The Pocket Smell Test
Abstract
The present study extended previous work on olfactory dysfunction (odor identification deficits) by using the Pocket Smell Test (PST) to discriminate between groups of patients with Alzheimer's disease (AD), vascular dementia (VaD), and major depression (MD). Sixty patients meeting the DSM-IV criteria for either AD, VaD, or MD (20 per group) underwent assessment with the PST, a three-item screening measure of odor identification, and the Mini-Mental State Examination (MMSE). Patients with AD scored significantly lower than patients with either VaD or MD on the PST, even after controlling for MMSE scores. A PST score of ≥1 (i.e., 1 or 0 correct) discriminated between patients with and without AD with a classification accuracy of 95% (sensitivity 100%, specificity 92.5%). Olfactory assessment may be of diagnostic utility in the differential diagnosis of AD versus VaD versus MD in elderly patients.
Olfactory dysfunction in general, and impaired odor identification in particular, have been noted in a number of neuropsychiatric conditions, including Alzheimer's disease (AD),1–5 Parkinson's disease (PD),6,7 Huntington's disease,8,9 Korsakoff's amnestic syndrome,10 human immunodeficiency virus (HIV) infection,11 amyotrophic lateral sclerosis,12 motor neuron disease,13 schizophrenia,2 and advanced anorexia.14 Olfaction, however, has been shown to remain relatively intact in healthy younger adults1–3 as well as depressed adults15–17 and older adults.5 Recently, the assessment of olfactory functioning has been used to discriminate between patient and nonpatient groups. For example, investigators have reported odor identification differences between AD patients and elderly nondemented control subjects,1–3 and these differences may prove clinically useful at the level of differential diagnosis.
Solomon et al.5 used a screening measure of odor identification, the Pocket Smell Test (PST),18 to discriminate between elderly patients with AD and major depression (MD). The PST, which is derived from the University of Pennsylvania Smell Identification Test,19 was administered to a group of patients with AD and a group of patients with MD. On this three-item test, a cutoff score of two or more errors correctly classified 90% of the sample, with the AD patients being more impaired than the depressed patients. Despite the clinically significant and diagnostically useful findings of this study, the authors noted two limitations. First, there was no assessment of the subjects' cognitive functioning, and second, the effects of demographic variables (e.g., age, education, gender) were not fully examined.
In an attempt to cross-validate and extend the previous work, McCaffrey et al.20 administered the PST and a cognitive screening measure, the Mini-Mental State Examination (MMSE), to a group of patients with AD and a group of patients with MD. Additionally, the effects of age, gender, and education on the PST and MMSE in both groups were assessed. Results were similar to previous findings: AD patients scored significantly below MD patients, and the PST discriminated between the two groups with a classification accuracy of 97.5% (better than the MMSE's accuracy of 90%). Unlike the MMSE, the PST did not correlate significantly with any of the demographic variables in either of the groups. Although the discrimination between AD and MD in older adults may prove useful for the differential diagnosis of these conditions, additional comparisons (e.g., between different types of dementias) are still needed.
To our knowledge, Knupfer and Spiegel21 conducted the only comparison of the effect of different types of dementia on olfactory functioning. A series of experimental olfactory tests (e.g., olfactory thresholds, smell recognition, naming of smells) was used to compare healthy elderly control subjects with vascular dementia (VaD) patients and patients with AD. The AD patients scored significantly worse on these measures than the VaD patients, who scored below the elderly control subjects. Although this study supports the use of olfactory assessment in the differential diagnosis of AD versus VaD, its use of nonstandardized measures, which may be unavailable or impractical for clinicians, calls into question its clinical usefulness. The present study was conducted to build on our previous work with the PST, which is available and practical, in discriminating between elderly patients with AD, VaD, or MD.
METHODS
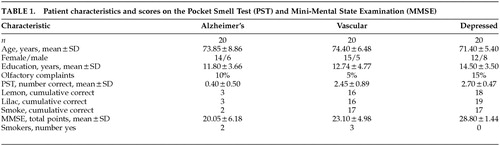
Patients were 55 years of age or older, met DSM-IV22 criteria for a diagnosis of AD, VaD, or MD, and gave informed consent. Diagnoses had been established by board-certified (adult and/or geriatric) psychiatrists, neurologists, or neuropsychologists who had the opportunity to follow these patients longitudinally. Patients were excluded if they had a history of neurologic, psychiatric, or medical disorder that could affect olfaction adversely (e.g., traumatic brain injury, schizophrenia, PD, HIV-positive status, upper respiratory illness). Demographic characteristics of the patient groups are presented in Table 1. Patients were also questioned about their smoking status and any recent change in their sense of smell. The presence/absence of current anticholinergic medication use was noted.
