Behavioral and Electrophysiological Indices of Temporal Processing Dysfunction in Schizophrenia
Abstract
Timing deficits in schizophrenia have been noted in several behavioral studies. However, the involvement of mediating factors, such as inattention, has not been ruled out as contributing to these effects. Mismatch negativity (MMN), an electrophysiological measure, may provide a more direct index of stimulus processing ability in individuals with schizophrenia. The current study explored the relationship between behavioral time judgments and a time-based MMN paradigm. Participants were administered two MMN paradigms consisting of an “easy” or “difficult” deviant and an analogous behavioral measure of time processing. Matched against a healthy comparison group, patients exhibited decreased MMN amplitude on the “difficult” deviant interval only. However, on the behavioral paradigm, the patients made significantly more errors across all conditions. These results suggest that behavioral measures of time processing may reflect different processes than those captured by preattentive physiological measures in this population.
Researchers have argued that time processing is a fundamental component of daily goal-oriented behaviors.1 More specifically, time processing has been associated with the ability to sequence, plan events, and understand “warning signals” that predict later events.2,3 Navon argued that our perception of the world consists of a hierarchy of dimensions and that time occupies the highest level of that hierarchy.4
Given the possible impact of temporal processing dysfunction on cognition and daily behavior, researchers have attempted to understand the role of timing dysfunction in clinical populations for a number of years. Early research assessing timing abilities in individuals with schizophrenia provided the first indicators that time estimation may be dysfunctional in this population.5 More recent findings suggest that individuals with schizophrenia are impaired on both auditory and visual temporal processing tasks.6,7 Limitations including small sample sizes and disparity in tasks described as “temporal processing” measures have made general assertions about time processing difficult. The degree of nontemporal information that has historically been included in timing tasks is a further limitation. Poynter and Homa point out that temporal perception tasks often require the subject to attend to nontemporal information, which likely affects temporal processing performance.8 Specifically, judging the duration of complex stimuli presentation (e.g., words and nonsense words) versus interstimulus intervals or simple stimuli (e.g., duration of blank screen between screens with a dot or cross) significantly affects one’s ability to accurately estimate elapsed time. Whether differences in timing performance are due to variations in attentional demands or other cognitive resources, the variability of stimuli utilized across temporal processing tasks may have led to disparate findings on tasks that are theorized to measure the same construct.
In view of these factors, our initial behavioral studies assessed temporal processing by manipulating interstimulus interval durations only, rather than by manipulating the duration of various types of stimuli.9,10 Significantly impaired performance was noted in the schizophrenia group across multiple conditions. Specifically, the patient group committed significantly more errors than comparison subjects on a variety of interstimulus interval durations ranging from approximately 15%–25% deviant from the standard interval duration over three interspike interval (ISI) duration conditions (e.g., 500, 1000, and 3000 msec ISI). While these initial findings may represent temporal deficits, they also may reflect other types of dysfunctional processes that contribute to poor performance on behavioral tasks in schizophrenia. Specifically, global deficits in motivation, attention to time-dependent features, and mediating cognitive processes may also affect behavioral performances in schizophrenia.11 To address these potential confounders and to explore the possibility of a physiological contribution to these deficits, a preattentive physiological measure of brain activity elicited by temporal variations in auditory stimuli was employed in the present study. In addition, participants completed an analogous behavioral task to assess whether behavioral performance is proportional to the physiological measures or whether there is a dissociation between tasks or differing relationships dependent on group membership. Analyzing these relationships may elucidate whether the preattentive processes involved in time processing are the same processes used when making a temporal judgment. Specifically, is the impairment in schizophrenia due to early physiological processing of time information or are deficits only apparent when these individuals are required to actively make decisions regarding their perception of time? Mismatch negativity (MMN) was utilized as a physiological measure of preconscious temporal processing. MMN is elicited in response to infrequent auditory deviant stimuli in the context of recurring standard stimuli.12 A benefit of MMN is the fact that it is generated in the absence of focused attention or motor response and therefore may be less affected by deficits in other cognitive processes.13 MMN represents the process of automatic detection of auditory changes in stimuli and exhibits maximal amplitude at frontal and central sites of the scalp, with the main generators located in the auditory and frontal cortices.12,14 The frontal contribution to this waveform is hypothesized to represent an involuntary switch of attention to specific changes in the sensory environment.15
