Psychiatric Features and Disturbance of Circadian Rhythm of Temperature, Pulse, and Blood Pressure in Wilson's Disease
Abstract
Wilson's disease (hepatolenticular degeneration), a disease of genetic origin, is due to abnormal copper metabolism affecting many organs and systems, especially the liver and the nervous system. The initial symptoms can be exclusively or predominantly psychiatric, including psychotic features. Three cases are reported in which the clinical picture at the beginning was compatible with a psychiatric diagnosis. During hospitalization, before treatment, there were abnormal and spontaneous changes in the circadian rhythm of temperature, pulse, and blood pressure, recorded every 6 hours, with febrile peaks in the absence of infectious focus. Because the hypothalamus is important in the regulation of these autonomic functions, the hypothesis of a possible hypothalamic dysfunction was made, justifying a wide clinical and laboratory investigation that allowed the diagnosis of Wilson's disease. Alertness to circadian rhythm abnormalities in such cases may help the psychiatrist avoid an erroneous diagnosis.
Wilson's disease (WD), or hepatolenticular degeneration, an inherited disorder of autosomal transmission, is due to an abnormality of copper metabolism that affects many organs and systems, especially the liver and the central nervous system. In most cases it begins in childhood or adolescence, rarely starting in adulthood; the onset is generally insidious, and the clinical course is difficult to predict, varying from weeks to years.1 According to Bearn,2 in 40% of the cases the first symptoms are related to the liver (jaundice, hepatomegaly); in 40% they are neurologic (rigidity, athetosic movements, tremors, abnormal postures, dysarthria, dysphagia, epileptic seizures); and in 20% of the cases the illness begins with behavioral disorders or psychiatric diseases. Akil et al.,3 in a study of 31 patients with WD, concluded that about 50% of them displayed some evidence of psychopathology, and about 20% had seen a psychiatrist before the diagnosis was reached. Similar results were found by Dening and Berrios,4 who verified, in a study of 195 cases of WD, that an organic condition had not been suspected in 50% of 20 patients who were first seen by a psychiatrist. The initial clinical picture may be similar to schizophrenia, depression, or behavioral disorders.1,4–7 Cartwright8 reminds us that when psychiatric symptoms exist in WD, if neuroleptics are prescribed, abnormal liver function and motor disorders may be misinterpreted as side effects. Hoogenraad1 also notes that neuroleptic treatment is usually inadvisable for patients with WD because even in small doses it can cause side effects.
Lishman9 states that in the beginning, symptoms such as tremors, athetosic movements, and rigidity can be influenced by emotion, leading to an erroneous impression of conversion hysteria. The diagnosis of WD is confirmed when the total serum level of copper or ceruloplasmin is low and the naked eye or an ophthalmologic examination detects the presence of the Kayser-Fleischer ring in the cornea; however, in the earlier stages these findings may be absent or spontaneous remission may be seen, and even thereafter, marked fluctuations in severity may occur. Intellectual deterioration is common in the later stages, and the prognosis is worse the younger the age at onset; death usually occurs from liver failure, rupture of esophageal varices, inhalation, or intercurrent infections.9
Electroencephalographic changes are common in WD, taking the form of decreased alpha activity and increased beta and theta activities, with a low voltage background.10 Computed tomography (CT) and magnetic resonance may indicate enlarged ventricles and both cortical and brainstem atrophy.6
Malaspina et al.11 estimate the frequency of WD at 10 in 100,000. Despite the severity and importance of mental symptoms in WD, most of the psychiatric textbooks, including those dealing with child and adolescent psychiatry,12,13 give brief or even no information about this disease. On the other hand, in the bibliographic review the author did not find any reference to abnormal circadian rhythms of temperature, pulse, and blood pressure as possible symptoms of Wilson's disease. However, abnormal changes in these functions were found in the three cases that will be described, and these changes may be of help in making an early diagnosis of WD.
