Prefrontal Cortical Dysfunction in Abstinent Cocaine Abusers
Abstract
The anterior cingulate cortex (ACC) and lateral prefrontal (LPFC) cortex are brain regions important to executive cognitive functions (ECF). We determined ACC and LPFC function in 23-day abstinent cocaine abusers using positron emission tomography (PET H215O) during performance of a modified version of the Stroop Task. Cocaine abusers showed less activation than non-drug-using comparison subjects in the left ACC and the right LPFC and greater activation in the right ACC. Average amount of cocaine used per week was negatively correlated with activity in the rostral ACC and right LPFC. Disruption of ECF in substance abusers could interfere with attempts to stop drug use and undermine treatment. Since impairment in ECF may be a common feature of various neuropsychiatric disorders, these findings have applicability beyond the neurobiology of addiction.
Cocaine abuse is a substantial public health concern, as recent estimates indicate that there are 1.5 million chronic cocaine users in the United States.1 Societal costs of drug abuse including cocaine abuse are estimated to be $62 billion.2 The use of cocaine produces changes in brain chemistry with attendant functional consequences. Neuroimaging studies have revealed metabolic3,4 and structural5 differences in prefrontal brain regions, including the anterior cingulate cortex (ACC) and lateral prefrontal cortex (LPFC) of cocaine and polydrug abusers relative to non-drug-using comparison subjects. The caudal-dorsal ACC has strong reciprocal interconnections with the LPFC, and this network operates during performance of tasks that involve high levels of mental effort.6 This neural network also appears to participate in executive cognitive functions (ECF) governing resolution of conflict, response inhibition, performance monitoring, implementation of control, and error monitoring.7–11 Disruption of these functions could impair an individual’s ability to monitor and inhibit inappropriate behavior. In the context of substance abuse, such disruption could impede the discontinuation of self-destructive, drug seeking behavior, thereby undermining treatment.
The aim of this investigation was to determine if cocaine abusers have impaired function of the ACC and LPFC. We used water method positron emission tomography (PET H215O) imaging and a cognitive activation task, a modified version of the Stroop Task.12–15 The Stroop is a task of cognitive control and performance monitoring that requires response inhibition and suppression of a more habitual response in favor of an atypical one.10,16,17 Positron emission tomography (PET) and frontal magnetic resonance imaging (fMRI) studies in normal volunteers demonstrate that the ACC and LPFC have complementary functions underlying the performance of different aspects of the Stroop Task.9,10,17,18 The LPFC plays a role in maintaining attentional demands of the Stroop Task whereas the ACC plays a role in conflict monitoring.10 It is important to study the functional integrity of the ACC and LPFC in cocaine abusers because dysregulation of this prefrontal neural network might contribute to the development and persistence of maladaptive behaviors (e.g., poor response selection) that could sustain addiction or impede abstinence from drug use.
In previous neurobehavioral studies of abstinent cocaine abusers, we have shown a dose-related relationship between the amount of cocaine used and performance on tasks associated with ACC function (Stroop) and LPFC (CALCAP—sequence of numbers match-to-sample task).19,20 Based on this work, we predicted that abstinent cocaine abusers would show impaired function of the ACC and LPFC, compared with a non-drug-using comparison group. We also reasoned that if abnormalities in brain functioning were directly related to cocaine use, changes in brain activation in these regions during performance on the task should be correlated with the amount of cocaine used.
METHODS
Participants
Comparison subjects and cocaine abusers were recruited using newspaper advertisements, and the study was approved by the institutional review boards of the National Institute on Drug Abuse (NIDA) Intramural Research Program and Johns Hopkins University Bayview Medical Center. All participants gave written, informed consent and received remuneration. Participant selection was based on drug use history obtained using the structured interview Drug Use Survey Questionnaire (DUSQ),21 Addiction Severity Index (ASI)22 and the Diagnostic Interview Schedule (DIS).23 All participants were right handed, and English was their native language.
Non-drug-using group.
Participants were included in the comparison group if they reported consuming fewer than 11 alcoholic drinks per week and using marijuana less than 8 days per month. Individuals were excluded if they currently used any illicit drug other than marijuana.
Cocaine group.