Previous MMN studies have typically involved deviations in pitch, intensity, frequency or duration of tones.16,17 However, MMN can also be elicited by changes in interstimulus interval duration, absent of any other changes in the properties of the tone itself.18 The finding that MMN amplitude is proportional to the relative discriminability of interstimulus interval deviations suggests that MMN corresponds to preattentive neural activity which is subsequently available for the conscious perception of time during temporal discrimination tasks.19
In schizophrenia, results have overwhelmingly suggested generalized MMN abnormalities. Utilizing various deviant stimulus dimensions, the vast majority of studies have reported reduced MMN amplitude in this patient population20 while only a few studies have not noted a deficit.21,22 It should be noted that in the studies for which there were no differences between groups, a deviant stimulus was utilized and was substantially more dissimilar from the standard than the deviant stimuli used in studies for which differences were noted (e.g., 50% and 60% difference in frequency from standard). This is consistent with the assertion that studies focusing on clinical populations may benefit from inclusion of a wide range of difficulty levels to detect whether dysfunctional performance reflects a gross inability to perform a specific type of task or whether deficits are only noted on more difficult conditions within the same task.23
The current study explored the relationship between a preattentive measure (MMN) of temporal processing and an analogous behavioral measure. Given that individuals with schizophrenia may be more susceptible to confounding effects of attentional and motivational deficits on behavioral measures, it was hypothesized that a less robust correlation would exist between MMN indices and behavioral performances in individuals with schizophrenia when matched against comparison subjects. Second, the vast majority of MMN studies have found reduced amplitudes in patients with schizophrenia, suggesting a general cortical auditory information processing deficit.24 Our study examined performances on a difficult-to-detect deviant and an easy-to-detect deviant to assess whether there is a general impairment in sensory processing of temporal information in schizophrenia or if there are specific MMN amplitude deficits that are only evident when a wider range of deviants is administered.
METHODS
Participants
Participants consisted of 15 subjects who met DSM–IV criteria for schizophrenia, confirmed via a structured interview.25 Thirty healthy comparison subjects were recruited from the community. Respondents were screened for psychiatric histories. Individuals were excluded for a current diagnosis of major depression, substance abuse, neurological disorders, head trauma, or for any personal or first-degree family member history of psychosis. All subjects gave written consent for participation, as approved by an appropriate Institutional Review Board, and were paid $30 for participation. Given that the use of typical neuroleptics may be associated with adverse effects on timing tasks, only subjects treated with atypical neuroleptics were selected.26 Fourteen subjects were treated with either olanzapine or risperidone; 1 subject was unmedicated.
Previous research suggests that there may be an effect of age on MMN amplitude.27 Mean years of age between groups was not significantly different (t=−1.37, p=0.18). The mean age of participants in the schizophrenia group was 39.3 years (SD=10.7, range=21–55 years). Mean age for comparison subjects was 34.3 years (SD=10.6, range=23–55 years).
Mismatch Negativity Procedure
Gold-plated electrodes were attached to the following 10–20 scalp locations: Fz, Cz, Pz, left mastoid, right mastoid. Brain activity evoked by auditory stimuli was referenced to an electrode attached to the nose, bandpass filtered from .05 to 30 Hz, and digitally sampled at 1000 Hz. A ground electrode was attached to the forehead. Eye movements were monitored with electrodes attached above and directly lateral to the left eye. Headphones were used to binaurally present pure tones (1000 Hz, 50 msec in duration) to the subjects. Brain evoked responses were collected in a “passive” condition. Subjects were asked to watch a closed-captioned movie. Participants began watching a movie during set-up and were instructed to continue watching the movie and ignore the tones during the recordings. It should be noted that recently researchers have argued that in some situations, MMN may be modulated by attention.28 Müller et al., suggest that concurrent tasks during MMN recordings that direct attention toward auditory stimuli affect the MMN amplitude. This effect does not appear when subjects are administered a concurrent visual task, such as a silent closed-captioned movie.