METHODS
In the Child and Adolescent Psychiatry Service (CAPS) of the Institute of Psychiatry, temperature (T), pulse (P), and blood pressure (BP) of the inpatients were recorded just once a day, in the morning, unless the child had an infection and was febrile. However, since 1964, as part of a research project on the etiology of mental diseases, it became a norm applied to all inpatients that T, P, and BP would be recorded every 6 hours and that, unless urgent therapeutic measures were necessary, no treatment would be prescribed for at least 2 weeks after hospitalization, inasmuch as there could be a spontaneous remission. This would help to ensure that the correct diagnosis was made before introducing treatment. Recording of T, P, and BP every 6 hours revealed abnormal circadian rhythm of these autonomic functions in some patients; febrile peaks also appeared in the absence of infection and ceased spontaneously without use of antipyretics. Because the hypothalamus plays an important role in the regulation of blood pressure, heart rate, and temperature,14,15 when the abnormalities in these autonomic functions were detected the hypothesis of a possible hypothalamic dysfunction was adopted, justifying deeper laboratory investigation that led to the diagnosis of encephalopathies of different etiologies, including WD.
The author made a search of the diagnoses of the patients who have been hospitalized in CAPS, to look for those that received the diagnosis of WD. Three cases were found, and the symptoms that justified the initial diagnosis of a psychiatric disease, and later of WD, are described below.
RESULTS
Case 1. A., 15 years old, had a normal development and behavior until the age of 15, when, after an emotionally traumatic experience, she manifested quick involuntary movements, first of the right arm and progressively extending to other parts of the body, which disappeared spontaneously after 2 months. About 3 months later, she started avoiding social contact, had no spontaneous speech, and often reported auditory hallucinations. The diagnosis of schizophrenia was made and she was treated with chlorpromazine by a psychiatrist, with slight improvement in the first months, but later the symptoms reappeared. Eight months after the beginning of the disease, she was examined in CAPS and was hospitalized in April 1963. In the ward, she maintained the clinical picture described above. However, the intensity of the symptoms was reduced when she was alone and increased significantly in the presence of members of CAPS' staff. In addition to the involuntary movements, neurological examination revealed only a mild generalized hypertonia, and these symptoms were attributed to the recent use of neuroleptic. Even so, A. was administered some laboratory examinations, including EEG and cerebrospinal fluid (CSF), and the results were normal. The daily temperature, recorded only in the morning, remained in the range of 36.4° to 37.8° Celsius; P ranged from 80 to 100 per minute, and BP remained between 120/70 and 140/80. On the basis of these results, the characteristics of her behavior, and the fact that the disease had appeared after an emotional trauma, diagnosis of hysterical conversion was made. She was treated with psychotherapy and tranquilizers and showed great improvement. Involuntary movements seldom appeared in the arms; she had normal behavior, no longer reported hallucinations, seemed happy, and was discharged after 2 months of hospitalization.
In October 1964, A. returned to CAPS outpatient service, and her parents reported that she had been well until the preceding week, when the symptoms had reappeared. Once again she was hospitalized. On this occasion, she was dysarthric, had involuntary movements in the upper and lower limbs, and walked with difficulty, tracking to the left side; she exhibited hypertonia alternating with normal tonus in several muscular groups, and Babinski sign in the right foot. A discrete cognitive impairment in comparison with the preceding examination was detected. No hallucinations were then reported. Autonomic functions were recorded every 6 hours and revealed abnormal changes: BP oscillated between 100/60 and 150/100; T and P changes are shown in Figure 1.
Pneumoencephalography of the brain was then performed, revealing a ventricular dilatation and cortical atrophy. This time, EEG showed diffuse slow-wave discharges. The total serum copper level was low (30 μg/dl, vs. normal 70–160 μg/dl), and the ophthalmologic examination detected the presence of Kayser-Fleischer rings. The diagnosis of Wilson's disease was now evident, but her physical condition quickly deteriorated; she became semicomatose and died a few days later, in December 1964. During the days preceding death, BP oscillated between 140/100 and 180/120. Even though no infectious focus was detected, she had almost continuous high fever that did not cease with antipyretics, and the changes in T and P also increased, as shown in Figure 2.
