Deficits in Social Knowledge Following Damage to Ventromedial Prefrontal Cortex
Abstract
Patients with damage to the frontal lobes frequently exhibit impaired social behavior, but it is not clear which specific processes are disrupted. The authors investigated the ability to interpret nonverbal emotional expression in patients with lesions involving ventromedial (N=20) or dorsolateral prefrontal cortex (N=9) and in healthy volunteers (N=23). As hypothesized, only patients with ventromedial prefrontal lesions showed impaired task performance relative to normal comparison subjects. These results suggest that deficits in social knowledge, namely difficulty interpreting nonverbal emotional expression, contribute to the aberrant social behavior observed following ventromedial prefrontal cortex lesions.
Disturbances of higher cognition and social behavior have long been recognized as common sequelae of lesions of prefrontal cortex. These behavioral changes have been linked with damage specifically involving orbitofrontal or ventromedial prefrontal cortex, but the specific emotional, cognitive, and/or physiologic processes that may be disrupted have not been well elucidated. Several mechanisms have been proposed to explain the observed deficits in social behavior following ventromedial prefrontal cortex lesions: impaired decision making due to lack of a “somatic marker,”1,2 the inability to alter behavior appropriately in response to a change in reinforcement contingencies,3,4 deficits in the ability to represent the mental states of others or “theory of mind,”5 or the inability to access social knowledge.6,7 Regarding the latter hypothesis, some authors have argued that patients with ventromedial prefrontal cortex lesions have intact social knowledge,8 but these claims have been based on single case studies or very small sample sizes. Therefore, we investigated whether patients with ventromedial prefrontal cortex lesions show greater deficits in one aspect of social knowledge; namely, the ability to interpret and utilize nonverbal emotional expression, relative to healthy comparison subjects.
To test this hypothesis, we compared the performance of patients with ventromedial prefrontal cortex or dorsolateral prefrontal cortex lesions with healthy volunteers on the Tests of Social Intelligence (TSI).9 The TSI includes a series of drawings and cartoons that require subjects to use nonverbal cues to interpret social and emotional situations. Examples of tasks include choosing the correct panel to match the emotions of cartoon characters expressed using facial expression or body language or completing a cartoon strip that depicts events within a social interaction. One of the tasks includes language, but subjects must “read between the lines” to understand the social meaning of the verbal expression.
Based on previous studies of frontal lobe patients,1,2,4,5,7,10–21 we hypothesized that patients with ventromedial prefrontal cortex lesions, but not those with lesions involving dorsolateral and other prefrontal cortex areas outside of ventromedial sectors, would be impaired on the TSI, relative to healthy volunteers. In addition, to better understand any observed deficits in social cognition, we compared performance on the TSI with a standard task from the performance (nonverbal) scale of the Wechsler Adult Intelligence Scale (WAIS-R and WAIS-III),22,23 as well as an observer-rated measure of real-life behavioral functioning, the Neurobehavioral Rating Scale.24
METHODS
Subjects
Twenty-nine consecutive outpatients (26 males, three females) with nonprogressive prefrontal cortex lesions and 23 normal comparison subjects (21 males, two females) matched for age and education level were studied. The ventromedial group included 20 patients (18 males, two females), while the dorsolateral group comprised nine patients (eight males, one female). There were no significant differences among the two patient groups or the normal comparison subjects in terms of age (ventromedial: mean=53.1 years, SD=6.8; dorsolateral: mean=50.3 years, SD=9.3; normal comparison: mean=50.1 years, SD=11.5) (F=0.56, df=2, 49, p=0.57) or number of years of education (ventromedial: mean=14.3, SD=2.7; dorsolateral: mean=13.8, SD=2.5; normal comparison: mean=14.2, SD=1.8) (F=0.18, df=2, 49, p=0.84).
Lesion Analysis
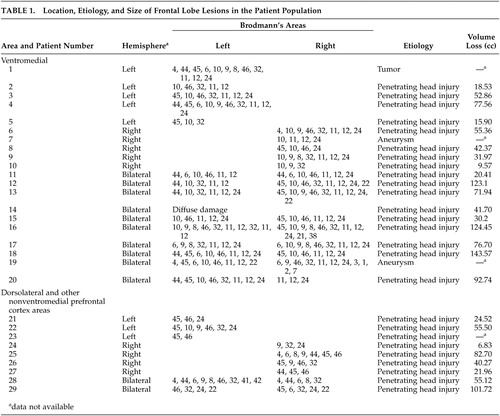
Details of prefrontal cortex lesion data for patients are presented in Table 1. All had lesions confined to the frontal lobes, with the majority secondary to penetrating head injury from missiles or shrapnel incurred during the Vietnam War (N=26). Other etiologies were similarly nonacute and included tumor resection (N=1; 9-month-old injury) and stroke from aneurysmal subarachnoid hemorrhage (N=2; 6- and 11-year-old injuries).