Each participant was administered two conditions utilizing either an “easy-to-detect” deviant or “difficult-to-detect” deviant. Each condition consisted of 4000 tones with a 500 msec standard interval between tones and a deviant interval occurring on average every 20th interval. In the easy condition, the deviant ISI was 250 msec, representing a 50% change from the standard. In the difficult condition, the deviant ISI was 425 msec, representing a 15% change from the standard. Administration order of “easy” and “difficult” deviant condition were counterbalanced.
MMN Data Analysis
Continuously recorded signals were separated into 500-ms epochs with a 100-ms prestimulus interval relative to timing pulses. Tone onset served as time 0. Baseline correction involved subtracting average voltage of the 100 msec prestimulus interval and was applied to each single trial. Trials for which any channel exceeded (SD=75microV) were removed from further analysis. The number of sweeps remaining after artifact rejection did not differ significantly between comparison subjects and schizophrenic subjects for either standard or deviant waveforms (mean total trials for both conditions: comparison subjects = 4707; schizophrenia subjects = 4800) (Figure 1). Following rejection of artifact, standard and deviant evoked responses were averaged separately off-line for each subject. Standard evoked response averages were subtracted from deviant evoked response averages, and the MMN amplitude was defined as the peak negativity between 140 and 210 msec poststimulus latency range in “ subtraction” waveforms (deviant minus standard) (Figure 2).
Behavioral Procedure
All participants completed a behavioral measure of temporal processing following MMN recordings. In short, subjects compared interval durations in successive pairs of tones. A total of two hundred sets of tones were administered. The first pair of tones in each set was used as a standard interval. Each tone was 50 msec in duration (similar to the MMN paradigm), and the initial time between the first pair of tones was consistently 500 msec. Five hundred milliseconds after the initial pair, the subject heard a second experimental pair of tones. In 100 of the trials, the second interval was similar to the difficult interval used in the MMN paradigm, 15% different from the standard. However, in the MMN paradigm, only deviants 15% shorter than the standard are assessed (e.g., 425 msec compared to 500 msec standard). On the behavioral paradigm, half of the difficult deviants were 75 msec shorter than the standard (e.g., 425 msec) and half of the deviants were 75 msec longer (e.g., 575 msec) than the standard. On the other one hundred trials, the second interval was matched to the easy deviant in the MMN paradigm, 50% different than the standard. Again, unlike the MMN paradigm, in which only deviants 50% shorter than the standard are assessed (e.g., 250 msec), the behavioral paradigm assessed deviant intervals that were both shorter (e.g., 250 msec) and longer (e.g., 750 msec) than the standard. The presentation order for the deviant interval durations was randomized over 200 trials. The subject was asked to manually respond by pressing the “S” key if the second interval was “shorter” than the first and the “L” key if the second interval was longer than the first.10
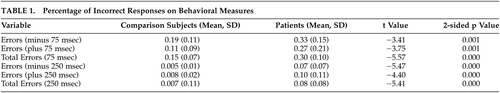
Statistical results were analyzed using Student’s t tests to determine between-group differences in mean errors on all conditions (e.g., plus or minus 75 msec, plus or minus 250 msec) (Table 1). The schizophrenia group committed significantly more errors across all conditions with no significant differences due to the direction of the temporal deviant (e.g., shorter intervals versus longer intervals).
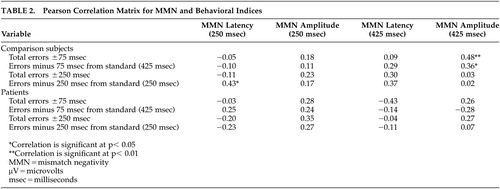
The behavioral results are presented in relationship to MMN amplitude in Table 2.