All patients were evaluated with PST and the Mini-Mental State Examination.23 The PST is a three-item microencapsulated “scratch and sniff” measure. On each item, the examiner releases an odor by scratching the encapsulated odor patch with a pencil; the patient then smells the odor and chooses one of the four response alternatives (one correct response and three distractors). In an effort to minimize the impact of other sensory or cognitive deficits (e.g., visual acuity or verbal memory impairment), the response alternatives were read to the patient continuously until a response was made. Patients were encouraged to guess if they were not sure. Correct responses are lemon, lilac, and smoke. The MMSE is a widely used screening measure of cognitive functioning that taps orientation, attention, short-term memory, language, and visuocontruction abilities. With a maximum score of 30 and an “impairment” cutoff score of approximately 23, the MMSE has been shown to be sensitive to the cognitive deficits in AD.
RESULTS
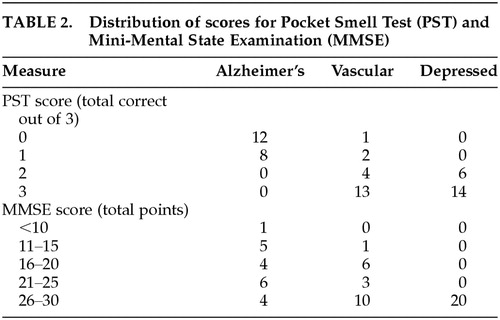
Descriptive statistics for the PST and MMSE are presented in Table 1. The distribution of PST and MMSE scores is presented in Table 2. The three groups did not significantly differ on age, gender, education, smoker status, subjective reporting of smell changes, or percentage taking anticholinergic medications. Because all three groups did differ on their performance on the MMSE (F=18.19, df=2,57, P<0.001), these scores were used as a covariate in the analyses of the PST scores. Analyses of covariance revealed that the AD patients scored significantly below the VaD and MD patients on the PST (F=55.89, df=2,56, P<0.001).
Based on a PST cutoff score of one or fewer (zero) correct items,5,20 sensitivity was 100%, specificity was 92.5%, and overall classification accuracy was 95%. This ≤1 correct cutoff score yielded no false positives or negatives in the AD group and 3 false positives in the non-AD groups (i.e., 3 VaD cases classified as AD). The remaining cutoff scores yielded less optimal hit rates.
Whereas none of the demographic variables (age, gender, education) significantly correlated with the PST for any of the groups, education significantly correlated with the MMSE for both AD patients (r=0.68, df=20, P<0.01) and VaD patients (r=0.59, df=20, P<0.01). Within the VaD group, the PST correlated with patients' reports of changes in their sense of smell (r=0.65, df=20, P<0.01) and the MMSE correlated with patients' smoking status (r=0.53, df=20, P<0.05). Within the AD group, the PST correlated with patients' anticholinergic medication status (r=–0.47, df=20, P<0.01). No other significant correlations were observed.
DISCUSSION
The results of the present study are similar to previous findings5,20 in supporting the use of the PST as a screening tool in differentiating between elderly patients with AD and those with MD. Additionally, the PST discriminates between patients with AD and those with VaD. As in earlier works,1–5 significant deficits in olfaction were observed in the AD group but not in the major depression group. The present study also observed findings similar to those reported by Knupfer and Spiegel,21 in that AD patients performed worse than VaD patients on measures of olfactory functioning.
Odor identification testing may be sensitive to AD because of early neurofibrillary tangles in the entorhinal cortex,24,25 β-amyloid deposits in the olfactory bulb regions,26 or reduced numbers of mitral cells in the olfactory bulb.27 Pathological functioning of “upstream” structures (e.g., nasal epithelium) are not specific to AD patients,28,29 and “downstream” areas (e.g., mesial temporal lobe) are only beginning to receive attention for their role in impaired olfaction.30 Conversely, there is no current neuropathological evidence to suggest that the entorhinal cortex or other olfactory system components would be affected in MD. Although the entorhinal cortex or other parts of the olfactory system theoretically could be affected in VaD, they are not consistent sites for vascular damage.