The cocaine group claimed that cocaine was their drug of choice, used cocaine by any route for at least 2 years, self-administered cocaine at least four times per month, had two urine toxicology screens at least 48 hours apart that were positive for cocaine metabolites, and reported alcohol consumption of fewer than 11 alcoholic drinks per week and marijuana use less than 8 days per month. The positive screens confirmed cocaine use during the 24 to 72 hours prior to admission into the inpatient unit and ensured that all participants were abstinent for a uniform period. Participants were excluded if they met the DSM –IV criteria as assessed on the DIS for current or past dependence on any other psychoactive substance other than cocaine, including alcohol, or if their urine toxicology screen was positive for substances other than cocaine and its metabolites.
Exclusion criteria for all participants.
Volunteers were excluded for a past or current Axis I disorder other than nicotine dependence by DSM-IV criteria using the DIS (e.g., PTSD, major depressive disorder). Volunteers were also excluded for a past or current history of any neurological illness, use of psychoactive medication, an abnormal neurological examination, pregnancy, color-blindness, or left handedness.
Data Collection
At the initial visit to the Clinical Inpatient Research Unit (CIRU) at NIDA-IRP, all participants had a medical evaluation, including a neurological exam, urine toxicology screening, and pregnancy test (for women). Cocaine abusers were then admitted to the CIRU for approximately 23 days, which allowed us to examine persistent effects without the confounding influence of acute effects of cocaine on the brain. Random drug screens were performed during the inpatient stay to ensure abstinence. No participant was in drug abuse treatment, psychotherapy, or on any medication at the time of screening or when admitted to the study, and no treatment or medications were given over the 23-day stay. The residential research setting permitted us to monitor participants closely for 23 days prior to testing to ensure proper medical, environmental, and nutritional control. The comparison subjects stayed at the General Clinical Research Center (GCRC) for three days.
Design: Research participants were instructed to abstain from cigarettes and caffeine for 3 hours prior to the PET session. Subjects participated in one PET session (on day 23 of the residential stay for cocaine abusers and day 3 of the residential stay for comparison subjects), during which they received six injections of H215O (10 mCi each). Injections were followed by a 1-min acquisition scan. Three cognitive conditions were studied: Rest (eyes on a fixed target), a no conflict condition, and a conflict condition. Two scans were acquired for each of the three conditions (rest, no conflict, conflict), yielding a total of 6 scans in a testing session. The rest condition occurred at the beginning and end of the session. The order of the no conflict and conflict conditions was counterbalanced within and between participants. Tasks began 1 minute prior to injection of the tracer and continued until the end of the task (approximately 3 minutes).
Measures
Modified Stroop Task.
A modified version of the computer administered Automated Neuropsychological Assessment Metrics (ANAM) version of the Stroop Task was used.24 As in Golden’s version25 of the Stroop Task that is used clinically, the participants were required to correct each mistake before proceeding to the next stimulus (self-paced). Unlike Golden’s version, the modified task required a manual rather than vocal response, and stimuli were presented as a single trial rather than as an array. Responses were recorded using a “3 key-button box” (KPX-17 Parallel keypad, ALPS electronic), with separate buttons corresponding to red, green and blue. All responses were made with the first three fingers of the right hand. Two minutes of practice trials were allotted to train the participant to respond to the correct keys on the keypad while in the scanner. Stimuli were presented for a total of 2 minutes for each condition. We modified the classic Stroop Task to enhance the differences between conditions by introducing a greater difference in the amount of conflict. The brain image was acquired during the first minute of stimulus presentation.
Conflict (color naming with word-interference-incongruent stimulus dimensions).
This condition consisted of conflicting stimulus dimensions, and the task demand was to suppress a typical response. The Conflict condition displayed the words red, green, or blue in colors that did not match the meanings of the words (e.g., red displayed in the color blue). For example, a correct response was a press of the blue key when viewing the word red written in the color blue.
No conflict (word naming without word-interference-congruent stimulus dimensions).