RESULTS
Mismatch Negativity
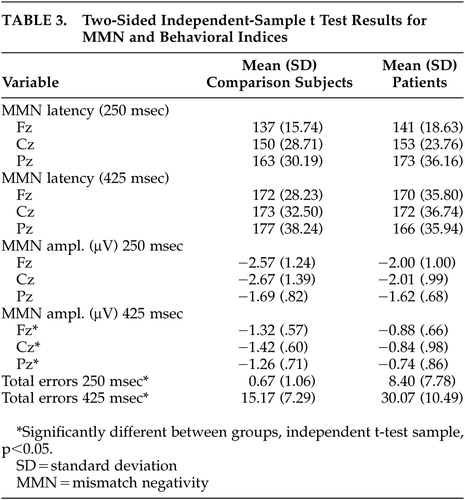
To test for possible group (comparison group; schizophrenia patients) differences in the MMN waveform, amplitude and latency were separately analyzed by Greenhouse-Geisser corrected repeated measures ANOVA with electrode (Fz; Cz; Pz) and difficulty (“easy,” 250 msec deviant; “difficult,” 425 msec deviant) as within-subject factors. Significant main effects were found for electrode (F=10.87, p<0.001) and condition (F=38.30, p<0.001), where MMN amplitude was generally largest at frontocentral sites (Cz, Fz) and during the easy condition (Table 3). The latter finding is consistent with previous studies demonstrating that a larger temporal deviance in the ongoing stimulus train produces a larger MMN waveform.29 A significant effect was also found for group (F=5.792, df=1, 43, p=0.02), where comparison subjects generally exhibited larger mean MMN amplitudes (marginal mean=1.82 μV) than patients (1.35 μV). Planned t tests conducted separately across electrodes and conditions revealed significant between-group differences for the difficult (425 msec deviant) condition at Fz (t=−2.28, p=0.02), Cz (t=2.44, p=0.02), and Pz (t=−2.18, p=0.04) but not for the easy (250 msec) condition: Fz (t=−1.54, p=0.13), Cz (t=−1.63, p=0.12), Pz (t=−0.30, p=0.77).
Main effects for MMN latency were also found for electrode (F=10.46, p<0.001) and condition (F=9.64, p=0.003), where waveforms tended to peak earlier at frontocentral sites and in the easy condition (Table 3). No effect of group was found for this variable (F=0.012, df=1, 43, p=0.91). This lack of significant group difference in latency suggests that the differences in MMN amplitude noted between groups reflect the ERP activity associated with detection of the deviant stimuli rather than a general deficit related to processing the auditory stimuli.30
Behavioral Performance Data
Comparison subjects performed considerably better than the patients on both conditions (p <0.000). However, a proportional relationship between MMN amplitude elicited by temporal variation and perceptual threshold for temporal cues measured via behavioral response was noted only on the difficult deviant. Additionally, this linear relationship only existed for the comparison group on the total behavioral errors for the difficult deviant and the analogous minus 75 msec behavioral deviant (r=0.36; p<0.05). Specifically, reduced MMN amplitude is related to lower percentage of correct behavioral responses on the difficult deviant. However, in the schizophrenia group, the relationship between behavioral performance and MMN amplitude was not as clear (r=0.28; r=0.11).
There were no statistically significant correlations between MMN amplitude and behavioral errors for the easy deviant condition in either group. The relationship between MMN amplitude and behavior on the easy deviant in the schizophrenia group appeared to be more robust than the relationship on the difficult deviant (Table 2). However, the correlation between errors and MMN amplitude did not reach statistical significance for either the analogous paradigm measuring errors minus 250 msec from the standard or for total errors either plus or minus 250 msec from the standard. It is important to note that the correlation (r=0.28) between behavioral performance and MMN amplitude on the easy condition approached statistical significance in the patient population. Therefore, it cannot be ruled out that in a larger sample of participants with schizophrenia, a stronger trend toward significance on the easy condition would be revealed. In our sample, however, the data suggest that a minority of individuals (N=3) in the schizophrenia group exhibited a linear relationship between behavioral errors and MMN amplitude similar to the comparison group. The majority of individuals made significant behavioral errors even though MMN amplitudes were similar to comparison subjects.