Case 2. B. had normal behavior, was healthy, and was a good student until the beginning of her illness. When she was 13 years old, there appeared a slow and progressive change of behavior: she became very quiet, stopped speaking spontaneously, gave laconic answers in a very low voice, had unmotivated crises of laughing, crying, or aggressiveness, was inattentive, and had almost no initiative. Four months later she was examined by a psychiatrist, who diagnosed schizophrenia and prescribed chlorpromazine. A few days later B. developed difficulty swallowing and tremors in the extremities, which were attributed to a side effect of the neuroleptic.
As she did not improve with treatment, she was brought to CAPS in 1969 and hospitalized 10 months after the beginning of the disease. In the ward, she maintained the same behavior described above. On clinical and neurological examination the only abnormalities were a discrete hypertonia of the extremities and slight reduction of the stretch reflexes, which could be attributed to the recent use of neuroleptic. However, BP, recorded every 6 hours, oscillated between 100/60 and 140/100; T and P also showed abnormal changes, and febrile peaks appeared in absence of infection, as shown in Figure 3.
These abnormalities suggested a hypothalamic dysfunction and the need of a broader laboratory investigation. A hemogram demonstrated eosinophilia. All other results, including EEG, CSF with protein electrophoresis, urine and feces analyses, glycemia, and levels of bilirubin, antistreptolisin, and immunoglobulin (IgG, IgA, IgM), were normal. However, an ophthalmic examination detected the presence of Kayser-Fleischer rings, the total serum copper level was low (50 μg/dl), and a diagnosis of Wilson's disease was made. For this reason, she was transferred to the Neurologic Clinic for treatment.
Case 3. C. had a normal development, was calm and sociable, and was a good student. When she was 12 years old, her behavior changed, with a progressive reduction of initiative, daily activity, and verbal communication, in addition to crises of aggressiveness. Two months later, she experienced difficulty in swallowing and developed hand tremors. She was examined by a clinician who prescribed glutamic acid and phosphate. According to the parents' information, dysphagia and tremors disappeared but psychiatric symptoms persisted. For this reason, she was brought to CAPS 10 months after the beginning of the disease. The psychiatric examination indicated reduced attention, slow movements with tendency to immobility, and inexpressive mimicry. She did not speak spontaneously and gave laconic answers to some questions, in a very low voice. Sometimes she cried without tears, but this ceased abruptly. No delusions or hallucinations were reported. Her nutritional condition was good, and neurological examination detected only slight intentional tremors of both hands.
In 1975, C. was hospitalized and maintained the same behavior described above, which was compatible with the diagnosis of schizophrenia. However, during her first days of hospitalization, and without any treatment, instability of autonomic functions was detected: BP oscillated between 90/60 and 160/120; changes in T and P were also abnormal, as shown in Figure 4.
Because of a hypothesized hypothalamic dysfunction, laboratory examinations were then done: the hemogram revealed eosinophilia. Feces, urine, EEG, and CSF examinations gave normal results. However, computed tomography showed a cortical atrophy and enlarged ventricular system. The total serum copper level was below normal (30 μg/dl), and the ophthalmologic examination detected the presence of Kayser-Fleischer rings. While lab results were pending, the patient developed dysphagia and involuntary tremors of the extremities. The diagnosis of Wilson's disease was made, and the patient was transferred to the Neurologic Clinic for treatment.
DISCUSSION
The cases herein reported confirm the description of Wilson's disease, in which the initial symptoms may be behavioral and may even mimic psychiatric diseases. Making the correct diagnosis is therefore difficult, particularly because laboratory and clinical examination may be normal at the beginning of the disease.
In Case 3, the improvement in the initial phase of the disease could hardly be related to the use of glutamic acid or phosphate prescribed by a clinician; probably it was coincident with a phase of transitory and spontaneous remission of the motor symptoms, a possibility mentioned in the literature.9
In Cases 2 and 3, when only psychiatric symptoms existed and were compatible with the diagnosis of schizophrenia, the instability of the circadian rhythms of T, P and BP, when recorded several times a day, alerted medical personnel to the possibility of an encephalopathy and to the need of a deeper laboratory and clinical investigation, thus averting an incorrect psychiatric diagnosis and revealing that both patients had WD.