The present study also replicates the findings of Solomon et al.5 and McCaffrey et al.20 in that a cutoff score of two or more errors on the PST yielded the optimal classification rate, correctly classifying 100% of the AD and depressed subjects. Such a finding, however, does not support the use of the PST as the sole indicator in the differential diagnosis of AD vs. VaD vs. MD. Rather, it does support the use of the PST as a screening measure that may augment a clinician's assessment and test battery if such differential diagnosis questions arise. Unfortunately, little research has been reported indicating the value of olfactory screening for such differential diagnosis purposes. For example, a recent review of the differential diagnosis between AD, VaD, and MD (as well as other types of dementias) in the elderly reported the differences between these groups across several neuropsychological domains (e.g., memory, language, visuospatial abilities); however, information concerning the differences between these groups in odor identification was neglected.31
Results were consistent with prior research5,20 in that the effects of age, gender, and education showed minimal impact on the PST for all three patient groups, yielding no statistically significant correlations. The findings suggest that these demographic variables do not systematically affect PST performance. Significant correlations were noted, however, between education and MMSE scores in the AD and VaD groups. These correlations indicate that education accounted for approximately 35% to 46% of the variance in MMSE scores for the AD and VaD groups. Therefore, interpretations of MMSE scores are not as straightforward as interpretations of PST scores with these patient groups.
The potential impact of medication and smoking on odor identification skills has been addressed in various patient populations, but with few definitive findings. Whereas the majority of studies have found no relationship between medication usage and odor identification in various patient groups (e.g., AD,32 nondemented elderly,33 schizophrenia34), Gross-Isseroff et al.35 found improved olfactory sensitivity (not odor identification) in depressed patients after 6 weeks of antidepressant pharmacotherapy. In the current study, of the three groups, only the AD group showed a significant relationship between medication usage and PST scores, in that lower PST scores were related to less anticholinergic medication use. This unexpected finding may arise from the medication variable's having been dichotomized (yes/no) rather than viewed continuously (e.g., dosage of medications). No significant relationships were observed between patients' smoking status and PST scores in any of the groups, consistent with previous work.2,11,34–36 Future studies should include smoking and medication variables (e.g., dosages of medications, other non-anticholinergic medications) to further assess these possible multivariate relationships.
As noted in Solomon et al.5 and McCaffrey et al.,20 only 10% of the total sample in the present study reported any awareness of olfactory decline. Olfactory testing (PST), however, indicated objective evidence of odor identification deficits (i.e., PST score of <2 correct) in 100% of the AD patients, 15% of the VaD patients, and 0% of the depressed patients. Across groups, AD patients tended to show the poorest insight into their olfactory functioning. Only 10% of AD patients reported olfactory functioning consistent with objective testing results, compared with 90% of VaD and 85% of MD patients. As concluded in prior studies, patient report of olfactory change may be unreliable, and formal testing is warranted.
Although building on the works of Solomon et al.5 and McCaffrey et al.,20 the present study has a number of other limitations that should be noted. First, the cognitive evaluation of the patients used in the study was limited to a screening device (MMSE). A more thorough neuropsychological evaluation would have better characterized the status of each group as to level of impairments. Neuropsychological testing might also have yielded more impressive diagnostic accuracy. Similarly, the PST is a screening measure, and a more thorough olfactory assessment (e.g., UPSIT, olfactory threshold, olfactory memory testing) might have led to better classification accuracy. A second shortcoming was the lack of a more objective quantification of the VaD and MD groups. For example, Hachinski ratings or Hamilton Rating Scale for Depression scores would have been useful for the vascular dementia and major depression groups, respectively. Future studies should more clearly define groups by using such measures. Again, we must emphasize that all patients in the AD group are cases of Probable AD; neuroradiological evidence (e.g., positron emission tomography) and neuropathological confirmation (e.g., autopsy results) is lacking. Any misdiagnoses could certainly alter the results of this study. Finally, whereas the present study explored the relationship between olfactory and cognitive functioning, comparisons of the PST with other types of functioning that are also impaired in AD patients could be of additional value to the clinician. For example, mood, activities of daily living, and physical functioning and their relationship to olfactory capacities may further empirically discriminate AD groups from non-AD groups.