DISCUSSION
In the past, temporal processing has been measured behaviorally in individuals with schizophrenia. Various types of measures assessing temporal perception, temporal estimation and time reproduction have all identified differences in the ability to process time between patients with schizophrenia and healthy comparison subjects.7,9,10,31 A concern that has arisen regarding behavioral measures of cognition in the study of schizophrenia is that due to possible confounding effects, such as poor motivation or attention, performance on cognitive measures in this population may not truly reflect specific task-related dysfunction.32 Rather, sustaining attention and motivation throughout the task may affect the outcome on behavioral measures.33,34 In an attempt to control for these possible confounders, MMN was utilized to assess temporal processing. To address the differing patterns of results noted in the MMN literature in schizophrenia, we assessed MMN amplitude in response to a “difficult” duration deviant (15%) versus an “easy” duration deviant (50%).
Results suggest that individuals with schizophrenia do exhibit a deficit in temporal processing measured via MMN, consistent with previous research.35 However, a generalized early processing deficit was not noted. On the electrophysiological measures, poor temporal processing was only noted for the more difficult paradigm. Regarding the relationship between behavioral performance and MMN amplitude, a dissociation was noted between groups. In comparison subjects, a proportional relationship between behavioral temporal processing performance and MMN was noted on the more difficult deviant. However, there was not a clear relationship between behavior and MMN amplitude on the easy deviant. An analysis of the data suggests a ceiling effect regarding the comparison subjects’ behavioral performances. While there was a range of amplitude values in this group, there was not a range of errors on the behavioral measure. Of the 30 comparison subjects, 26 either made no errors or a single error out of 200 trials. The remaining comparison subjects made no more than four total errors. Therefore, it is possible that the improved performance on the behavioral task compared to the physiological measure, which is apparent in a number of the comparison subjects, is due to mediating cognitive factors that can be employed on the behavioral task. It may be that increased vigilance to the task or the development of response strategies may improve behavioral performance in this population.
Behavioral performances by the individuals with schizophrenia appeared to be impaired, regardless of their MMN amplitude, for both the easy and difficult deviants. While there was variability in MMN amplitude within this group, behavioral performances appeared impaired even in individuals with relatively higher MMN amplitudes. Performance on the easy deviant was significantly better than performance on the difficult deviant within the schizophrenia group. However, even though the easy deviant MMN amplitudes were similar to comparison subjects, the number of behavioral errors persisted in this group independent of MMN amplitude. While comparison subjects exhibit improved behavioral performance relative to MMN indices, it may be that behavioral measures are disproportionately affected in the opposite direction in schizophrenia by mediating cognitive variables that may be impaired in this population.
Salisbury et al. suggest that the use of traditional behavioral measures of cognition in conjunction with electrophysiological measures of brain activity allows a unique multifaceted examination of behavior and the underlying processes.36 Differing patterns of performance on electrophysiological measures and behavioral measures have been noted previously in individuals with schizophrenia.17,37 However, using an interstimulus interval deviant on both a behavioral index and an analogous MMN measure has not previously been assessed. The combination of methods in the current study has shed additional light on temporal processing in schizophrenia.
The relationship between MMN and behavioral performance is of particular interest due to the putative lack of confounding cognitive factors associated with MMN measures. Our findings suggest that assessing cognition via behavioral measures and assessing preattentive stimulus processing by MMN measures may lead to disparate findings. Specifically, a dual assessment of electrophysiological indices and behavioral measures may provide insight into how the brain processes information and how the individual interprets that information. While MMN may provide useful insight into the physiological early processing of temporal information, the behavioral assessment complements that knowledge by showing us how temporal processing deficits are manifested on behavioral tasks that may better reflect everyday functioning for the patient.
The current results also suggest that MMN may be ideal when measuring processing on graded tasks. Specifically, a wide range of difficulty levels may allow for examination of differential thresholds in cognitive processes between comparison subjects and schizophrenia patients. Our results do not suggest a generalized MMN generation deficit regarding temporal processing, but rather a select impairment in MMN generation dependent on the level of duration detection difficulty.