This condition presented no conflict in the dimensions of the stimulus. The no conflict condition displayed the words red, green, or blue in colors that matched the written words (e.g., red displayed in the color red). Therefore, this task was matched with the conflict condition for response type (button press) and stimulus types (color, word meaning, and number of letters) making the visuomotor task demands identical to those of the conflict condition. We designed this no conflict condition to be less cognitively demanding than the conflict condition. Therefore this modification enhanced the differences between the conflict and no conflict conditions by increasing the amount of conflict. The no conflict condition was easy since the word (e.g., red) matched the color (e.g., red) and was the dominant tendency. However, the conflict condition was more difficult since the stimulus dimension was incongruent requiring inhibition of the impulse to read the word (e.g., red) while naming the incongruent color stimuli (e.g., the word red presented in the color blue). Our pilot work showed that the mean average response time for the conflict condition was significantly slower (mean response time: 864 [SD 314]) msec than the mean response time for the no conflict condition (mean response time: 572 [SD 90] msec; Wilcoxin Signed Rank Test, Z = −3.11; p < 0.01). These data (i.e., slower reaction time) provide evidence that the conflict condition was more cognitively demanding and required more effort than the no conflict condition.
Scans: Scans were acquired with a Siemens ECAT EXACT HR + in 63 planes with a 15.5 cm field of view in 3D mode. Images were reconstructed using a Hann filter with 0.5 cut off frequency. The average transverse resolutions [full width half maximum (FWHM)] of the scanner at 1 cm and 10 cm from the center of the field of view measured in 3D mode and determined using a Fluorine-18 line source and a ramp filter (with a 0.5 cutoff frequency), were 4.66 mm and 5.45 mm, respectively. Axial resolutions of the scanner (FWHM) that were measured using a point source of F 18 and the same reconstruction algorithm were 4.21 mm and 5.0 mm at 0 cm and 10 cm from the center, respectively. In case of application of a Hann filter with a 0.5 cut off frequency, used for reconstruction of brain images, the average transverse resolutions were 6.52 mm and 7.16 mm, respectively. For the same reconstruction algorithm, the average axial resolution at 0 cm from the center was 3.72 mm and at 10 cm, 5.64 mm.
Image Processing and Statistical Analyses: Position emission tomography images were aligned, spatially normalized into the Montreal Neurological Institute (MNI) coordinate system, and smoothed with a 12× 12×12 mm Gaussian kernel using Statistical Parametric Mapping Software (SPM99; Wellcome Department of Cognitive Neurology). A two-stage procedure was implemented for statistical analyses. In the first stage, PET images from each participant were used to create an adjusted mean image, representing the relative change in brain activity between the no conflict condition and the conflict condition (all scans from the conflict minus all scans from the no conflict condition). Thus, the adjusted mean image reflected the change in brain activity between the easier no conflict condition and the more difficult conflict condition. This “conflict effect” was assumed to be directly related to the difference in cognitive effort required by the two conditions. In the computation of these adjusted images, within-session variations in global activation were adjusted using proportional scaling. There were no significant differences in the correlation between the global activation (i.e., the sum of all intracerebral voxels) and the conditions of interest (i.e., the sum of all activated voxels) for the no conflict condition and the conflict condition scans.26-29 All coordinates were converted from MNI space to Talairach space using a nonlinear transformation.
In order to identify the brain regions activated during performance of our modified Stroop Task conditions (differences in activation between the two conditions), we first tested a group of comparison subjects (n = 10) who were not included in this study but who performed the identical modified Stroop Task conditions. Three regions were identified using a fixed effects analysis: the left ACC (x = −6, y = 14, z = 44; [BA 32]) and the bilateral prefrontal cortex (left: -30,43,14 [BA 10]; right: 38,36,18; [BA 10]). In the second stage of the analyses, the single combined adjusted image from each of the 13 comparison subjects and 13 cocaine abusers was entered into a two-sample t test (with 24 degrees of freedom) employing a random effects model. Three regions of interest were examined (6-mm spheres, left ACC and left lateral prefrontal cortex, right lateral prefrontal cortex), centering on the coordinates that showed greater activation for the conflict condition than the no conflict condition in the pilot study. To correct for multiple comparisons, we employed the “small volume correction” method that was implemented in SPM99,29 with the experiment-wise false positive rate (Type I error) for a particular region of interest at the α < 0.05 level.