We used templates from Damasio and Damasio25 to delineate the location of lesions. Patients were classified according to the presence of ventromedial prefrontal cortex damage (ventromedial group, N=20), or absence, the latter of which included primarily dorsolateral and dorsomedial prefrontal cortex areas (dorsolateral group, N=9). Ventromedial prefrontal cortex was defined as including any of Brodmann’s areas 11, 12, 13, 14, 47, and ventral area of 10 based on CT or MRI scans. Representative ventromedial prefrontal cortex and dorsolateral lesions are illustrated in Figure 1. Lesion size was available for 17 of the ventromedial patients and eight of the dorsolateral patients and did not differ between the two groups (ventromedial prefrontal cortex: mean=60.5 cc, SD=41.1; dorsolateral: mean=48.6 cc, SD=32.0) (t=0.72, df=32, p=0.48).
All participating subjects understood study procedures and gave their written informed consent to participate in the study. This work was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke, Bethesda, Maryland.
Materials
TSI.
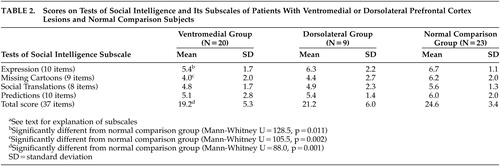
The TSI consists of four different subtests (Expression, Missing Cartoons, Social Translations, and Cartoon Predictions) designed to measure the ability to understand the thoughts, feelings, and intentions of other people as expressed in behavior.9 Split-half reliability is reported at 0.72 for the TSI, and performance on the TSI correlates with indices such as job success, peer ratings of social preference, and performance in interpersonal training courses.9 To minimize the influence of verbal abilities, memory, and general intelligence on performance, cartoons and drawings are primarily used, with the exception of the Social Translations subtest, which requires subjects to interpret verbal phrases in a given social context.
The original TSI included 30 items in the Expression set, 27 in the Missing Cartoons set, 24 in the Social Translations set, and 30 in the Cartoon Predictions set. To prevent fatigue, we shortened the original subtests by randomly assigning test items to form three evenly divided blocks of items. For example, the 30 items in the Expression grouping were randomly assigned to three separate blocks with 10 items in each block. Subjects completed a 10-item block from the Expression set, a 9-item block from the Missing Cartoons set, an 8-item block from the Social Translations test, and a 10-item block in the Cartoon Predictions Set, for a total of 37 items for the entire TSI task. Blocks for each of the subtests were randomly assigned to subjects, but all subjects completed the subtests in the following order: Expression, Missing Cartoons, Social Translations, and Cartoon Predictions. The total possible score on our modified version of the TSI was 37.
Our modified versions of the TSI were not likely to bias the results since we randomly allocated blocks of test items to subjects. In addition, rather than comparing performance on the TSI with published normative data on the original TSI, we recruited a group of matched healthy volunteers to establish new norms for the revised test. Importantly, the performance of healthy volunteers on the revised TSI (mean score of 26.6 on modified 37-item TSI=66.4%) was comparable to norms reported in the scoring manual for the original test (mean score of 80.2 on original 121-item TSI=66.3%).9
TSI Subtests.
1. Expression. This subtest presents subjects with drawings of hand gestures, body postures, and facial expressions that show the same thought, feeling or intention. Subjects must choose one of four alternative drawings that express the same emotion as the preceding drawings.
2. Missing Cartoons. Subjects are instructed to choose one of four alternative cartoon panels to complete a series of panels depicting characters interacting in an everyday social setting. Correct selection of the missing panel requires subjects to accurately interpret the character’s thoughts and feelings so that the completed cartoon story is coherent.