Temporal processing is a type of cognition that has been studied, for the most part, at a behavioral level. In other clinical populations, researchers have asserted that time processing likely influences performance on a number of higher level cognitive processes and contributes to behavioral problems that are attributed to other characteristics, such as inattention or impulsivity.36,38 Given the possible importance of temporal processing deficits in daily functioning and on behavior, it appears that research assessing temporal processing in schizophrenia is warranted. Our findings suggest that temporal processing is impaired at a very early processing level in schizophrenia. However, the degree of impairment is not clear. Based on performance assessed at two very different levels of difficulty, results suggest that behavioral deficits are present across conditions while physiological processing of the “easy” temporal stimuli is not significantly different from comparison subjects.
One implication is that employing behavioral and physiological measures of cognition may provide greater information about different thresholds in different populations when multiple levels of difficulty are employed. Also, the role of attention, motivation, and/or other mediating cognitive factors cannot be minimized when examining behavioral indices of cognition in populations that may be more affected by impairments in these areas. In addition, utilizing both methodologies points out the importance in acknowledging that there may be a difference between effects that are assessed physiologically and those that are seen in everyday living.
While the current results shed some light on time processing in schizophrenia, further research is needed that focuses on the specific types of time processing deficits in this population, the physiology related to these deficits and the implications for daily living.
ACKNOWLEDGMENTS
Portions of this paper were presented at the Annual Meeting of the Cognitive Neuroscience Society, San Francisco, April 18, 2004.
This study was supported in part by the U.S. Department of Veterans Affairs and the following grants: Developmental Psychobiology Endowment Fund and Public Health Services Grants MH-56539 and MH-152442.
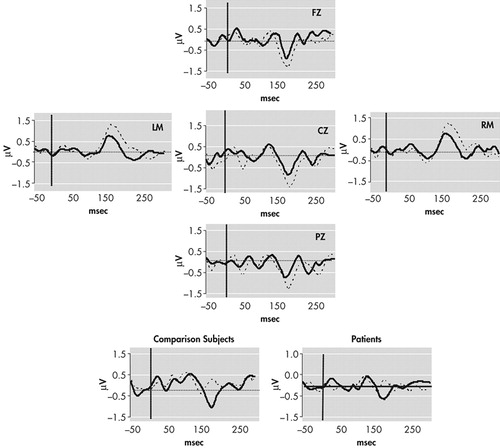
FIGURE 1. Grand Average MMN Waveforms for Comparison Subjects (dotted line) and Patients (solid line) Evoked for 425 msec Deviant Interval Compared to the 500 msec Standard Interval
Negativity is plotted down. The insets show the standard (dotted line) and deviant (solid line) group average waveforms at FZ. Please note that a different scale (SD 1.5 μVs) was used in Figure 1 compared to Figure 2 (SD 3.0 μVs) to accentuate the presentation of the waveforms.
μV=microvolts
msec=milliseconds
MMN=mismatch negativity
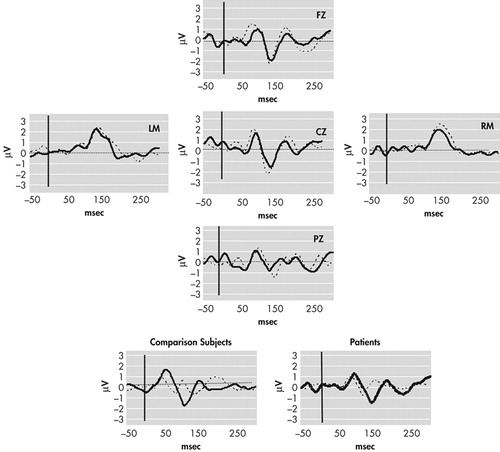
FIGURE 2. Grand Average MMN Waveforms for Comparison Subjects (dotted line) and Patients (solid line) Evoked for 250 msec Deviant Interval Compared to the 500 msec Standard Interval
Negativity is plotted downward. The insets show the standard (dotted line) and deviant (solid line) group average waveforms at FZ.