Because we were interested in both the right and left ACC and did not hypothesize a lateralized effect, we identified a fourth region of interest (6-mm sphere) in the right ACC (x = 10,y = 8, z = 48) based on findings reported in the literature using a similar modified Stroop Task (congruent versus incongruent).14 The coordinates were transformed from Talairach space to MNI space before being analyzed in SPM99. A post hoc exploratory analysis using a threshold of p < 0.001, with an extent threshold of 50 voxels and corrected for the search volume of the whole brain, was also conducted to search for additional regions that may be affected by cocaine abuse other than our regions of interest. We also performed correlation analyses (n=13) between intensity of cocaine use (grams of cocaine used per week) and brain activity during performance of the task.
RESULTS
Demographics and Drug Use (Table 1).
The comparison group (n = 13) was matched to the cocaine group (n = 13) on intelligence quotient (IQ) and sex. All participants were right handed. No group differences were found in years of education, maternal education, Shipley IQ, or Hollingshead Index of socioeconomic status. The cocaine group was older (36 years versus 30 years). Smoking was the main route of cocaine self-administration (100% smoked, and 84% smoked exclusively). Estimates were made of overall average amount and frequency of cocaine use (grams per week). Grams per week were estimated from participants’ self-reports of how much money was spent each week and U.S. Drug Enforcement Agency estimates for the Baltimore area ($100 per gram for 50% purity). Cigarette smoking was more common in the cocaine group (11/13) than the comparison group (7/13). None of the comparison subjects had used marijuana in the 3 months prior to admission into the study.
Consistent with our a priori hypotheses, the cocaine abusers had less conflict-related activation (conflict minus no conflict condition) than comparison subjects in the left ACC (peak located at –6, 18, 41, [BA 32]; t(24)=3.10, p < 0.03, corrected for multiple comparisons; cluster of 28 contiguous voxels at a t(24)=1.71 or greater) and right LPFC (peak located at 38, 34, 20, [BA 10]; t(24)=2.33, p < 0.05, corrected for multiple comparisons; cluster of 17 contiguous voxels at a t (24)=1.71 or greater) (Figure 1A). No significant group differences were observed in the left LPFC region of interest. Other investigators reported activation in the right ACC when comparing congruent to incongruent Stroop Task conditions, and we used the coordinates of this effect in the right ACC (x = 10, y = 8, z = 48) to test for group differences.14 Cocaine abusers had significantly greater activation than comparison subjects in the right ACC (peak located at 10, 11, 34, [BA 32]; t(24)=3.34, p < 0.02, corrected for multiple comparisons; cluster of 30 contiguous voxels) (Figure 1B). Exploratory whole brain analysis did not reveal other brain regions that showed statistically significant group differences.
Since we did not perform absolute quantification of cerebral blood flow, we examined whole brain activity differences between the groups for the mean of the two resting scans and the mean of the two scans obtained during the no conflict condition. No significant group differences in activation were found between groups, even at a threshold of p < 0.05 –uncorrected, for either the resting or no conflict condition. We also addressed the possibility that group differences in vascular reactivity may be responsible for the observed effects in the ACC and LPFC by examining changes in brain activity in the motor cortex, where we expected normal levels of activation in both groups.30 Using coordinates suggested by previous studies,31 we found no group differences in the motor cortex (-26, -44, 65). This finding suggests that the cocaine abusers activate the motor cortex in a fashion similar to non-drug-using comparison subjects while they fail to activate the left ACC and right LPFC and over-activate the right ACC. Thus, the observed group differences in brain activity in the left and right ACC and right LPFC are not due to global group differences in vascular reactivity. No between-group differences for the resting or no conflict conditions suggest that cocaine abusers did not have a different baseline level of activation than the comparison subjects. Rather, they failed to activate to the same degree as the comparison group during the more cognitively demanding conflict condition in the left ACC and right LPFC, while showing greater activation in the right ACC.