3. Social Translations. This task assesses the ability to interpret the meaning of verbal statements in different social contexts. Subjects are instructed to match different social situations that would convey the same meaning for a verbal phrase. For example, the expression “please” when used by an employer to employee, would have a similar meaning when used by a father to a son. In contrast, the same expression would have a different meaning when used by a beggar to a stranger or a chauffeur to his employer. Subjects choose from three possible response options.
4. Cartoon Predictions. This subtest measures the ability to predict social consequences by interpreting the intention and feelings of characters. Subjects must choose the correct panel from three alternatives that depict the event that would follow, based on the character’s emotional reaction to a situation.
Neuropsychological.
We determined the association between performance on the TSI and scores on the Picture Arrangement subtest of the WAIS-R or WAIS-III.22,23 Subjects enrolled prior to 1997 (N=6) received the WAIS-R while the remaining (N=23) received the more recent version (WAIS-III). There were no differences in performance on the TSI for subjects who received earlier versus more recent versions of the intelligence tests (t=0.23, df=27, p=0.82). We focused on the picture arrangement task because it primarily tests the ability to plan, interpret, and accurately anticipate consequences in nonverbal interpersonal situations.26 Subjects are required to rearrange cards that depict social situations into a cohesive story, but do not choose from possible alternatives to complete the story. Therefore, we expected that skills used to complete the Picture Arrangement task would contribute to performance on the TSI.
Behavioral.
To determine whether performance on the social cognition tasks was indicative of observable, everyday behavior, patients were assessed using the Neurobehavioral Rating Scale.24 The Neurobehavioral Rating Scale is a validated measure of neuropsychiatric symptomatology resulting from head injury, such as loss of insight, disinhibition, and impaired attention. The behavior of patients with prefrontal cortex lesions was rated by the research assistant during neuropsychological testing. Data were available for 25 of the 29 subjects. There were no differences in performance on the TSI for subjects who had a Neurobehavioral Rating Scale score and those who did not (t=0.45, df=27, p=0.45).
All tasks were administered individually. No constraints on time were imposed to complete the TSI.
Statistical Analysis
We compared differences in TSI performance between each patient subgroup and the normal comparison group using the nonparametric statistical equivalent of a planned independent t test, the Mann-Whitney U test, because the TSI data (particularly subscale scores) were not normally distributed. We used overall scores on the TSI (“total TSI score”) and scores on each of the TSI subtests as dependent measures for these analyses. Analysis of variance and t tests were used to examine group differences on demographic and clinical variables. Pearson’s correlation was used to determine associations between performance on the TSI and other neuropsychological and behavioral measures. The alpha level was set at 0.05, two-tailed probability was used for all analyses.
RESULTS
Effects of Lesion Location on TSI Performance
Mean scores on the TSI and its subscales for both patient groups and normal comparison subjects are reported in Table 2.
All subjects performed at above chance levels, indicating that errors were not due to simply guessing at random (score 10.75 out of 37 if performing at chance). This is particularly important in the case of ventromedial patients, who may make errors due to difficulties in inhibiting responses, rather than to a true deficit in interpreting and using nonverbal emotional cues. As indicated previously, the performance of healthy volunteers in our study (mean score of 26.6 on modified 37-item TSI=66.4%) was comparable to norms reported in the scoring manual (mean score of 80.2 on original 121-item TSI=66.3%).9
As hypothesized, ventromedial patients had lower total TSI scores as compared with the normal comparison group (Mann-Whitney U=88.0, p=0.001), while dorsolateral patients were not significantly impaired relative to normal comparison subjects (Mann-Whitney U=71.0, p=0.18). Ventromedial patients were specifically impaired on the Expression and Missing Cartoons subtests (U=128.5, p=0.011; U=105.5, p=0.002, respectively). In other words, ventromedial patients had difficulty choosing drawings to match an emotion expressed through facial expression, gestures, or body posture (Expression subtest) or to complete a series of cartoon panels depicting a social interaction (Missing Cartoons subtest).
We wished to evaluate the possibility of a failure to find differences in performance on the TSI between the dorsolateral and normal comparison groups due to low statistical power given the smaller number of dorsolateral patients (N=9). Thus, we reanalyzed the TSI data by dividing the ventromedial group according to whether lesions were unilateral (N=10) or bilateral (N=10), since these sample sizes were more comparable to that of the dorsolateral patients. For these analyses, total TSI scores were used. Both ventromedial groups performed more poorly on the TSI as compared to the normal comparison group, although the group difference for patients with unilateral ventromedial prefrontal cortex lesions trended only toward statistical significance (normal comparison, mean=24.6, SD=3.4; unilateral ventromedial prefrontal cortex: mean=20.6, SD=6.0; Mann-Whitney U=69.5, p=0.074; bilateral ventromedial prefrontal cortex: mean=17.8, SD=4.3; Mann-Whitney U=18.5, p<0.001). Thus, it is unlikely that low statistical power alone accounted for the failure to find statistically significant differences between dorsolateral and normal comparison groups.