μV=microvolts
msec=milliseconds
 |
 |
 |
1 Mangels JA, Ivr RB: Time perception, in The Handbook of Cognitive Neuropsychology, Edited by Rapp B. Philadelphia, Psychology Press, 2001, pp 467-493Google Scholar
2 Tracy JI, Monaco C, McMichael H, et al: Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Percept Mot Skills 1998; 86:515–526Crossref, Medline, Google Scholar
3 Volz H, Nenadic I, Gaser C, et al: Time estimation in schizophrenia: an FMRI study at adjusted levels of difficulty. Neuroreport 2001; 313-316Google Scholar
4 Navon D: On a conceptual hierarchy of time, space, and other dimensions. Cognition 1978; 6:223–228Crossref, Google Scholar
5 Rabin AI: Time estimation of schizophrenics and non-psychotics. J Clin Psychol 1957; 13:88–90Crossref, Medline, Google Scholar
6 Schwartz BD, Mallott DB, Winstead DK: Preattentive deficit in temporal processing by chronic schizophrenics. Biol Psychiatry 1988; 23:664–669Crossref, Medline, Google Scholar
7 Tysk L: Estimation of time by patients with positive and negative schizophrenia. Percept Mot Skills 1990; 71:826Crossref, Medline, Google Scholar
8 Poynter WD, Homa D: Duration judgment and the experience of change. Perception & Psychophysics 1983; 33:549–560.Crossref, Google Scholar
9 Davalos DB, Kisley MA, Ross RG: Deficits in auditory and visual temporal perception in schizophrenia. Cognitive Neuropsychiatry 2002; 7:273–282Crossref, Google Scholar
10 Davalos DB, Kisley MA, Ross R: Effects of interstimulus interval manipulation on auditory temporal processing in schizophrenia. Brain and Cognition 2003; 52:295–301Crossref, Medline, Google Scholar
11 Brebion G, Amador X, Smith M, Malaspina D, Sharif Z, Gorman JM: Depression, psychomotor retardation, negative symptoms, and memory in schizophrenia. Neuropsychiatry, Neuropsychology & Behavioral Neurology 2000; 13:177–183Medline, Google Scholar
12 Näätänen R: Attention and Brain Function. Hillsdale, NJ, Erlbaum, 1992Google Scholar
13 Sussman E, Winkler I, Wang W: MMN and attention: competition for deviance detection. Psychophysiology 2003; 40:430–435Crossref, Medline, Google Scholar
14 Pulvermuller F, Shtyrov Y: Automatic processing of grammar in the human brain as revealed by the mismatch negativity. Neuroimage 2003; 20:159–172Crossref, Medline, Google Scholar
15 Deouell LY, Bentin S, Giard M: Mismatch negativity in dichotic listening: evidence for interhemispheric differences and multiple generators. Psychophysiology 1998; 35:355–365Crossref, Medline, Google Scholar
16 Baldeweg T, Klugman A, Gruzelier JH, Hirsch SR: Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. Int J Psychophysiology 2002; 43:111–122Crossref, Medline, Google Scholar
17 Todd J, Michie PT, Jablensky AV: Association between reduced duration mismatch negativity (MMN) and raised discrimination thresholds in schizophrenia. Clin Neurophysiology 2003; 114:2061–2070Crossref, Medline, Google Scholar
18 Näätänen R, Jiang D, Lavikainen J, Reinikainen K, Paavilainen P: Event-related potentials reveal a memory trace for temporal features. Neuroreport 1993; 5:310–312Crossref, Medline, Google Scholar
19 Kisley MA, Davalos DB, Layton HS, Pratt D, Ellis JK, Seger CA: Small changes in temporal deviance modulate mismatch negativity amplitude. Neuroscience Letters 2004; 358:197–200Crossref, Medline, Google Scholar
20 Michie PT: What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiology 2001; 42:177–194Crossref, Medline, Google Scholar