Correlation analyses between brain activity and grams per week of cocaine use revealed two significant clusters. The first cluster was located in the rostral Anterior Cingulate Cortex (rACC) (peak located at x = 2, y = 33, z = 8, [BA 24]; t(12) = 6.12; cluster of 1132 voxels contiguous, p < 0.01, corrected) (Figure 1D). The second cluster was located in the right LPFC (peak located at x=38, y= 28, z = 10, [BA13]; t(12)=5.66; cluster of 856 contiguous voxels, p < 0.05, corrected for multiple comparisons). The greater the amount of cocaine used (grams per week) the lower the activity at the epicenter of each cluster [rACC (r = −0.88) and right LPFC (r = −0.86)] (Figure 2).
Task Performance Analyses.
No significant group differences were observed in the reaction times (RT) for the no conflict or conflict conditions, the RT difference scores (mean RT conflict condition minus mean RT no conflict condition), the interference scores,25 the number correct, or the error scores.
Discussion
Abstinent cocaine abusers showed less activation than comparison subjects in the left ACC and right LPFC and more activation in the right ACC when performing a modified Stroop Task, suggesting a conflict-related effect. In addition, the amount of cocaine used prior to the 23-days of enforced abstinence was negatively correlated with large areas of activation in the rostral-ventral (infragenual) ACC and right LPFC. These findings suggest that 23-day abstinent cocaine abusers exhibit persistent functional brain abnormalities in prefrontal brain circuits involved in executive cognitive function (ECF) and that the effects are related to cocaine use since those with the highest cocaine use also showed the lowest activation in prefrontal regions. Compromised prefrontal brain circuitry, and thus difficulty with ECF may favor susceptibility to addiction or resistance to treatment.
Although we cannot determine causality from this study, the dose-related association between the amount of cocaine administered and activations in the rostral-ventral ACC and right LPFC supports the hypothesis that chronic, heavy use of cocaine contributes, at least partly, to persistent prefrontal cortical dysfunction. One possible mechanism for cocaine-induced prefrontal cortical dysfunction relates to the vasoconstrictive actions of cocaine and the purported associated ischemic changes.32 However, we believe that reduced activation in the left ACC and right LPFC and increased activation in the right ACC in cocaine abusers is a specific effect rather than a general phenomenon since no between-group differences were found in brain activity in the resting scans, no conflict scans, or in the pattern of activation in the left motor cortex. Another possible mechanism for dysfunction of the ACC and LPFC may relate to tissue volume loss in these specific brain regions. In the identical sample of cocaine users and comparison participants that were described in this study, the cocaine group showed smaller brain volumes in the ACC and LPFC, as tested with voxel-based morphometry (VBM).33 It may be impossible, however, to separate cerebrovascular from neuronal mechanisms to explain the alterations in brain activity in cocaine abusers.
Although the two groups studied differed in mean age and proportion of individuals who were smokers, we found no differences in activation between smokers and nonsmokers. Also, no significant correlations were observed between age and activation in the ACC or LPFC in our entire group of 26 participants.
Grams of cocaine used were negatively correlated with activation in the rostral-ventral division of the ACC (infragenual) (Figure 1D), whereas group differences in activation during task performance were found in divisions of the caudal-dorsal (midcingulate) ACC (Figure 1C). The rostral-ventral division of the ACC is believed to play a role in emotional processing, whereas the caudal-dorsal division is involved in cognitive processing.6,34 It therefore, appears that the amount of cocaine intake influenced function of the emotional—rostral—ventral division of the ACC. Blunting of activation in a brain region associated with emotion may have important ramifications for the development and persistence of drug dependence, especially since higher amounts of cocaine use were associated with lower activation in the emotional division of the ACC. More cocaine use was also associated with lower activation in the right LPFC. Similar negative associations between cocaine use and brain activity in these two brain regions likely reflect the reciprocal connectivity between these two regions.9,15
Cocaine abusers did not show poorer behavioral performance than non-drug-using comparison subjects on either condition of our modified Stroop Task. One explanation for this finding is that cocaine abusers may utilize different neural pathways than those used by the comparison subjects to perform our task. This hypothesis is supported by our finding of higher activation in the cocaine users in the right ACC, possibly reflecting a compensatory mechanism. Nevertheless, reports on the laterality of activation in the ACC during performance on other versions of the Stroop Task are mixed. Some studies report only right-sided activation. Other studies report left-sided activation, and some report bilateral activation.35 In this study, we found both right and left ACC group differences in activation in the same caudal-dorsal division of the ACC as observed by others in normal subjects and schizophrenics.11,14,15 Our neuroimaging findings are, therefore, biologically plausible and consistent with those of others. Further speculation about the significance of the differences in function between the right and left ACC would be premature since little has been described about the functional asymmetry of the ACC. The present data, however, suggest that an asymmetry exists.