In Case 1, the morning temperature during the first hospitalization was always under 37° C, and the clinical picture induced to the diagnosis of hysteria. Febrile peaks occurred at 6:00 a.m. only during the second hospitalization, when neurological signs were already evident. It is probable that if the CAPS procedure during the first hospitalization had included recording T, P, and BP not only in the morning, but four times a day, the abnormal changes in these autonomic functions would have been seen to exist already, and once they had been detected, the hypothesis of an encephalopathy would have been investigated and WD diagnosed. In this case, an early and correct treatment could have been prescribed, probably averting the patient's death.
Abnormal changes of T, P, and BP have not been observed in patients hospitalized in CAPS for treatment of endogenous psychosis or severe behavior disorders; however, they were present not only in WD, but also in other encephalopathies, such as acute sclerosing panencephalitis, neurocysticercosis, and encephalitis related to immune process, all of which began with the manifestation of psychiatric symptoms.
Wilson's disease requires lifelong therapy, and the resources for treatment are expanding,1,16–19 mainly through the use of chelating agents. However, as Chow and Cummings20 comment, WD is an inevitably progressive fatal disease; the earlier treatment is initiated, the better for preventing or reducing damage. This paper may be a contribution to an earlier diagnosis of this severe disease, especially when it starts with psychiatric symptoms.
ACKNOWLEDGMENTS
The author is grateful to Dr. Cesar Timo-Iaria, Professor of Physiology, for his help in interpreting the results of this research.
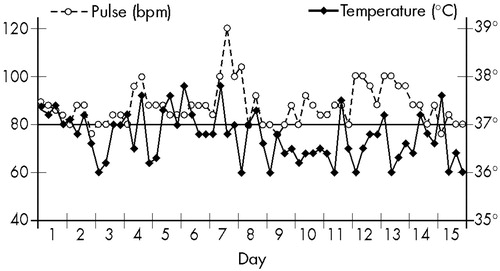
FIGURE 1. Wilson's disease patient, 15 years old (Case 1): temperature and pulse, recorded at 6, 12, 18, and 24 hours, at her second hospital admission and during the first 15 days without treatment thereafter.
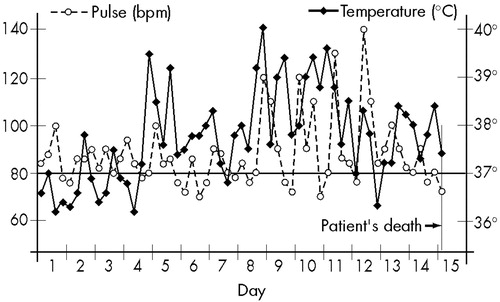
FIGURE 2. Wilson's disease patient, 15 years old (Case 1): temperature and pulse, recorded at 6, 12, 18, and 24 hours, during the 15 days preceding her death. No infectious focus was detected. Antipyretics were given every 4 hours.
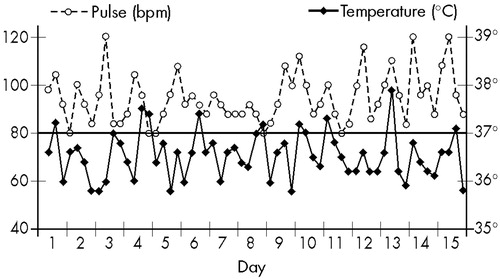
FIGURE 3. Wilson's disease patient, 13 years old (Case 2): temperature and pulse, recorded at 6, 12, 18, and 24 hours, during 15 days without treatment.
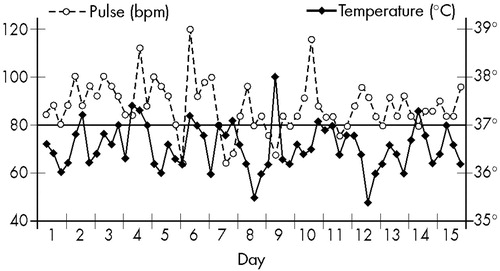
FIGURE 4. Wilson's disease patient, 12 years old (Case 3): temperature and pulse, recorded at 6, 12, 18, and 24 hours, during 15 days without treatment.