21 Kathmann N, Wagner M, Rendtorff N, Engel RR: Delayed peak latency of the mismatch negativity in schizophrenics and alcoholics. Biological Psychiatry 1995; 37:754–757Crossref, Medline, Google Scholar
22 O’Donnell BF, Hokama H, McCarley RF, Smith RS, Salisbury DF, Mondrow E, Nestor PG, Shenton ME: Auditory ERPS to non-target stimuli in schizophrenia: relationship to probability, task-demands, and target ERPS. Int J Psychophysiology 1994; 17:219–231Crossref, Medline, Google Scholar
23 Fletcher PC, McKenna PJ, Frith CD, Grasby PM, Friston KJ, Dolan RJ: Brain activations in schizophrenia during a graded memory task studied with functional neuroimaging. Archives of General Psychiatry 1998; 55:1001–1008Crossref, Medline, Google Scholar
24 Umbricht D, Koller R, Schmid L, Skrabo A, Grubel C, Huber T, Stassen H: How specific are deficits in mismatch negativity generation to schizophrenia? Biological Psychiatry 2003; 53:1120–1131Crossref, Medline, Google Scholar
25 Endicott J, Spitzer RL: A diagnostic interview: The schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35:837–844Crossref, Medline, Google Scholar
26 Meck WH: Selective adjustment of the speed of internal clock and memory processes. J Experimental Psychol: Animal Behavioral Processes 1983; 9:171–201Crossref, Medline, Google Scholar
27 Pekkonen E: Mismatch negativity in aging and in alzheimer’s and parkinson’s disease. Audiology & Neuro-Otology 2000; 5:216–224Crossref, Medline, Google Scholar
28 Müller BW, Achenbach C, Oades RD, Bender S, Schall U: Modulation of mismatch negativity by stimulus deviance and modality of attention. Neuroreport 2002; 13:1317–1320Crossref, Medline, Google Scholar
29 Kisley MA, Davalos DB, Layton H, Ellis J, Pratt J, Seger C: Small changes in stimulus deviance modulate mismatch-negativity amplitude. Neurosci Lett 2004; 358:197–200Crossref, Medline, Google Scholar
30 Catts SV, Shelley AM, Ward PB, et al: Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry 1995; 152:213–219Crossref, Medline, Google Scholar
31 Rammsayer T: Temporal discrimination in schizophrenic and affective disorders: evidence for a dopamine-dependent internal clock. Int J Neuroscience 1990; 53:111–120Crossref, Medline, Google Scholar
32 Brown RG, Pluck G: Negative symptoms: the pathology of motivation and goal-directed behaviour. Trends in Neurosciences 2000; 23:412–417Crossref, Medline, Google Scholar
33 Lieb K, Merklin G, Rieth C, Schuttler R, Hess R: Preattentive information processing in schizophrenia. Schizophrenia Research 1994; 14:47–56Crossref, Medline, Google Scholar
34 Savage RM, Jackson WT, Sourathathone CM: A brief neuropsychological testing battery for evaluating patients with schizophrenia. Community Ment Health J 2003; 39:253–262Crossref, Medline, Google Scholar
35 Davalos DB, Kisley MA, Ross R: Mismatch negativity in detection of interval duration deviation in schizophrenia. Neuroreport 2003; 14:1283–1286Crossref, Medline, Google Scholar
36 Salisbury DF, Shenton ME, Nestor PG, McCarley RW: Semantic bias, homograph comprehension, and event-related potentials in schizophrenia. Clin Neurophysiology 2002; 113:383–395Crossref, Medline, Google Scholar
37 Cappella B, Gentile R, Juliano DB: Time estimation by hyperactive and normal children. Percept Mot Skills 1977; 44:787–790Crossref, Medline, Google Scholar
38 Van Ingelghem M, Van Wieringen A, Wouters J, Vandenbussche E, Onghena P, Ghesquiere P: Psychophysical evidence for a general temporal processing deficit in children with dyslexia. Neuroreport 2001; 12:3603–3607Crossref, Medline, Google Scholar