Another possible reason for the lack of group differences in performance is the weaker sensitivity of behavioral assays compared with neuroimaging for detecting abnormalities or differences in brain functioning.31 Indeed, on the standardized Stroop Task used for clinical purposes, lower performance was associated with increasing cocaine use in 56 cocaine abusers.20 Thus, between-group performance differences may become apparent with a larger sample size.
Although our data does not show behavioral evidence of impairment in ECF, we do show abnormalities in the brain regions responsible for the complex mental process of disassociation between intact behavior/performance and abnormal metabolic functioning, which may occur because regions downstream and parallel to the affected brain areas might subsume the role of dysfunctional regions. Cortico-cortical connections and distributed connections in the various cortical brain regions36 may make such a compensatory mechanism viable. During periods of stress, however, when it might be important to inhibit certain behaviors (e.g., drug self-administration), cocaine abusers might find it difficult to use compensatory neural mechanisms to overcome faulty self-monitoring, especially in situations involving conflict (e.g., attempting to refrain from drug use in the presence of drug-related cues). Finally, impairments in ECF may be a common denominator in the evolution of maladaptive behaviors, not only in substance abuse but also in other neuropsychiatric disorders such as posttraumatic stress disorder,37 obsessive-compulsive disorders,38 and attention-deficit disorder.39 An understanding of the neural mechanisms involved in executive cognitive control, especially under conditions of conflict, may elucidate the neural substrates involved in a variety of neuropsychiatric disorders.
ACKNOWLEDGMENTS
This study was supported by NIH grants DA 11426 (KB), the JHBMC-GCRC (MO1 RR02719), and the DHH NIDA Intramural Research Program.
The authors thank the nurses and clinical staff at NIDA-IRP, the Brain Imaging Center, and the Bayview GCRC who contributed to this project. We especially thank Debra Hill, B.A. for computer and database support and Kathy Winters, Ph.D. for the programming of the modified Stroop Task.
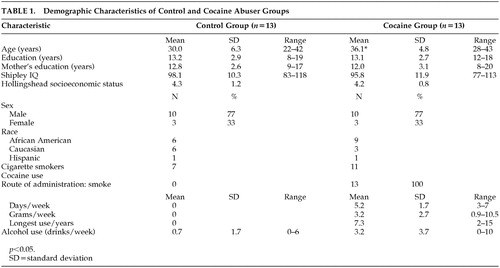 |
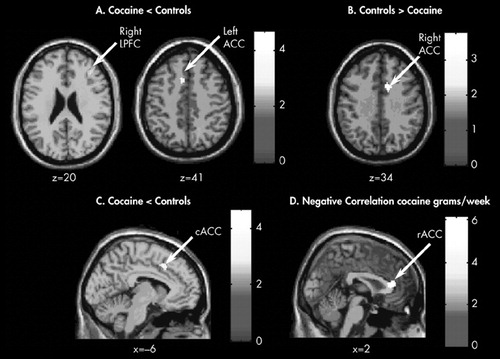
FIGURE 1. For Illustrative Purposes Figures are Presented at a Threshold of p < 0.05, Uncorrected and Nonapriori Regions Have Been Excluded. All Images are in Neurological Orientation (Right is Right). Color Bars Indicate t Test Values
(A) Brain regions showing significantly less activation in the cocaine abusers than control participants with the modified Stroop paradigm (Conflict condition minus No Conflict condition); right LPFC (x = 38, y = 34, z = 20) and left ACC (x = −6, y = 18, z = 41).
(B) Brain regions showing greater activation in the cocaine abusers than control participants in the right ACC (x = 10, y = 11, z = 34).
(C) Caudal ACC (cACC) showing less activation in the cocaine abusers than the controls (x = −6, y = 18, z = 41) during performance of the modified Stroop Task.