Relationship Between Measures of Social Cognition and Neuropsychological Tests
We examined the association between performance on the TSI and the Picture Arrangement subtest of the WAIS-R/WAIS-III, a task requiring similar cognitive abilities. In dorsolateral patients, total TSI and Picture Arrangement scores were strongly correlated (r=0.81, N=9, p=0.009), but this was not the case for patients with ventromedial prefrontal cortex lesions (r=0.21, N=20, p=0.38). These results suggest that the deficits observed in ventromedial patients on the TSI were specific to the emotional elements inherent in the TSI, rather than to a more general cognitive difficulty in arranging images to create a logically coherent story.
Relationship With Neurobehavioral Rating Scale
As shown in Figure 2, performance on the TSI was negatively and moderately correlated with ratings of behavior on the Neurobehavioral Rating Scale in both the entire patient group (r=−0.47, p=0.018) and among ventromedial patients only (r=0.44, p=0.078). These results indicate an association between social cognitive impairment and greater observed psychopathology.
DISCUSSION
The primary aim of this study was to examine the effects of ventromedial prefrontal cortex lesions on the ability to use nonverbal cues to interpret emotional expression and social interaction. Consistent with our hypotheses, ventromedial patients showed deficits on the TSI, with specific impairments in the ability to use nonverbal cues to understand emotional expression (Expression subtest), as well as the ability to complete a socially meaningful story by interpreting the feelings and behavior of characters (Missing Cartoons subtest). No differences in performance between patients and comparison subjects were found on the subtests that assess the ability to interpret the meaning of verbal statements in different social contexts (Social Translations subtest) and to predict social consequences by interpreting the intention and feelings of others (Cartoon Predictions). We found that deficits on the TSI were not related to more general cognitive impairment on another nonverbal task, the Picture Arrangement test from the Performance subscale of the WAIS, and TSI deficits were correlated with ratings of real-life behavior as determined by the Neurobehavioral Rating Scale.
Our findings are broadly consistent with the experimental literature showing deficits in socially relevant behavior following ventromedial prefrontal cortex lesions.1,2,4,5,7,10–21 In particular, previous studies have demonstrated impairment in identifying emotional expression using face or voice stimuli following ventral frontal damage7,27 and deficits in representing the mental states of others on verbal theory of mind tasks.5,10 These findings are in line with our results which demonstrate deficits in perception of nonverbal emotional cues (Expression subtest) and in interpreting the thoughts, feelings, and behavior of characters to create a story (Missing Cartoons subtest) in ventromedial patients.
Two case reports also included portions of the TSI as a measure of social cognition, with variable results. Saver and Damasio8 found that E.V.R., a patient with ventromedial prefrontal cortex bifrontal injuries, performed within the normal range on the Cartoon Predictions subtest of the TSI, which tests the ability to predict the most likely consequences of a social situation, consistent with our results. They did not administer either of the two subtests for which we found ventromedial patients were impaired (Expression and Missing Cartoons). Three of the TSI subtests were used in another case study of a woman with traumatic orbitofrontal brain injury by Cicerone et al.28 Similar to our ventromedial group, this patient showed deficits in interpreting thematic relationships involving nonverbal interpersonal interactions (Missing Cartoons). However, in contrast to the study reported by Saver and Damasio8 and our findings, this patient was found to be impaired on the Cartoon Predictions subtest and in interpreting the meanings of verbal, social exchanges in different contexts (Social Translations). We did not find deficits in performance on the Social Translations subtest in our ventromedial prefrontal cortex subjects, and speculate that the use of verbal rather than nonverbal, stimuli may not have adequately tapped into the social cognitive impairment observed following ventromedial prefrontal cortex damage. For example, it may be that ventromedial patients partially compensate for their social cognitive deficits by recruiting verbal strategies to perform well on the Social Translations subtest. We also did not detect statistically significant differences between ventromedial patients and healthy volunteers on the Cartoon Predictions subtest, a task similar to the Missing Cartoons subtest. This may be due, in part, to the greater variability in performance in the ventromedial group in using nonverbal cues to predict the behavior of characters. The overall inconsistency of these results across studies highlights the difficulty in interpreting results from single case reports and points to the need for studies of adequate samples of ventromedial patients. Although requiring replication, our results suggest that lesions of ventromedial prefrontal cortex are associated with deficits in social knowledge; namely, the inability to understand nonverbal behavioral cues of emotional expression.