1 Hoogenraad T: Wilson's Disease. London, WB Saunders, 1996, pp 71-108Google Scholar
2 Bearn AG: Wilson's disease, in The Metabolic Basis of Inherited Diseases, 3rd edition, edited by Stanbury JB, Wyngaarden JB, Fredrickson DS. New York, McGraw-Hill, 1972, pp 563-565Google Scholar
3 Akil M, Schwartz JA, Dutchak D, et al: The psychiatric presentations of Wilson's disease. J Neuropsychiatry Clin Neurosci 1991; 3:377-382Link, Google Scholar
4 Dening TR, Berrios GE: Wilson's disease: psychiatric symptoms in 195 cases. Arch Gen Psychiatry 1989; 46:1126-1134Crossref, Medline, Google Scholar
5 Davis EJB, Bored M: Wilson's disease and catatonia. Br J Psychiatry 1993; 162:256-259Crossref, Medline, Google Scholar
6 Daniel DG, Egad MF, Wolf SS: Neuropsychiatric aspects of movement disorders, in Kaplan and Sadock's Comprehensive Textbook of Psychiatry, 7th edition, vol 1, edited by Sadock BJ, Sadock VA. Philadelphia, Lippincott Williams and Wilkins, 2000, pp 285-299Google Scholar
7 Dening TR: The neuropsychiatry of Wilson's disease: a review. Int J Psychiatry Med 1991; 21:135-148Crossref, Medline, Google Scholar
8 Cartwright GE: Diagnosis of treatable Wilson's disease. N Engl J Med 1978; 298:1347-1350Crossref, Medline, Google Scholar
9 Lishman A: Organic Psychiatry: The Psychological Consequences of Cerebral Disorders, 3rd edition. Oxford, UK, Blackwell Scientific, 1998, pp 639-687Google Scholar
10 Nazer A, Ede RJ, Mowat AP, et al: Wilson's disease in childhood: variability of clinical presentation. Clin Pediatr 1983; 22:755-757Crossref, Medline, Google Scholar
11 Malaspina D, van Kammen M, Johnson J, et al: Epidemiologic and genetic aspects of neuropsychiatric disorders, in The American Psychiatric Press Textbook of Psychiatry, 3rd edition, edited by Yudofsky SC, Hales RE. Washington, DC, American Psychiatric Press, 1997, pp 271-329Google Scholar
12 Rutter M, Taylor E, Gottersov L (eds): Child and Adolescent Psychiatry. London, Blackwell Scientific, 1994Google Scholar
13 Zametkin AJ, Andreason P, Markus JPK: Laboratory and diagnostic testing, in Textbook of Child and Adolescent Psychiatry, edited by Wiener JM. Washington, DC, American Psychiatric Press, 1991, pp 121-127Google Scholar
14 Patton HD: Higher control of autonomic outflows: the hypothalamus, in Neurophysiology, 19th edition, edited by Ruch TC, Patton HD, Woodbury JW, et al. Philadelphia, WB Saunders, 1965, pp 238-251Google Scholar
15 Gelb D: Abnormalities of thermal regulation and the nervous system, in Neurology and General Medicine, 2nd edition, edited by Aminoff MJ. New York, Churchill Livingstone, 1995, pp 931-932Google Scholar
16 Denny-Brown D, Porter H: The effect of BAL(2,3-dimercaptopropanol) on hepatolenticular degeneration (Wilson's disease). N Engl J Med 1951; 241:917-925Crossref, Google Scholar
17 Adams DA, Goodman R, Maxwell MH, et al: Nephrotic syndrome associated with penicillamine therapy of Wilson's disease. Am J Med 1964; 36:330-336Crossref, Medline, Google Scholar
18 Sternlieb I, Scheinberg IH: Penicillamine therapy in hepatolenticular degeneration JAMA 1964; 189:748-754Google Scholar
19 Starosta-Rubenstein S: Treatment of Wilson's disease, in Treatment of Movement Disorders, edited by Kurlan R. Philadelphia, Lippincott, 1995, pp 663-664Google Scholar
20 Chow TW, Cummings L: Neuropsychiatry: clinical assessment and approach to diagnosis, in Kaplan and Sadock's Comprehensive Textbook of Psychiatry, 7th edition, vol 1, edited by Sadock BJ, Sadock VA. Philadelphia, Lippincott Williams and Wilkins, 2000, pp 221-241Google Scholar