(D) Activation of the rostral ACC (rACC) (x = 2, y = 33, z = 8) where activation was negatively correlated with the number of grams of cocaine used per week ( r = −0.88).
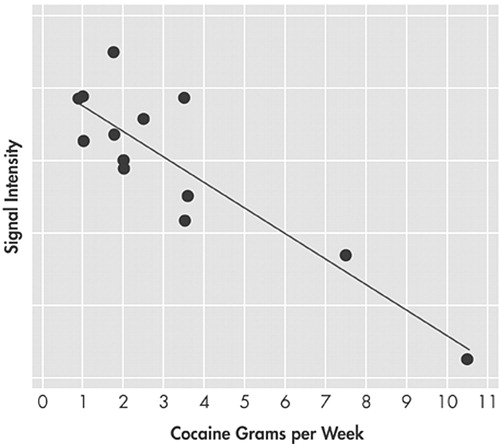
FIGURE 2. Regression Between Activation at the Epicenter of the Rostral ACC (x = 2, y = 33, z = 8) and Number of Grams of Cocaine Used Per Week (r = −0.88)
1 National Household Survey on Drug Abuse: U.S. Department of Health and Human Services Substance Abuse and Mental Health Services Administration Office of Applied Studies. Washington, D.C.: U.S. Government Printing Office 1999Google Scholar
2 National Drug Control Strategy FY 2001 Budget Summary, pg. 2. Office of National Drug Control PolicyGoogle Scholar
3 Stapleton JM, Morgan MJ, Phillips RL, et al.: Cerebral glucose utilization in polysubstance abuse. Neuropsychopharmacology 1995; 13:21–31Crossref, Medline, Google Scholar
4 Volkow ND, Mullani H, Gould KL, et al. : Cerebral blood flow in chronic cocaine abusers: A study with positron emission tomography. British Journal of Psychiatry 1988; 152:641–648Crossref, Medline, Google Scholar
5 Liu X, Matochik JA, Cadet J-L, et al.: Smaller volume of prefrontal lobe in polysubstance abusers: A magnetic resonance imaging study. Neuropsychopharmacology 1998; 18:243–252Crossref, Medline, Google Scholar
6 Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences 2000; 4:215–222Crossref, Medline, Google Scholar
7 Carter CS, Braver TS, Barch DM, et al.: Anterior cingulate cortex, error detection, and the online monitoring of performance. Neuroscience 1998; 280:747–749Google Scholar
8 Kiehl KA, Liddle PF, Hopfinger JB: Error processing and the rostral anterior cingulate: An event related fMRI study. Psychophysiology 2000; 37:216–223Crossref, Medline, Google Scholar
9 Leung H-C, Skudlarski P, Gatenby JC, et al.: An event-related functional MRI study of the Stroop color word interference task. Cerebral Cortex 2000; 10:552–560Crossref, Medline, Google Scholar
10 MacDonald AW, Cohen JD, Stenger VA, et al.: Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288:1835–1838Crossref, Medline, Google Scholar
11 Nordahl TE, Carter CS, Salo RE, et al.: Anterior cingulate metabolism correlates with Stroop errors in Paranoid Schizophrenia patients. Neuropsychopharmacology 2001; 25:139–148Crossref, Medline, Google Scholar
12 Roy CW, Sherrington CS: On the regulation of the blood supply of the brain. Journal of Physiology (London) 1890; 11:85–108Crossref, Medline, Google Scholar
13 Dolan RJ, Friston KJ: Positron Emission Tomography in psychiatric and neuropsychiatric disorders. Seminars in Neurology 1989; 9:330–337Crossref, Medline, Google Scholar
14 Carter CS, Mintun MA, Cohen JD: Interference and facilitation effects during selective attention: An H215O PET Study of Stroop Task performance. NeuroImage 1995; 2:264–272Crossref, Medline, Google Scholar
15 Carter CS, Mintun MA : Anterior cingulate gyrus dysfunction and selective attention deficits in Schizophrenia: H2015 PET study during single-trial Stroop Task performance. American Journal of Psychiatry 1997; 154:1670–1675Crossref, Medline, Google Scholar
16 Spreen O, Strauss E: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press 1991Google Scholar
17 Pardo JV, Pardo PJ, Janer KW, et al.: The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Procedures of the National Academy of Sciences 1990; 87:256–259Crossref, Medline, Google Scholar
18 Bench CJ, Frith CD, Grasby PM, et al.: Investigations of the functional anatomy of the attention using the Stroop Test. Neuropsychologia 1993; 31:907–922Crossref, Medline, Google Scholar