We also found that the deficits on the TSI were not associated with performance on the Picture Arrangement subtest of the WAIS. Since both tasks present nonverbal stimuli in which subjects must use the behavior of characters to interpret a social situation, we initially hypothesized a relationship between scores on the Picture Arrangement and the TSI. However, we found a strong association between the two tasks in the dorsolateral group, but not in the ventromedial group. These results indicate that similar cognitive and emotional processing skills are used in the Picture Arrangement and TSI by patients with dorsolateral lesions, and that the deficits observed in ventromedial patients appear to be specific to the TSI (e.g., a social cognitive component), rather than to general impairment in performing a nonverbal task involving a sequence of actions. It may be that the TSI differs from the Picture Arrangement in that it emphasizes the ability to infer the thoughts and feelings of characters, similar to theory of mind tasks in which verbal stimuli are presented. This could be empirically validated by comparing the performance of ventromedial patients on the TSI and on typical verbal theory of mind tasks.5
Importantly, we found that deficits in social knowledge as assessed by the TSI were associated with increased ratings of neuropsychiatric disturbance on the Neurobehavioral Rating Scale in the patient group as a whole and a near-significant trend in the ventromedial prefrontal cortex sample alone. This finding suggests that impaired ability to interpret nonverbal cues of emotional expression and social interaction may contribute to the socially aberrant behavior described in case reports of ventromedial patients such as Phineas Gage29,30 and E.V.R.31 In addition, the observed association between the TSI and Neurobehavioral Rating Scale suggests that behavioral disturbances can be predicted by performance on objective social-cognitive tasks. Thus, the TSI, or a similar task, may be useful in clinical settings to predict and monitor social and behavioral dysfunction following neuropsychiatric injury.
One limitation of our study relates to differences in sample size between the two patient groups, with the ventromedial prefrontal cortex sample comprised of twice the number of patients as the dorsolateral group. It may be argued that low statistical power accounted for the failure to find significant differences in task performance between the smaller dorsolateral group and normal comparison subjects. However, when we reanalyzed a subset of the data to compare ventromedial prefrontal cortex subgroups (unilateral or bilateral lesions) of similar sample sizes (N=10) to that of the dorsolateral group (N=9), the finding of impaired TSI performance in ventromedial patients persisted. In addition, our finding of social cognitive deficits in ventromedial prefrontal cortex, but not dorsolateral patients, is consistent with the few studies that have directly compared the two patient groups. Patients with dorsolateral lesions have been shown to perform normally on theory of mind tasks,5 implicit association tasks,19 and the Iowa Gambling Task.16 However, one study provided contrary results. Manes and colleagues20 found that patients with dorsolateral lesions (N=4) and dorsomedial lesions (N=5) showed some impairment on decision-making tasks relative to comparison subjects. We found no evidence supporting a role for dorsolateral prefrontal cortex areas in interpreting nonverbal cues of emotional expression.
Additionally, previous studies of patients with focal brain damage suggest the contribution of the right hemisphere to emotional expression,32–34and emotional perception35–37 and a specific role for right ventromedial prefrontal cortex areas in mediating altered emotion, behavior, and decision making.38 Tranel and his colleagues38 found that while patients with right-sided ventromedial prefrontal cortex lesions (N=4) were more impaired on the gambling task relative to patients with left-sided ventromedial prefrontal cortex lesions (N=3), the latter group did not perform as well as normal comparison subjects. Patients with right-sided ventromedial prefrontal cortex damage showed similar impairments to those with bilateral ventromedial prefrontal cortex lesions, with a tendency to choose from disadvantageous card decks and generating smaller anticipatory skin conductance responses. We were unable to examine laterality by lesion location effects in our study because of inadequate numbers within each cell.