It should be noted that despite these encouraging results, the PST has not yet faced the experimental challenge of discriminating previously undiagnosed clinical cases (e.g., cases of depressive pseudodementia, Lewy body disease, or dementia of mixed etiology). In this study and in previous research,5,20 the PST has been used to differentiate well-established cases of AD, VaD, and MD. Our procedure, however, follows the standard for developing a diagnostic test in medicine, which is to see how well the test discriminates between clear-cut instances of potentially similar/different conditions. This study supports the potential utility of the PST. The next step would be to test the PST with clinical cases presenting with mixed affective and cognitive symptoms, as well as with various types of dementia, by comparing them with the findings of the established cases in the present study. Similarly, future studies might follow patients longitudinally to assess PST performance in the different groups over time.
The results of the present study provide neuropsychologists, neuropsychiatrists, neurologists, and primary care physicians with a helpful diagnostic indicator in the differential diagnosis of Alzheimer's disease versus vascular dementia versus major depression. The PST is a brief, portable, and user-friendly screening measure that has been used successfully to discriminate between these three groups, which often present with similar clinical pictures. PST performance has direct implications for safety, independent functioning, and quality of life. Interpretation of PST scores appears straightforward, since the impact of age, gender, and education are negligible for these patient groups. The assessment of olfactory functioning continues to provide valuable information for the clinician in the differential diagnosis of AD versus VaD versus MD and in the delineation of subsequent treatment interventions.
ACKNOWLEDGMENTS
Support for this research came in part from an unrestricted research grant from Pfizer Pharmaceutical and Eisai, Inc. Portions of this paper were presented at the 20th Annual Meeting of the National Academy of Neuropsychology, Orlando, FL, November 14–18, 2000.
 |
 |
1 Mesholam RI, Moberg PJ, Mahr RN, et al: Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol 1998; 55:84-90Crossref, Medline, Google Scholar
2 Moberg PJ, Doty RL, Mahr RN, et al: Olfactory identification in elderly schizophrenia and Alzheimer's disease. Neurobiol Aging 1997; 18:163-167Crossref, Medline, Google Scholar
3 Serby M, Larson P, Kalkstein DS: The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry 1991; 148:357-360Crossref, Medline, Google Scholar
4 Solomon GS: Anosmia in Alzheimer disease. Percept Mot Skills 1994; 79:1249-1250Crossref, Medline, Google Scholar
5 Solomon GS, Petrie WM, Hart JR, et al: Olfactory dysfunction discriminates Alzheimer's dementia from major depression. J Neuropsychiatry Clin Neurosci 1998; 10:64-67Link, Google Scholar
6 Doty RL: The Smell Identification Test Administration Manual, 3rd edition. Haddon Heights, NJ, Sensonics, 1995Google Scholar
7 Hawkes CH, Shepard BC, Daniel SE: Olfactory dysfunction in Parkinson's disease. J Neurol Neurosurg Psychiatry 1997; 62:436-446Crossref, Medline, Google Scholar
8 Bylsma FW, Moberg P, Doty RL, et al: Odor identification in Huntington's disease patients and asymptomatic gene carriers. J Neuropsychiatry Clin Neurosci 1997; 9:598-600Link, Google Scholar
9 Moore AB, Paulsen JS, Murphy C: A test of odor fluency in patients with Alzheimer's and Huntington's disease. J Clin Exp Neuropsychol 1999; 21:341-351Crossref, Medline, Google Scholar
10 Jones B, Moskowitz H, Butters N: Olfactory discrimination in alcoholic Korsakoff patients. Neuropsychologia 1975; 13:173-179Crossref, Medline, Google Scholar
11 Westervelt HJ, McCaffrey RJ, Cousins JP, et al: Longitudinal analysis of olfactory deficits in HIV infection. Arch Clin Neuropsychol 1997; 12:557-565Crossref, Medline, Google Scholar