19 Bolla KI, Cadet J-L, London ED : The neuropsychiatry of chronic cocaine use. Journal of Neuropsychology and Clinical Neurosciences 1998; 10:280–289Link, Google Scholar
20 Bolla KI, Funderburk FR, Cadet J-L: Differential effects of cocaine and cocaine + alcohol on neurocognitive performance. Neurology 2000; 54:2285–2292Crossref, Medline, Google Scholar
21 Smith SS: Addictive drug survey manual. Baltimore, NIDA Addiction Research Center 1991Google Scholar
22 McLellan AT, Luborsky L, Woody GE, et al.: An improved evaluation instrument for substance abuse patients: the Addiction Severity Index. Journal of Nervous and Mental Disorders 1980; 168:26–33Crossref, Medline, Google Scholar
23 Robins LN, Helzer JE, Croughan J, et al. : National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Archives of General Psychiatry 1981; 38:381–389Crossref, Medline, Google Scholar
24 Reeves DL, Schlege R, Gilliland K: The UTCPAB and the NATO AGARD STRES Battery: Results from standardization studies. Medical Defense Biosciences Review 1991Google Scholar
25 Golden CJ: Stroop Color and Word Test: A manual for clinical and experimental uses. Wood Dale, Il: Stoelting Company 1978Google Scholar
26 Aguirre GK, Zarahn E, D’Esposito M : The variability of human, BOLD hemodynamic responses. NeuroImage 1998; 8 360–369Google Scholar
27 Desjardins AE, Kiehl KA, Liddle PF: Removal of confounding effects of global signal in functional magnetic resonance imaging analyses. NeuroImage 2001; 13:751–758Crossref, Medline, Google Scholar
28 Andersson JL: How to estimate global activity independent of changes in local activity. NeuroImage 1997; 6:237–244Crossref, Medline, Google Scholar
29 Worsley KJ, Marrett S, Neelin P, et al.: A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping 1996; 4:58–73Crossref, Medline, Google Scholar
30 Callicott JH, Ramsey NF, Tallent K, et al.: Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 1998; 183:186–196Crossref, Google Scholar
31 Kiehl KA, Liddle PF: An event-related functional magnetic resonance imaging study of auditory oddball task in schizophrenia. Schizophrenia Research 2001; 48:159–171Crossref, Medline, Google Scholar
32 Herning RI, King DE, Better WE, et al.: Neurovascular deficits in cocaine abusers. Neuropsychopharmacology 1999; 21:110–118Crossref, Medline, Google Scholar
33 Matochik JA, London EL, Eldreth DA, et al.: Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage 2003; 19:1095–1102Crossref, Medline, Google Scholar
34 Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118:279–306Crossref, Medline, Google Scholar
35 Barch D, Braver T, Akbaduk E, et al.: Anterior cingulated cortex and response conflict: Effects of response modality and processing domain. Cerebral Cortex 2001; 11:837–848Crossref, Medline, Google Scholar
36 Mesulam MM: Principles of Behavioral and Cognitive Neurology (2nd Edition). Oxford University Press. Inc. New York, USA (2000)Google Scholar
37 Shin LM, Whalen PJ, Pitman RK, et al.:An fMRI study of Anterior Cingulate function in Posttraumatic Stress Disorder. Biological Psychiatry 2001; 50:932–942Crossref, Medline, Google Scholar
38 Schmidtke K, Schorb A, Winkelmann G, et al. Cognitive frontal lobe dysfunction in Obsessive-Compulsive Disorder. Biological Psychiatry 1998; 43:666–673Crossref, Medline, Google Scholar
39 Walker AJ, Shores EA, Trollor JN, et al.: Neuropsychological functioning of adults with Attention Deficit Hyperactivity Disorder. Journal of Clinical and Experimental Neuropsychology 2000; 22:115–124Crossref, Medline, Google Scholar