In summary, we found objective evidence of deficits in social knowledge in patients with ventromedial prefrontal cortex damage as compared with normal comparison subjects. We propose that the inability to access social knowledge, which serves to guide appropriate behavior, may be one mechanism whereby patients with ventromedial prefrontal cortex lesions exhibit aberrant social behavior. Few studies of ventromedial patients7 have evaluated for deficits in social knowledge using nonverbal stimuli, as was done in our study. The majority have focused on abnormalities of behavior in response to outcome contingencies2,4 or have employed verbal tasks assessing theory of mind or moral judgment.5,8 Other findings7,20,38 implicate the role of the right ventromedial prefrontal cortex in social cognitive abilities. If social cognitive ability is more lateralized towards the right hemisphere, it is possible that nonverbal stimuli would better evaluate social cognitive deficits in ventromedial patients. Future studies using comparable verbal and nonverbal tasks of social cognition are required to empirically validate this hypothesis.
ACKNOWLEDGMENTS
The authors thank Charlotte F. Manly, Ph.D., for guidance in analyzing the data and for helpful comments on a previous version of this manuscript. A portion of this work has been presented at the 14th Annual Meeting of the American Neuropsychiatric Association, Honolulu, Hawaii, February 2–4, 2003.
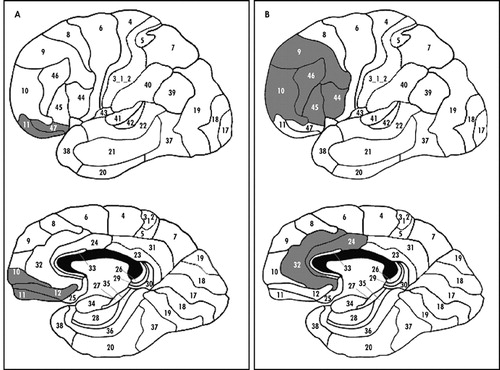
FIGURE 1. Representative Ventromedial and Dorsolateral Prefrontal Cortext Lesions
Figure 1 as shown is only numbers, not lesions.
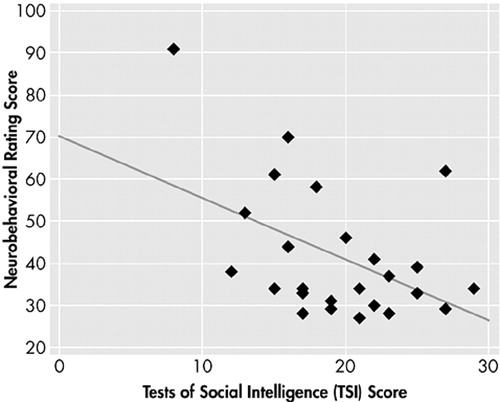
FIGURE 2. Relationship Between Social Cognitive Impairment and Observed Psychopathology
 |
 |
1 Damasio AR, Tranel D, Damasio H: Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res 1990; 41:81–94Crossref, Medline, Google Scholar
2 Bechara A, Damasio AR, Damasio H, et al: Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994; 50:7–15Crossref, Medline, Google Scholar
3 Rolls ET: The orbitofrontal cortex. Phil Trans R Soc Lond B Biol Sci 1996; 351:1433–1443; discussion 1443–1444Crossref, Medline, Google Scholar
4 Rolls ET, Hornak J, Wade D, et al: Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 1994; 57:1518–1524Crossref, Medline, Google Scholar
5 Stone VE, Baron-Cohen S, Knight RT: Frontal lobe contributions to theory of mind. J Cogn Neurosci 1998; 10:640–656Crossref, Medline, Google Scholar
6 Grafman J, Schwab K, Warden D, et al: Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 1996; 46:1231–1238Crossref, Medline, Google Scholar
7 Hornak J, Rolls ET, Wade D: Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 1996; 34:247–261Crossref, Medline, Google Scholar
8 Saver JL, Damasio AR: Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia 1991; 29:1241–1249Crossref, Medline, Google Scholar
9 O’Sullivan M, Guilford J: Four Factor Tests of Social Intelligence (Behavioral Cognition): Manual of Instructions and Interpretations. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
10 Rowe AD, Bullock PR, Polkey CE, et al: “Theory of mind” impairments and their relationship to executive functioning following frontal lobe excisions. Brain 2001; 124(part 3):600–616Google Scholar
11 Happe F, Malhi GS, Checkley S: Acquired mind-blindness following frontal lobe surgery? a single case study of impaired “theory of mind” in a patient treated with stereotactic anterior capsulotomy. Neuropsychologia 2001; 39:83–90Crossref, Medline, Google Scholar
12 Shammi P, Stuss DT: Humour appreciation: a role of the right frontal lobe. Brain 1999; 122 (part 4):657–666Google Scholar
13 Dimitrov M, Grafman J, Hollnagel C: The effects of frontal lobe damage on everyday problem solving. Cortex 1996; 32:357–366Crossref, Medline, Google Scholar
