 2009; 50:878–886Crossref, Medline, Google Scholar
37 : Manual for Beck Depression Inventory-II (BDI-II). San Antonio, Tex, Psychology Corporation, 2001Google Scholar
38 : BAI, Beck Anxiety Inventory: Manual. San Antonio, Tex, Psychological Corporation, 1990Google Scholar
39 : Association of brain amyloidosis with the incidence and frequency of neuropsychiatric symptoms in ADNI: a multisite observational cohort study. BMJ Open 2019; 9:
40 : Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int J Geriatr Psychiatry 2017; 32:455–463Crossref, Medline, Google Scholar
41 : Mild behavioral impairment is associated with β-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement 2020; 16:192–199Crossref, Medline, Google Scholar
42 : Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry 2018; 175: 530–537Crossref, Medline, Google Scholar
43 : Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/amyvid) positron emission tomography. Eur J Nucl Med Mol Imaging 2014; 41:714–722Crossref, Medline, Google Scholar
44 : Amyloid-beta and depression in healthy older adults: a systematic review. Aust N Z J Psychiatry 2015; 49:36–46Crossref, Medline, Google Scholar
45 : Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol Aging 2012; 33:2334–2342Crossref, Medline, Google Scholar
46 : Amyloid burden and incident depressive symptoms in preclinical Alzheimer’s disease. J Affect Disord 2018; 229:269–274Crossref, Medline, Google Scholar
47 : Depressive symptoms and biomarkers of Alzheimer’s disease in cognitively normal older adults. J Alzheimers Dis 2015; 46:63–73Crossref, Medline, Google Scholar
48 : Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002; 58:1791–1800Crossref, Medline, Google Scholar
49 : Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer’s disease: implications for amyloid imaging. J Alzheimers Dis 2012; 28:869–876Crossref, Medline, Google Scholar
50 : Alzheimer disease, biomarkers, and clinical symptoms: quo vadis? Reply. JAMA Neurol 2020; 77:394Crossref, Medline, Google Scholar
51 : Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Dement (Amst) 2017; 7:69–87Crossref, Medline, Google Scholar
52 : Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015; 138:761–771Crossref, Medline, Google Scholar