12 Sajjadian A, Doty RL, Gutnick DN, et al: Olfactory dysfunction in amyotrophic lateral sclerosis. Neurodegeneration 1994; 3:153-157Google Scholar
13 Elian M: Olfactory impairment in motor neuron disease: a pilot study. J Neurol Neurosurg Psychiatry 1991; 54:927-928Crossref, Medline, Google Scholar
14 Fedoroff I, Stoner SA, Andersen AE, et al: Olfactory dysfunction in anorexia and bulimia nervosa. International Journal of Eating Disorders 1995; 18:71-77Crossref, Medline, Google Scholar
15 Amsterdam JD, Settle RG, Doty RL, et al: Taste and smell perception in depression. Biol Psychiatry 1997; 22:1477-1481Google Scholar
16 Kopala LC, Clark C, Hurwitz T: Olfactory deficits in neuroleptic-naïve patients with schizophrenia. Schizophr Res 1992; 8:245-250Crossref, Google Scholar
17 Warner M, Peabody C, Cserninsky J: Olfactory functioning in schizophrenia and depression. Biol Psychiatry 1990; 27:457-467Crossref, Medline, Google Scholar
18 Sensonics, Inc.: The Pocket Smell Test. Haddon Heights, NJ, Sensonics, Inc., n.d.Google Scholar
19 Sensonics, Inc.: The Smell Identification Test. Haddon Heights, NJ, Sensonics, Inc., n.d.Google Scholar
20 McCaffrey RJ, Duff K, Solomon GS: Olfactory dysfunction discriminates probable Alzheimer's dementia from major depression: a cross-validation and extension. J Neuropsychiatry Clin Neurosci 2000; 12:29-33Link, Google Scholar
21 Knupfer L, Spiegel R: Differences in olfactory test performance between normal aged, Alzheimer and vascular-type dementia individuals. Int J Geriatr Psychiatry 1986; 1:3-14Crossref, Google Scholar
22 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
23 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
24 Braak H, Braak E: Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 1991; 82:239-259Crossref, Medline, Google Scholar
25 Pearson RC, Esiri MM, Hiorns RW, et al: Anatomical correlates of the distribution of the pathologic changes in the neocortex in Alzheimer's disease. Proc Natl Acad Sci USA 1985; 82:4531-4534Crossref, Medline, Google Scholar
26 Kovacs T, Cairns NJ, Lantos PL: Beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer's disease. Neuropathol Appl Neurobiol 1999; 25:481-491Crossref, Medline, Google Scholar
27 Davies DC, Brooks JW, Lewis DA: Axonal loss from the olfactory tracts in Alzheimer's disease. Neurobiol Aging 1993; 14:353-357Crossref, Medline, Google Scholar
28 Feldman JI, Murphy C, Davidson TM, et al: The rhinologic evaluation of Alzheimer's disease. Laryngoscope 1991; 101:1198-1202Crossref, Medline, Google Scholar
29 Tabaton M, Cammarata S, Mancardi GL, et al: Abnormal tau-reactive filaments in olfactory mucosa in biopsy specimens of patients with probable Alzheimer's disease. Neurology 1991; 41:391-394Crossref, Medline, Google Scholar
30 Kareken DA, Doty RL, Moberg PJ, et al: Olfactory-evoked regional cerebral blood flow in Alzheimer's disease. Neuropsychology 2001; 15:18-29Crossref, Medline, Google Scholar
31 Rosenstein LD: Differential diagnosis of the major progressive dementias and depression in middle and late adulthood: a summary of the literature of the early 1990s. Neuropsychol Rev 1998; 8:109-167Crossref, Medline, Google Scholar
32 Kesslak JP, Cotman CW, Chui HC, et al: Olfactory tests as possible probes for detecting and monitoring Alzheimer's disease. Neurobiol Aging 1988; 9:399-403Crossref, Medline, Google Scholar
33 Ship JA, Pearson JD, Cruise LJ, et al: Longitudinal changes in smell identification. J Gerontol A Biol Sci Med Sci 1996; 51A:M86-M91Google Scholar
34 Kopak L, Good K, Honer WG: Olfactory hallucinations and olfactory identification ability in patients with schizophrenia and other psychiatric disorders. Schizophr Res 1994; 12:205-211Crossref, Medline, Google Scholar
35 Gross-Isseroff R, Luca-Haimovia K, Sasson Y, et al: Olfactory sensitivity in major depressive disorder and obsessive compulsive disorder. Biol Psychiatry 1994; 35:798-802Crossref, Medline, Google Scholar
36 Houlihan DJ, Flaum M, Arnold SE: Further evidence for olfactory identification deficits in schizophrenia. Schizophr Res 1994; 12:179-182Crossref, Medline, Google Scholar