14 Bechara A, Tranel D, Damasio H, et al: Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 1996; 6:215–225Crossref, Medline, Google Scholar
15 Bechara A, Damasio H, Tranel D, et al: Deciding advantageously before knowing the advantageous strategy. Science 1997; 275:1293–1295Crossref, Medline, Google Scholar
16 Bechara A, Damasio H, Tranel D, et al: Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 1998; 18:428–437Crossref, Medline, Google Scholar
17 Bechara A, Tranel D, Damasio H: Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000; 123(part 11):2189–2202Google Scholar
18 Bechara A, Damasio H, Damasio AR, et al: Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 1999; 19:5473–5481Crossref, Medline, Google Scholar
19 Milne E, Grafman J: Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. J Neurosci 2001; 21:RC150Google Scholar
20 Manes F, Sahakian B, Clark L, et al: Decision-making processes following damage to the prefrontal cortex. Brain 2002; 125(part 3):624–639Google Scholar
21 Rogers RD, Everitt BJ, Baldacchino A, et al: Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 1999; 20:322–339Crossref, Medline, Google Scholar
22 Wechsler D: Wechsler Adult Intelligence Scale, 3rd ed (WAIS-III). San Antonio, Tex, Psychological Corp, 1997Google Scholar
23 Wechsler D: Wechsler Adult Intelligence Scale—Revised (WAIS-R). San Antonio, Tex, Psychological Corp, 1981Google Scholar
24 Levin HS, High WM, Goethe KE, et al: The Neurobehavioral Rating Scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry 1987; 50:183–193Crossref, Medline, Google Scholar
25 Damasio H, Damasio A: Lesion Analysis in Neuropsychology. New York, Oxford University Press, 1989Google Scholar
26 Groth-Marnat G: Handbook of Psychological Assessment. New York, John Wiley & Sons, 1997Google Scholar
27 Blair RJ, Cipolotti L: Impaired social response reversal: a case of “acquired sociopathy.” Brain 2000; 123(part 6):1122–1141Google Scholar
28 Cicerone K, Tanenbaum L: Disturbance of social cognition after traumatic orbitofrontal brain injury. Arch Clin Neuropsychol 1997; 12:173–188Crossref, Medline, Google Scholar
29 Harlow JM: Passage of an iron rod through the head. Boston Med & Surgical J 1848; 39:389–393Crossref, Google Scholar
30 Harlow JM: Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society 1868; 2:239–347Google Scholar
31 Eslinger PJ, Damasio AR: Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology 1985; 35:1731–1741Crossref, Medline, Google Scholar
32 Ross ED, Mesulam MM: Dominant language functions of the right hemisphere? prosody and emotional gesturing. Arch Neurol 1979; 36:144–148Crossref, Medline, Google Scholar
33 Buck R, Duffy RJ: Nonverbal communication of affect in brain-damaged patients. Cortex 1980; 16:351–362Crossref, Medline, Google Scholar
34 Weintraub S, Mesulam MM: Developmental learning disabilities of the right hemisphere: emotional, interpersonal, and cognitive components. Arch Neurol 1983; 40:463–468Crossref, Medline, Google Scholar
35 Kolb B, Taylor L: Affective behavior in patients with localized cortical excisions: role of lesion site and side. Science 1981; 214:89–91Crossref, Medline, Google Scholar
36 Borod JC, Cicero BA, Obler LK, Welkowitz J, Erhan HM, Santschi C, Grunwald IS, Agosti RM, Whalen JR: Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology 1998; 12:446–458Crossref, Medline, Google Scholar
37 Heilman KM, Bowers D, Speedie L, et al: Comprehension of affective and nonaffective prosody. Neurology 1984; 34:917–921Crossref, Medline, Google Scholar
38 Tranel D, Bechara A, Denburg NL: Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex 2002; 38:589–612Crossref, Medline, Google Scholar



